Genetic Analysis and Gene Fine Mapping of a Midrib-deficient Mutant dl-6 in Rice
2015-11-08JunWANGJingWANGJieYANGXiangqiangZHAOJinyanZHUFangjunFANWenqiLlFangquanWANGWeigongZHONG
Jun WANG,Jing WANG,Jie YANG,Xiangqiang ZHAO,Jinyan ZHU,Fangjun FAN,Wenqi Ll,Fangquan WANG,Weigong ZHONG*
1.Institute of Food Crops,Jiangsu Academy of Agricultural Sciences,Nanjing Branch of Chinese National Center for Rice Improvement,Nanjing 210014,China;
2.School of Life Sciences,Nantong University,Nantong 226007,China
Genetic Analysis and Gene Fine Mapping of a Midrib-deficient Mutant dl-6 in Rice
Jun WANG1,Jing WANG2,Jie YANG1,Xiangqiang ZHAO2,Jinyan ZHU1,Fangjun FAN1,Wenqi Ll1,Fangquan WANG1,Weigong ZHONG1*
1.Institute of Food Crops,Jiangsu Academy of Agricultural Sciences,Nanjing Branch of Chinese National Center for Rice Improvement,Nanjing 210014,China;
2.School of Life Sciences,Nantong University,Nantong 226007,China
To clone the DL-6 gene,positive and negative cross combinations were developed between dl-6 and 9311;based on the genetic analysis,it was found that drooping leaf was controlled by one recessive nuclear gene DL-6,DL-6 was primarily mapped on the short arm of chromosome 3 with SSR markers,finally the DL-6 gene was fine mapped in the 85 kb section between markers I3-5 and I3-8 using newly developed InDel marker;the open reading frames (ORFs)in the section were analyzed and found that YABBY gene coded by ORF9 might be a drooping leaf-related gene.YABBY genes in mutant dl-6 and in wild type were sequenced,and the sequencing results were compared with Nipponbare sequence,and showed that 1 bp mutation was found in first exon of YABBY gene in the mutant dl-6,which caused the coded cysteine of the wild type become the arginine of the mutant;at the same time,8 bp deletion was also found at 3’end of ORF9 gene.These two mutations which one was the functional mutation of dl-6 has been still uncertain and needed further research.
Rice;Midrib-deficient;Gene mapping;dl-6
R ice (Oryza sativa L.)is the most important crop in China,and two thirds of the population take it as the staple diet.With the increasing number of the population,improving rice yield is of great significance to food safety in China.90%-95%dry matter accumulation of rice is from photosynthesis,the leaf is the main organ for photosynthesis,the researches carried out by IRRI showed that CO2amount assimilated by all green leaves occupies 93.6%of plant assimilation amount,leaf sheath and stem occupies 4.3%,the ear only occupies 2.1%[1].Wu Dian et al.[2]carried out a correlation analysis on the yield and blade profile characteristics of 12 rice varieties and showed that the correlation coefficients between the yield and each blade profile character all reached significant or extremely significant level.Different rice planting regions have their own ideal types of rice,and also have different requirements to the characters of leaf shape,however,the ideal type of rice designed by almost all researchers at home and abroad was erect leaf[3-8].
The leaf of rice is long lanceolate,and a lot of longitudinal vascular bundles are evenly distributed in it,the leaf vein composed by longitudinal vascular bundles is also called parallel vein,and longitudinal vascular bundles are connected by connected longitudinal vascular bundles.The main vein in the middle of rice leaf is called midrib,the midrib is formed by cell proliferation in the middle area of the leaf,its structure is rigid and supports the leaf,thereby making the leaf erect and not droop,and playing the role of nutrient substance and water transportation[9]. Therefore,the formative molecular mechanism of leaf vein should be made clear to provide a theoretical basis and technical support for the ideal type of rice,thereby giving full play to the maximum output potency of rice.
For midrib-deficientmutant,its midrib is not formed,thereby causing the leaf drooping,this is a kind of relatively rare mutant.Therefore,the study on midrib-deficient mutant is of great significance to understanding the development of rice leaf.Now,a lot of midrib-deficient mutants of rice have been mapped or separated,and they have been all on the short arm of chromosome 3,it was also found that these genes had various effects on the development of leaf midrib and flower organs[10-12].In this study,a midrib-deficient mutant was found in the japanica rice Wuyunjing 7,the mutant lacked midrib,but the flower organs were normal,these were not uniformly the same as the previous reports on the phenotype of midrib-deficient mutants,the mutant was temporarily named as dl-6.The phenotype identification and physiological analysis on the mutant were carried out,and the fine mapping and cloning research on the gene using map-based cloning were conducted,so as to lay a foundation for deeply studying the molecular mechanism of vein development.
Materials and Methods
Experimental materials
The midrib-deficient mutant of rice was from the naturalmutation of japanica rice Wuyunjing 7,after many generations of self-cross breeding and selection,the direct phenotype of the mutant was leaf drooping and no obvious midrib structure,the phenotype had already been stable,thus it was named as dl-6.The other parent of genetic analysis and target group was indica rice 9311.
The observation of the microstructure of midrib-deficient mutant
Making the leaf and observing: the 1stleaves from the top of the mutant and the wild type were respectively selected during the tillering stage,the small fragments with the width of 1 cm and the length of 0.2 cm from the midribs of the two leaves were selected,then FAA stationary liquid(contained 5 ml formalin,5 ml glacial acetic acid and 90 mE 70%ethyl alcohol)was used to fix and ethyl alcohol was used to dehydrate;paraffin imbedding method was adopted,Leica RM2235 microtome was used,and the slice thickness was 8 m,the counterstaining of red and fast green (1%red and 0.5% fast green)was carried out,thereby the permanentslice was made;the leaf midribs of the mutant and the wild type were respectively carried out microscopic examination and taken photos for observing.During flowering period,the floral organs of midrib-deficient mutants were dissected and taken photos using the microscope for observing.
Genetic analysis and the construction of target group
In summer of 2011,positive and negative cross combinations were developed between dl-6 and 9311 in Nanjing,and the F1was planted in Hainan in the same year,and the seeds of F2were obtained.In summer of 2012,each parent and F2population were planted in Nanjing,the parent and the population both were individual planting;30 d after transplanting,the investigation and statistics on the normal plant and leaf drooping plant of F2population were conducted for genetic analysis.
DNA extraction and SSR analysis
During tillering stage, tender leaves were selected,genomic DNA of the parent and F2segregation population were selected using Dellaporta et al.[13].The microsatellite markers published by the predecessors were selected for carrying out gene mapping of midrib-deficient genes,SSR primer sequence wasquoted from http:// www.gramene.org/,the primer was compounded byInvitrogenTrading(Shanghai)Co.,Ltd..20 μl PCR reaction system contained 2.0 μl PCR buffer(contained Mg2+),2.0 μl dNTP(2.5 mmol/L),2.0 μl Primer(15 ng/μl),2 μl template DNA (about 15 ng/μl),0.2 μl Taq enzyme(5 U/μl)and 11.8 μl sterile double distilled water.Then it was amplified on Eppendorf’s fast PCR instrument,the reaction condition were 94℃5 min,94℃1 min,53℃ 1 min and 72℃ 1.5 min,there were totally 32 cycles;extending for 10minunder72℃,3.5%AGE(agarose gel electrophoresis)was used for detecting.
The development of lnDel marker
According to the research results of Shen et al.[14],the deleted or inserted loci(InDel)of 9311 and Nipponbare in the object regions were found,Primer Premier 5.0 was used to select≥10 bp deleted or inserted loci and design the upper and lower primers of PCR products with the amplified fragment length of 100-200 bp.The primer was compounded byInvitrogenTrading(Shanghai)Co.,Ltd.
The location method of midrib-deficient gene DL-6
The midrib-deficient gene DL-6 was mapped using BSA method proposed by Michelmore et al.[15].The normal plant and midrib-deficient plant in F2segregation population were divided into two groups,the DNAs of 10 plants in each group were mixed,and 2 gene pools of normal plant and midrib-deficientplantwere formed. The molecular marker was used to seek the primers with amplification differences in the two pools,then the midrib-deficient plant from the separation descendant was used to test and verify whether the primer linked to the target gene or not and the linkage distance. MAPMAKER (EXP3.0b)mapping software[16]was used to establish the physical map of the molecular linkage in the target gene region.
Results and Analyses
The phenotype of midrib-deficient mutant dl-6
The development of leaf midrib in midrib-deficient mutant dl-6 was inhibited,thereby lacking normal midrib midrib and can not support the self weight of the leaf,thus showing leaf drooping;the midrib of wild Wuyunjing 7 was normal,the leaf showed erect shape(Fig.1A).During flowering period,10 mutants were selected,10 glumous flowers from each plant were randomly selected for observing,and found that the development of the stamen and pistil of mutant dl-6 was normal(Fig.1B and C);for other agronomic traits,the mutant had not obvious difference with the wild type.
The microstructure of midrib of midrib-deficient mutant dl-6
During tillering stage,the leaves of wild Wuyunjing 7 and mutant dl-6 was carried out paraffin section and microstructure observation.The results showed that the leaf midrib of wild Wuyunjing 7 had 5 air cavities which were also called clear cells,they were formed by lots of dead cells after the sequencing in the central region of the leaf,the 5 air cavities formed a thin and hollow cylindrical structure from the base to the top of the leaf,there were many vascular bundles along the fringe which was in the front of the central fibrovascular bundle (Fig.2A);in the midrib-deficient mutant,there were a lot of vascular bundl-es in the vein,but the midrib was short of clear transparent cells and central fibrovascular bundle cells,the structures of central vein and lateral vein were completely similar(Fig.2B),these showed that the mutant did not form obvious midrib structure.
The genetic analysis of midrib-deficient mutant dl-6
The positive and negative cross combinations were developed between dl-6 and 9311,the F1showed the phenotype of normal parent,indicating that the midrib-deficient character was recessive inheritance,the midrib-deficient character was recessive for normal leaves.The F2segregation population was planted in Nanjing experimental field of Jiangsu Academy of Agricultural Sciences,the leaves were observed during the tillering stage,midrib-deficient leaves and normalleaves separated obviously and had not intermediate form,showing that the midrib-deficient character was controlled by major genes.The segregation ratio of normal leaves to midrib-deficient leaves in F2was 3∶1(Table 1),showing that the midrib-deficient character was controlled by a pair of recessive main nuclear genes. DL-6 gene mapping and cloning
In order to map the genes controlling midrib-deficient characters to the chromosomes of rice,taking F2population as the target group,and using BSA method,the midrib-deficient pool and normal leaf pool were established.The polymorphic analysis was carried out for the two pools using 628 pairs of SSR primers evenly covering the whole genome,then the linkage analysis on the midrib-deficient plant in F2segregation population in the two pools was carried out using polymorphic primers.The polymorphic analysis results of the whole genome showed that SSR markers RM523 and RM218 on the chromosome 3 showed polymorphism in the two pools.The 2 SSR primers were used to further detect the midrib-deficient plant in the F2segregation population,and found that there were different degrees of linkages between midrib-deficient gene DL-6 and the 2 molecular markers,thereby verifying that the gene was on the chromosome 3.MAPMAKER 3.0 software was used to carry out linkage analysis and the results showed that DL-6 was between the two primers(Fig.3A).To further fine map the gene,multiple pairs of SSR and InDel markers were developed in the section between RM523 and RM218,then based on the marker with differences in two gene pools(Table 2),the DL-6 in the section of 85 kb between I3-5 and I3-8 of PAC clone AC135158 on chromosome 3 was fine mapped(Fig.3B).

Table 1 Segregation of dropping leaf in F2population
According to the gene annotation results on AC135158 sequence of PAC clone on the chromosome 3 of rice provided by TIGR website(http:// www.tigr.org),the candidate gene analysis on the section of 85 kb between markers I3-5 and I3-8 was carried out;in this region,there were totally 16 open reading frames(ORFs),and meanwhile 16 gene annotationresults of ORF were obtained(Fig.3C),in which ORF9 coded a YABBY gene,and YABBY gene was concerned with the development of vein and flower organs.To verify whether YABBY was the candidate gene of DL-6,this study designed 5 pairs of primers which could amplify the full length of DNA sequence of the gene(Table 2),the 5 pairs of primers were used to respectively carry out PCR amplification on the genes of midrib-deficient mutant and wild type,then the PCR products were delivered to Invitrogen Trading(Shanghai)Co.,Ltd.to sequence after the recycle ofagarose gelelectrophoresis(AGE).BioXM2.6 software was used to carry out sequence alignment between the sequence of midribdeficient mutant and wild type and YABBY gene of Nipponbare in the database,the results showed that 1 bp mutation was found at the 31stbasic group of the first exon of YABBY gene in the mutant dl-6 and 8 bp deletion was also found at 3’end of ORF9 gene,and wild-type sequence was completely the same as that of Nipponbare(Fig.3D).The mutation of the single base caused the mutation of cysteines(cys)belonging to wild type become arginine (arg)belonging to mutant.These two mutations which one was the functional mutation of dl-6 has been still uncertain and needed further research.
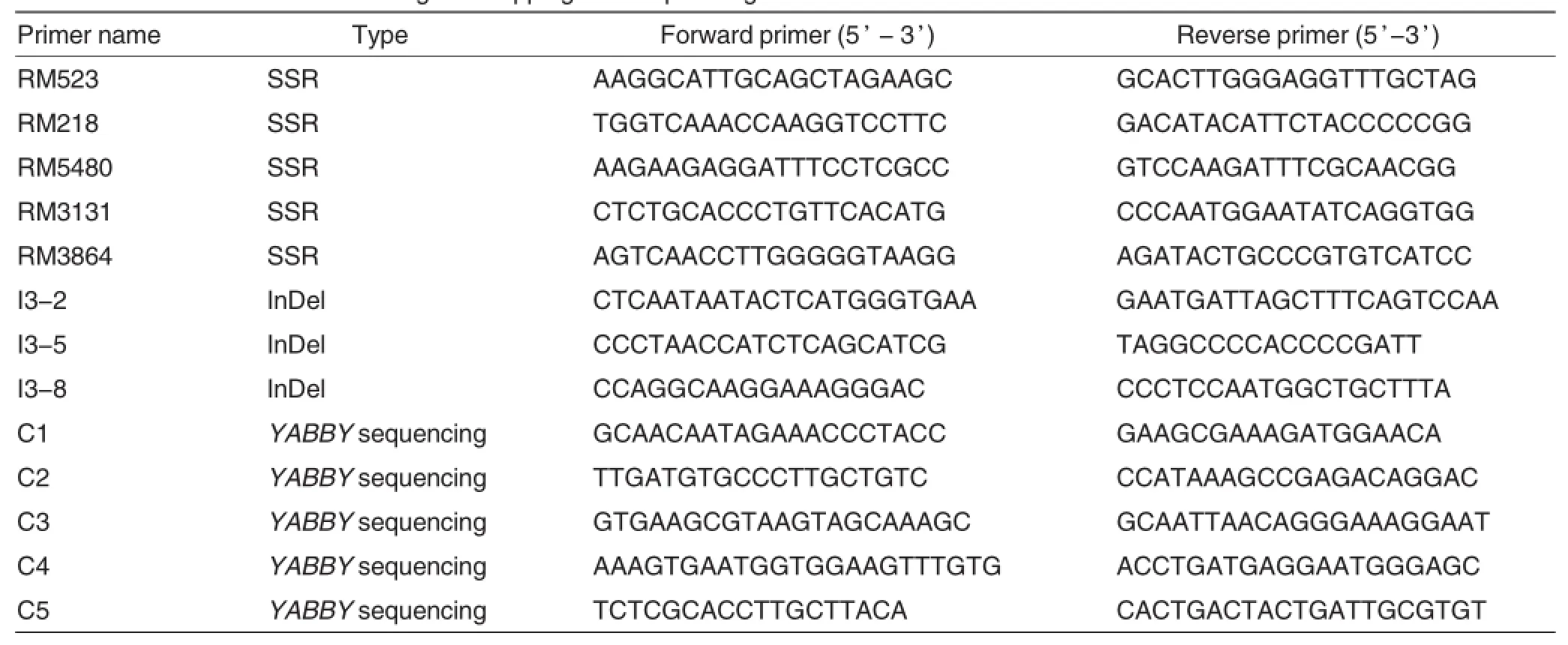
Table 2 The markers used for DL-6 gene mapping and sequencing
Discussions
Up to now,a lot of midrib-deficient mutants have been reported in China. Nagasawa et al.[10-11]successively segregated many midrib-deficient mutants(dl-sup1,dl-sup1,dl-1,dl-2),and the genes controlling these mutants were mapped on chromosome 3.The further gene cloning research showed that DL gene contains 7 exons and belongs to a member of YABBY gene family,it codes a spiral-cyclic-spiral protein structure domain and zinc finger,andhashighhomologywith CRABS CLAW(CRC)of YABBY gene belonging to Arabidopsis thaliana,as well as receives negative control[12]by SUPERWOMAN1(SPW1).Wang Bin et al.[17]found a midrib-deficient and glumous flower organ mutant DLR from maintainer line D62B,the genetic analysis showed thatthe mutant character was controlled by a pair of recessive genes,the mutant gene which was between molecular markers RM563 and RM282 on the chromosome3wasmappedusingSSR molecular marker,the gene was 5.56 cM away from RM282.Zhang Xiaoxiang et al.[18]obtained 3 midrib-deficient mutants with different genetic backgrounds,namely MR304,MR168 and MR312,in which the midrib-deficient character of MR304 was controlled by two pairs of recessive genes,the midrib-deficient characters of MR168 and MR312 were controlled by a pair of recessive genes.Wang Nan et al. found a midrib-deficient mutant dl-(t)from crossbreeding generations,the characters of floral organ were normal,and the gene was mapped between RM6038 and RM7576,and the gene was respectively 3.99 and 0.47 cM away from the two and co-segregated with RM1324[19].In this study,a naturalmutant dl-6 was found in the japanica rice Wuyunjing 7,and the development of the stamen and the pistil of dl-6 was normal.Using the method of map--based cloning,the dl-6 in the section of 85 kb between I3-5 and I3-8 of PAC clone AC135158 on chromosome 3 was fine mapped,the section contained YABBY gene reported by the predecessors[12];according to the results of YABBY gene sequencing in the mutant,it was found that 1 bp mutation was found in first exon of YABBY gene in the mutant dl-6,and 8 bp deletion was also found at 3’end of ORF9 gene,and it was such weak recessive mutation which led to leaf drooping,but the development of floral organ was normal,showing that DL-6 is a new allele for DL gene.
The popularization and planting of hybrid rice makes rice yield improved greatly,our country is in the international advanced level in the study and application of hybrid rice.The propagative and seed production techniques of hybrid rice are very complicated,in which the leaf-cutting treatment of the sterile line not only needs a lot of time and labour,but also causes a certain effect on the photosynthesis of the sterile line,thereby affecting the growth and development of rice.If guiding the midrib-deficient character into the sterile line,the panicle layer of the sterile line after earing is all on the leaf layer and not hindered by leaves,which is beneficial to the transfer of the pollen of hybrid rice during breeding and seed production,thereby improving the yield.This not only doesn’t need leaf cutting,but also doesn’t need gibberellin spraying[19],in addition,midrib-deficientcharacter also can be the marker character of purity identification,for example,if guiding the midrib-deficient character into the sterile line and maintainer line,the leaf of maintainer line will be erect,so the F1generation crossed by the sterile line and the maintainer line will be erect,thus it is very easy to get rid of sterile line and maintainer line from the F1generation during the seedl-ing stage,this is quick and convenient[21]. However,most reported midrib-deficient mutants showed not only leaf drooping,but also the malformation of floral organ,these were caused by the serious mutation of YABBY gene,thus it was very difficult to use in production practice.In this study,the YABBY gene of dl-6 had weak recessive mutation,and showed leaf drooping,but the development of floral organ was normal and had not abnormal glumes. Therefore,crossbreeding can be used to transfer the gene to the sterile line,which not only alleviates the working routine of the propagation and seed production of hybrid rice and field workload,but also provides good experimental materials for the innovation of germplasm resources.
[1]CAI J(蔡晶),WANG XG(王晓光),JI ZJ(季芝娟),et al.Research progress of genetic and molecular biology in rice leaves morphology(水稻叶片形态的遗传与分子生物学研究进展)[J].China Rice(中国稻米),2008,14(6):5-11.
[2]WU D(吴钿),GUO JF(郭建夫),ZHANG JZ(张建中).The canonical correlation analysis of paddy rice between leaf characters and individual plant yield(水稻叶型特征与产量的典型相关分析)[J]. Journal of Shihezi University(Natural Science)(石河子大学学报 (自然科学版)),2005,23(3):336-338.
[3]HUANG YX(黄耀详).Clustering breeding of rice(水稻丛化育种)[J].Guangdong Agricultural Sciences(广东农业科学),1983(1):1-6.
[4]YANG SR(杨守仁),ZHANG LB(张龙步),WANG JM (王进民).A preliminary discussion on the theory and method of ideal plant type breeding of rice(水稻理想株型育种的理论和方法初论)[J].Scientia Agricultura Sinica(中国农业科学),1984,17(3):6-13.
[5]ZHOU KD(周开达),MA YQ(马玉清),LIU TQ (刘太清),et al.The breeding of subspecific heavy ear hybrid rice-Exploration about super-high yield breeding of hybrid rice(杂交水稻亚种间重穗型组合选育-杂交水稻超高产育种的理论与实践)[J].Journal of Sichuan Agricultural University(四川农业大学学报),1995,13(4):403-407.
[6]YUAN LP(袁隆平).The breeding of super-high-yield hybrid rice(杂交水稻超高产育种)[J].Hybrid Rice(杂交水稻),1997,12(6):1-3.
[7]CHENG SH(程式华),CAO LY(曹立勇),CHEN SG(陈深广),et al.Conception of late-stage vigor super hybrid rice and its biological significance(后期功能型超级杂交稻的概念及生物学意义)[J].Chinese Journal of Rice Science(中国水稻科学),2005,19(3):280-284.
[8]KHUSH GS.Prospects and approaches to increasing the genetic yield potential of rice [C].Rice Research in Asia, Progress and Priorities,199-6:59-71.
[9]SAKAGUCHI J,FUKUDA H.Cell differentiation in the longitudinal veins and formation of commissural veins in rice(Oryza sativa)and maize(Zea mays)[J]. Journal of Plant Research,2008,121(-6):593--602.
[10]NAGASAWA N,MIYOSHI M,SANO Y,et al.DL regulates both leaf and pistil development in rice [J].Rice Genet Newsl,199-6,13:102-105.
[11]NAGASAWA N,MIYOSHI M,SANO Y,et al.SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice [J].Development(Cambridge,England),2003,130(4): 705-718.
[12]YAMAGUCHI T,NAGASAWA N,KAWASAKI S,et al.The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa[J].Plant Cell,2004,1-6(2):500-509.
[13]DELLAPORTA SL,WOOD J,HICKS JB.A plant DNA minipreparation:Version II[J].Plant Molecular Biology Reporter,1983,1(4):19-21.
[14]SHEN YJ,JIANG H,JIN JP,et al.Development of genome-wide DNA polymorphism database formap-based cloning of rice genes[J].Plant Physiology,2004,135(3):1198-1205.
[15]MICHELMORE RW,PARAN I,KESSELI RV.Identification of markers linked to disease-resistance genes by bulked segregant analysis:a rapid method to detect markers in specific genomic regions by using segregating populations[J].Proceedings of National Academy of Sciences of the U-nited States of America,1991,88(21): 9828-9832.
[16]LANDER ES,GREEN P,ABRAHAMSON J,et al.MAPMAKER:An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations[J].Genomics,1987,1(2):174-181.
[17]WANG B(王彬),HAN ZP(韩赞平),WU XJ(吴先军),et al.Identification of a mutant with drooping leaf(DLR)in rice(水稻颖花突变体DLR的鉴定)[J]. Seed(种子),2004,23(11):30-32.
[18]ZHANG XX(张小祥),ZHANG ZL(张忠林),HONG RK(洪汝科),et al.Phenotypic and genetic analysis in several drooping leaf mutants of rice(水稻几个披垂叶突变体的表型研究及其遗传分析)[J].Molecular Plant Breeding(分子植物育种),2005,3(4):63-68.
[19]WANG N(王楠),ZHAO FM(赵芳明),LING YH(凌英华),et al.Genetic analysis and gene mapping of a rice drooping leaf mutant(一个水稻披叶突变体的遗传分析和基因定位)[J].Molecular Plant Breeding(分子植物育种),2007,5(1):54-58.
[20]HUANG TX(黄庭旭),YOU QR(游晴如),YANG D(杨东),et al.Research progress in recessive marker traits of rice male sterile line(水稻雄性不育系隐性标记性状的研究进展)[J].Acta A-griculturae Jiangxi(江西农业学报),2009,21(1):13-16.
[21]YANG ZL(杨占烈),HUANG ZH(黄宗洪),XIANG GL(向关伦),et al.The breeding ofthermo-sensitive genic male sterile line G15-6S-a drooping leaf of rice(披叶标记水稻温敏核不育系G15-6S的选育)[J].Seed(种子),2005,24(12):105-107.
Responsible editor:Nanling WANG
Responsible proofreader:Xiaoyan WU
水稻中脉缺陷突变体dl-6的遗传分析与精细定位
王军1,王婧2,杨杰1,赵祥强2,朱金燕1,范方军1,李文奇1,王芳权1,仲维功1*(1.江苏省农业科学院粮食作物研究所,国家水稻改良中心南京分中心,江苏南京210014;2.南通大学生命科学院,江苏南通226007)
在粳稻品种武运粳7号中发现了一个中脉缺陷的自然突变体,经过连续多代自交形成了稳定的突变系,暂命名为dl-6,该突变体表现为叶片中脉发育受阻,叶片完全披垂。以dl-6与9311配制正反杂交组合进行性状分析发现,dl-6突变性状受1对隐性核基因控制,利用SSR分子标记将控制dl-6性状的基因DL-6初步定位在水稻第3染色体的短臂上,进一步利用新发展的InDel标记将DL-6基因精细定位在I3-5和I3-8之间的85kb的物理距离内。对该区段内存在的开放阅读框(ORF)进行分析,发现其中ORF9编码的YABBY基因是一个与叶脉发育相关的基因,对突变体dl-6和野生型中YABBY基因进行测序,将测序结果与数据中日本晴序列进行比对发现,突变体dl-6中YABBY基因的第1个外显子存在1个碱基突变,该突变导致野生型中编码的半胱氨酸突变为突变体中的精氨酸;同时突变体dl-6在ORF9基因的3端还在一个8个碱基的缺失。这2个突变位点哪个是导致dl-6突变的功能区目前还不确定,有待进一步研究。
水稻;中脉缺陷;基因定位;dl-6
国家科技支撑计划重大项目(2011BAD16B03);江苏省研究生创新工程项目(CXZZ12_0906);江苏省农业自主创新资金项目[CX(12)1003];江苏省自然科学基金项目(BK20130725);江苏省科技支撑计划项目(BE2012309)。
王军(1981-),男,江苏盐城人,副研究员,在读博士,主要从事水稻分子遗传育种研究,E-mail:wangjunjaas@aliyun.com。*通讯作者,研究员,主要从事水稻遗传育种研究,
2015-06-13
Supported by the Key Project of National Science and Technology Support Plan(2011BAD16B03); Postgraduate Innovation Project of Jiangsu Province(CXZZ12_0906);the Project of Agricultural Independent Innovation Fund in Jiangsu Province[CX(12)1003];Natural Science Fund in Jiangsu Province(BK20130725);the Project of Science and Technology Support Program in Jiangsu Province(BE2012309).
*Corresponding author.E-mail:wgzhong0503@aliyun.com
Received:June 13,2015 Accepted:November 19,2015
E-mail:wgzhong0503@aliyun.com。
修回日期 2015-11-19
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Study on Engineering Characteristics and Application of Sticky Rice
- Periodical Development Trend of Vertical Greening
- Advances in Microbial Remediation on the Application of Heavy Metal Pollution in Agricultural Water Resources
- Analysis on Status quo and Future Development of Fruit and Vegetable Protreatment
- Effect of Three Treatment Measures on Harmless Seedling Raising of Pinus sylvestris var. mongolica Litv.
- Discussions and Recommendations for Supervision of Vegetable Quality and Safety in Miyun County
