The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies
2015-10-31HaoqingShiXiangjieQiBinMaYanweiCaoLinaWangLijiangSunHaitaoNiu
Haoqing Shi*, Xiangjie Qi*, Bin Ma, Yanwei Cao, Lina Wang, Lijiang Sun, Haitao Niu
1Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China;2Department of Urology, People’s Hospital of Linzi District, Zibo 255400, China;3Medical College of Qingdao University, Qingdao 266021, China
*These authors contributed equally to this study.
Correspondence to: Haitao Niu. Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China. Email: Niuht0532@126.com;Lijiang Sun. Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China. Email: slijiang999@sohu.com.
The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies
Haoqing Shi1*, Xiangjie Qi2*, Bin Ma1, Yanwei Cao1, Lina Wang3, Lijiang Sun1, Haitao Niu1
1Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China;2Department of Urology, People’s Hospital of Linzi District, Zibo 255400, China;3Medical College of Qingdao University, Qingdao 266021, China
*These authors contributed equally to this study.
Correspondence to: Haitao Niu. Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China. Email: Niuht0532@126.com;Lijiang Sun. Department of Urology, Affiliated Hospital of Qingdao University, Qingdao 266003, China. Email: slijiang999@sohu.com.
In recent years, immunotherapy has been gradually established as the fourth frequently adopted antitumor therapy, following surgery, chemotherapy and radiotherapy, for advanced urologic malignancies with an improved understanding of theoretical basis, such as molecular biology and immunology. Thereinto, adoptive cellular immunotherapy (ACI) has become one of the hotspots, which comprises a variety of treatment approaches, such as TIL, CIK cell, γδ T cell, CAR-engineered T cell and Allogeneic stem cell transplantation (alloSCT). Although preclinical efficacy has been demonstrated remarkably,clinical trials could not consistently show the benefit due to multi-factors in complex immunosuppressive microenvironment in vivo compared to that of in vitro. Here we review some timely aspects of ACI for advanced urologic malignancies, and describe the current status and limitation of immunotherapy from the cellular level. It’s our expectation to provide prompting consideration of novel combinatorial ACI strategies and a resurgence of interest in ACI for advanced urologic malignancies.
Adoptive cellular immunotherapy (ACI); limitation; improvement; urologic malignancies
Introduction
In recent years, the incidence rates of urologic malignancies continued to increase. Surgery, chemotherapy and radiotherapy have shown mixed success for earlystage patients. However, the prognosis of patients with advanced-stage malignancies is still extremely poor. With the development of theoretical basis, such as molecular biology and immunology, immunotherapy has been gradually established as the fourth frequently adopted antitumor therapy. It comprises a variety of treatment approaches, such as antitumor monoclonal antibodies,genetic modification, targeted therapy, adoptive cellular immunotherapy (ACI), and cellular vaccines. It attempts to harness the power and specificity of the immune system to fight against malignancies. ACI as a promising method of immunotherapy, harnesses the cells that largely expanded in vitro and have anti-tumor activity to eradicate malignant cells by either active or passive immunotherapy.
However, immunotherapy had not yet been approved as a standard treatment for urologic malignancies, which were largely confirmed immunogenic tumor, due to some obstacles to achieving efficacy. Immunotherapy strategies are ineffective at tumor elimination in vivo but can exert specific functions outside the immunosuppressive and toleragenic tumor microenvironment. This is because the tumor microenvironment contains suppressive elements including regulatory T cell (Treg), myeloid-derived suppressor cell (MDSC) and tumor-associated macrophage(TAM); soluble factors such as interleukin 6 (IL-6), IL-10, vascular endothelial growth factor (VEGF), and transforming growth factor beta (TGF-β) (1). Gradually,enhancing the efficacy of ACI in vivo has becoming the current hotspot of cancer immunotherapy. In this review,we expounded ACI for urologic malignancies systematically,combined with the evaluation and improvement of clinical efficacy.
The status of adoptive cellular immunotherapy
Tumor-infiltrating lymphocytes (TILs)
TILs are a class of lymphocytes derived from primary or metastatic tumor tissue fragments, regionally tumordraining lymph nodes or malignant ascites, which were expanded in vitro in IL-2-supplemented media and enriched predominantly in CD8+ cytotoxic T lymphocytes (CTLs)in order to eradicate autologous tumor antigens in a MHC-restricted pattern. Sharma et al. (2) concluded that the extent of intratumoral CD8+ TILs is an important prognostic indicator in muscle-invasive urothelial carcinoma(MIUC) by showing that MIUC patients with higher numbers of CD8+ TILs had better disease-free survival and overall survival (OS) than patients with fewer intratumoral CD8+ TILs. Ju et al. (3) found that administration of 6-gingerol, which is a component of ginger, could enhance the number of CD8+ TILs, then inhibit tumor growth in renal cell carcinoma (RCC) murine model and reduce Tregs in addition. Clinical trials of TILs have been conducted, but many patients with cancer are ineligible for such treatment on account of the poor objective response rates except malignant melanoma. Although the difficulty in generating sufficient numbers of reactive T cells in vitro remains the main drawback of a successful TILs treatment, several other factors also contribute, such as losing expression of tumor associated antigens (TAAs) and/or MHC molecules.
Cytokine-induced killer (CIK) cells
CIK cells have been confirmed as one of the most widely clinically used therapeutic cells for patients with malignancies. They are a heterogeneous population of effector CD8+T cells with diverse TCR specificities,possessing non-MHC restricted cytolytic activities against tumor cells with the dual characteristics of T cells and NK cells, which could identify the target cells not only through the TCR and MHC, but also could through the NK cell activated receptor. Liu et al. (4) indicated that CIK cells immunotherapy could improve the prognosis of metastatic clear cell renal cell carcinoma (ccRCC), and increased frequency of CIK cells immunotherapy could result in enhanced beneficial effects by their randomized study. CIK cells directly kill target cells by releasing a variety of cytokines after activation, which could also activate the apoptosis genes, inducing tumor cell apoptosis and necrosis,and thus play a lasting oncolytic effect. Wang et al. (5)manifested CIK cells are feasible and effective in treating advanced RCC combined with dendritic cells and thus provide a new approach to cancer treatment.
γδ T cells
γδ T cells are a special type of immune cells which were considered to represent a link between specific immunity and non-specific immunity, because of its expression of both natural killer receptors and γδ T cell receptors (6). Utilizing these anti-tumor properties of γδ T cells, preclinical and clinical trials have been conducted to develop novel immunotherapies for malignancies. Stage III trials manifested that an increase in the proportion of peripheral γδ T cell is a favorable prognostic factor for patients with locally advanced RCC. A research from Japanese applied γδ T cells in the treatment of advanced RCC postoperative patients, and found that ACI using in vitro-activated autologous γδ T cells was well tolerated and induced anti-tumor effects. Siegers et al. (7)proclaimed that intravesical administration of γδ T cells significantly demonstrated antitumor activity against prostate cancer cells, resulting in prolonged survival. The same outcome was obtained in the experiment of bladder cancer.
Chimeric antigen receptor (CAR)-engineered T cells
CAR-engineered T cells combined TAA-recognized single-chain antibody with the activation motif of T cells,freeing antigen recognition from MHC restriction and thus breaking one of the barriers to more widespread application of ACI. It means combining the high affinity of antibody to TAA with the killing mechanism of T cells. It had been bolstered that CAR-engineered T cells exhibited antitumor function to prostate cancer and other advanced malignancies. However, the toxicity of CAR-engineered T cells was detected in some researches, such as healthy tissues that express the targeted antigen might undergo T cellmediated damage (8). Lamers et al. (9) administered CAR-engineered T cells to 12 patients with RCC. They observed that CAR-engineered T cells exerted antigen-specific effects in vivo and induced antigen-directed toxicity. Nonetheless,the toxicity could be prevented by monoclonal antibody which blocks tumor associated antigenic sites in off-tumor organs. Kloss et al. (8) transduced T cells not only CAR but also chimeric costimulatory receptor (CCR) that recognizesa second antigen in the aim of avoiding the unexpected toxicity. Then T cells destroyed tumors that express both antigens but do not affect tumors expressing either antigen alone. Furthermore, a fixed antigen specificity (10) of CARs had been mentioned that only one TAA can be targeted by CAR once. Generally speaking, breaking the obstacle of finding more true heterogeneous tumor-specific antigens had been identified as extremely urgent to broaden the applicability and avoid some of the side effects of targeted T-cell therapies.
Allogeneic stem cell transplantation (alloSCT)
alloSCT from a compatible donor peripheral blood has been utilized as ACI in advanced solid malignant tumors such as mRCC and castration resistant prostate cancer(CRPC). Several of correlative studies identified the population of donor derived lymphocytes as mediator of graft-versus-tumor (GVT) effects (11). Since the year 2000, several investigators have established that RCC is susceptible to GVT effect: they reported that patients with RCC may have partial or complete disease responses in the 20-40% range. Hess Michelini et al. (12) reported that tumor-directed vaccination cooperated with alloSCT could produce GVT response and prolong survival in prostate cancer mouse model. However, transplant-related side effects, such as graft-versus-host-disease (GVHD), is still in fraught incidence rate, even though there was a fraction of potential solvable approaches (13). The conclusion had been made that GVT reactivity after alloSCT may be unavoidably linked to GVHD in patients suffering from progressive metastatic ccRCC under a long-time study. Though the use had been decreased by monoclonal antibodies and small tyrosine-kinase inhibitor, the future development of alloSCT would require novel treatment protocols designed to augment and sustain post-transplant GVT effects against GVHD at the same time to generate renewed enthusiasm for this approach.
The limitation of adoptive immunotherapy
A variety of ACI strategies including what is said above,aimed at boosting the immune system, have been employed for the treatment of metastatic diseases. Despite the drawbacks associated with in vitro cell manipulation and upscaling, several such approaches have been assessed in the clinic. However, these approaches didn’t show consistent benefit when compared with in vitro experiment. In my opinion, the causes could be summarized into two divisions in major: immunological and non-immunological factors.
The non-immunological limitation of adoptive immunotherapy
Infusion pathway
ACI had been tested for cancer immunotherapy in general curative effect. However, little is known about the nonimmunological factors attached to the indistinctive in vivo effects, such as infusion pathways, source of cells and homing phenomenon. Skitzki et al. (14) reported that CIK cells could widely immigrate into most organs after intravenous transfusion; distribution was related to blood supply and immune properties of the organs as well as the order in which cells reached each organ. Du et al. (15) infused CIK cells via three different pathways into nude mice model of human gastric cancer. In the study, they found that, after intravenous transfusion, CIK cells distributed with the blood circulation and first arrived at the lungs though they could indeed arrive at the tumor tissue finally. Similarly, intraperitoneally infused CIK cells first gathered in the abdominal cavity, and then dissipated after the same course as the intravenous pathway. In contrast, peritumoral injection resulted in the maintenance of CIK cells in the tumor tissue for the maximum amount of time examined. These results indicated that peritumoral injection of immune effector cells may be able to represent the maximum effective delivery method of ACI.
Source of cells
Source of cells also impacted. Studies have shown that cord blood-derived CIK (CB-CIK) cells were more potent in cytotoxic activity against various tumor cells than other autologous ones with similar phenotype both in vitro and in vivo. Not coincidentally, Introna et al. (16)treated five patients with CB-CIK cells who had relapsed from aggressive acute leukemia, and acute or delayed adverse event was not found. These observations show the feasibility of this immunotherapy program for patients who could not otherwise benefit from donor lymphocyte infusions. However, there were only a few reports about the clinical application of CB-CIK cells in the treatment of patients with advanced malignancies. The present research evaluated that CB-CIK cells could be a possible enhancing therapeutic strategy of ACI.
Homing phenomenon
Circulating lymphocytes crossed the capillary highendothelial venule (HEV) selectively and trafficked into peripheral lymphoid organs or specific tissues under the interaction of homing receptor, such as CCR7 (17), and its ligands CCL19 and CCL21, this was so called lymphocyte homing (LH). HEV could be discovered in peripheral lymph node (PLN), mesenteric lymph node (MLN) and peyer’s patches (PPs), also possibility due to this, density of immunotherapy effector cells of the peritumoral area could not achieve the desired adequate effect in vitro. But interestingly, HEV could also be highlighted in human solid tumors. In view of this, we speculated that immunologic effector cells, represented by TIL, could be mediated into tumor tissues by adhesion molecules and chemotactic factors, in spite of negative factors derived from HEV (18). The challenge will be solved developing the means to increase lymphocyte infiltration into tumors by safely interfering with homing receptor/ligand axis while leaving the beneficial effects of enhancing antitumor immune mechanisms.
The immunological limitation of adoptive immunotherapy
Various types of ACI cells were gradually used for clinical application stage, and achieved initial results. However,antitumor effector cells in the tumor microenvironment could be normally induced into the “immune anergy”status, then lead to the embarrassing scenario of coexistence of effector cells and tumor cells. The actual overall efficiency did not exceed 30% when they were applied separately in clinical practice. It’s considered that the complex and volatile immunosuppressive network of tumor microenvironment restricted the function of immune system, wherein the inhibiting factors were the most dominating problems which including lack of costimulatory molecules on malignant cells, inhibition of T cell activation or function or weak tumor cell killing owing to suppressive cells and their secretion of suppressive cytokine.
Regulatory T lymphocytes
CD4+CD25+ Treg represent a unique population of lymphocytes that are thymus-derived, which were marked by forkhead box transcription factor (Foxp3), play a critical role in maintaining self-tolerance, suppress autoimmunity and regulate immune responses in organ transplantation and tumor immunity (19). It could be divided into two categories according to the origin: nTreg and iTreg. But recent studies have indicated that some Tregs do not express Foxp3, instead of the expression of CD127 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) (20). So there are many scholars regarding CD25 and CD127 as Treg markers. According to the identified Treg related protein, GITR and LAG-3 are currently available as Treg markers as well. Davidsson et al. (21) revealed that high Treg number was one of the significantly independent prognostic indicators of poor OS for CRPC by univariate analysis. The same conclusion was also elicited in mRCC. In addition, T eff/T reg ratio at the tumor site could predict therapeutic efficacy of antitumor immunotherapy. Suppressive capability is achieved through a variety of mechanism, like secretion of inhibitive cytokine such as TGF-β and IL-10; directing cell contact through binding cell surface molecules such as CTLA-4; interfering metabolism pathway of effector cells;embellishment of immature DCs. In addition, some scholars argued that Tregs played a crucial role in seperating GVHD from GVT effect after alloSCT as mentioned before (22).
Myeloid derived suppressor cells (MDSCs)
MDSCs were a group of heterogeneous cellular, which could be seen as hallmark of malignancy-associated inflammation and a major mediator for the induction of T cell suppression in cancers. It could be divided phenotypically into granulocytic (G-MDSC) and monocytic(Mo-MDSC) subgroups (23), maintaining Gr-1 (Ly-6G)and CD11b (Mac-1) as their common surface marker. Through tryptophan metabolic pathway (24), arginine metabolic pathway (23), phenylalanine metabolic pathway and homocysteine metabolism pathway, it developed inhibiting effect related to amino acid metabolism closely. Commonly, the effector T cells could be frustrated due to low-level arginine and tryptophan which were reduced by ARG1, IDO or other amino acid enzyme, with synergistic effect of the generation of reactive oxygen species (ROS). Wang et al. (25) treated 21 mRCC patients with an adoptive transfer of autologous CIK cells. Subgroup analysis indicated that patients with a relatively low proportion of MDSCs exhibited prolonged survival. So they suggested that MDSCs can serve as a potential marker for the prognosis of patients receiving a CIK-based therapy.
Tumor associated macrophage
TAMs derived from peripheral blood monocytes were multi-functional cells which exhibited different functions to different signals of microenvironment. Among cell types associated with tumor microenvironment, TAMs were the most influential for tumor progression. In response to microenvironmental stimuli, macrophages undergoM1 (classical) or M2 (alternative) activation. In most malignant tumors, TAMs have the phenotype and function of M2 macrophages, the process of its formation is closely promoted by the tumor microenvironment such as tumor extracellular matrix, tumor immune microenvironment,anoxic environment and cytokines secreted by tumor cells. Conversely, TAM could release a variety of cytokines,which promote the invasion and metastasis of malignant tumor, such as formation of new blood vessels, tumor basilar membrane damage, extracellular matrix remodeling,epithelial-mesenchymal transition in tumor cells. Adams et al. (26) confirmed that TAMs had a participatory role in tumor cell migration and they could be regarded as a potential diagnostic and prognostic biomarker in advanced solid tumors, such as prostate cancer. Mitchem et al. (27) discovered that targeting TAMs could relieve immunosuppression and improve chemotherapeutic responses. Based on this scenario, Santoni et al. (28)represented TAMs as a promising and effective target for cancer therapy in mRCC. In the current, transforming M2 macrophages into M1 macrophages becomes a challenge need to be solved urgently (Figure 1).
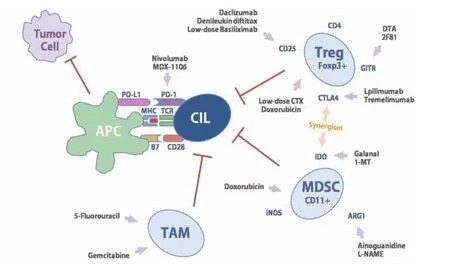
Figure 1 Mutiple immunologic mechanisms contribute to deplete or inhibit suppressive immunocytes, such as Treg, MDSC and TAM, in vivo in patients with cancer. MDSC, myeloid-derived suppressor cell; TAM, tumor-associated macrophage.
The Improvement of adoptive immunotherapy
The non-immunological measures
As previously mentioned, the non-immunological factors work. It could enhance therapeutic efficacy potentially in vivo, such as altering infusion pathways, ameliorating source of cells and interfering homing phenomenon. Studies had shown that peritumoral injection could be able to reserve the maximum density of adoptive immunologic effector cells (15). More promisingly, immunologic effector cells could be mediated into tumor tissues by modulating homing receptor ligands, such as adhesion molecules and chemotactic factors, which could combined to homing receptor (17). The feasibility had been confirmed by numerous preclinical trials (29) in immunotherapy of urologic malignancies.
Combined with chemotherapy
Chemotherapy is one of the most widely used approaches to advanced malignancies. What’s previously thought is that chemotherapy could led to the damage of immune system,then impede the immune response. However, recent studies have shown that traditional chemotherapy drugs are no longer purely cytotoxic, and gradually attention has been paid to their immunomodulatory effects. As is widely known that tumor cells killed by chemotherapy agents released large amounts of tumor antigens, increasing tumor antigens cross-presentation. The more exciting fact was that it could remarkably promote maturity of APCs and selectively decrease the number of immunosuppressive cells such asMDSCs and Tregs. Studies had shown that Treg were more sensitive than CTL and Th to cyclophosphamide (CTX)and low-dose CTX was not detrimental to the functional effector T cells (30). Walter et al. (31) claimed that in their randomized phase 2 trial they observed CTX could reduce the number of Tregs which could enhance the efficacy of IMA901, a therapeutic vaccine for RCC. Meanwhile, the same consequence was demonstrated that combining lowdose CTX with GVAX could enhance anti-prostate cancer immune effects. It had been confirmed that doxorubicin,another common agent for urologic malignancies, could enhance efficacy of antitumor immunotherapy by inhibited suppressive cells, such as Tregs and MDSCs, recruited to the tumor microenvironment and accelerated development of antitumor effector T cells responses (32). What’s more,fluorouracil and gemcitabine could also improve antitumor immunity by the similar rationale such as selectively inhibited suppressive immune cells.
Anti-CTLA-4 monoclonal antibody
CTLA-4 or CD152 is an inhibitory molecule on the surface of Tregs playing a critical role in the generation and maintenance of tolerance to self-antigens (33). It also could be seen as a key negative regulator in the well-defined B7:CD28/CTLA-4 pathway which is a complex integration of positive and negative co-stimulatory signal transduction pathway (34). This rationale had been exploited successfully for the generation of novel therapeutic strategies to augment antitumor immunity, exemplified by ipilimumab and tremelimumab, anti-CTLA-4 monoclonal antibody, which were evaluated extensively in melanoma without toxicity as others. Notably, ipilimumab was recently approved by FDA as monotherapy for the treatment of advanced melanoma. Meanwhile, tremelimumab was currently undergoing evaluation in phase III trials in CRPC (35). Hodi et al. (36)periodic infused anti-CTLA-4 antibodies after vaccination with irradiated, autologous tumor cells engineered to secrete GM-CSF. Excitingly, Holmgaard et al. (37) showed that CTLA-4 blockade strongly synergizes with IDO inhibitors to mediate rejection of immunogenic tumors, and CTLA-4 blockade restricted activity of IDO markedly.
Anti-PD-1 monoclonal antibody
Programmed death-1 (PD-1), a member of the B7-CD28 family, is a transmembrane receptor on the T-cell surface following TCR activation, whereas its ligands, PD-L1 and PD-L2, are present on the surface of the antigen presenting cells (APC). The binding of this to its cognate ligands, PD-1 promoted T-cell anergy and apoptosis, thus leading to immune suppression (38). It had been widely revealed that PD-1 positivity was one of the significantly independent prognostic indicators of poor OS, more distant metastatic relapse (DMR) and poor relapse-free survival(RFS) for mRCC by univariate analysis. PD-1 signaling in tumors is required for both suppressing effector T cells and maintaining tumor Tregs, and therefore disrupting PD-1/PD-L1 pathway could be a putative strategy to augment the antitumor immune response. Brahmer et al. (39)considered that blocking the PD-1 immune checkpoint with intermittent antibody dosing is well tolerated and associated with evidence of antitumor activity in their phase I study of anti-PD-1 agent (MDX-1106) in refractory solid tumors. Although obtained the most mature results in advanced melanoma patients, Nivolumab, one of the investigational fully human IgG4 antibody drug targeting PD-1 as a treatment, had been studied in phases III trial for mRCC (40), which demonstrating remarkable response rates, about 27% (9 of 33 patients), and high quality responses or prolonged duration (20 of 31 responses lasted 1 year or more) (41). It was also applied to prostate cancer in early experiments. Just because of these above, anti-PD-1 monoclonal antibody was considered “drug of the year” by the European Journal of Cancer.
Anti-GITR monoclonal antibody
Glucocorticoid-induced tumor necrosis factor receptor family related protein (GITR) is the 18thmember of the tumor necrosis factor receptor superfamily (TNFRSF18)and is known to interact with its cognate ligand GITRL(TNFSF18). It’s demonstrated that GITR are expressed mainly in CD4+CD25+Foxp3+ regulatory cells (Tregs). It is reasonable to believe that it plays a potential role in the immunosuppressive activation of Tregs. Ponte et al. (42)determined the ability of a rat anti-mouse GITR monoclonal antibody, 2F8, to stimulate murine humoral and cellular immunity which was greater than that obtained in mice dosed with standard adjuvants. Not coincidentally,Cohen et al. (43) investigated therapeutic efficacy of DTA-1, one of the anti-GITR monoclonal antibodies,reacted on melanoma tumor mice model. They suggested that DTA-1 could not only decrease intra-tumor Tregs accumulation due both to impaired infiltration, coupled with DTA-1-induced loss of Foxp3 expression in intra-tumor Tregs, but also enhanced tumor-specific CD8+ T cell activity. This finding shows that anti-GITR is a robust,versatile adjuvant that, unlike commonly used adjuvants,enhances both humoral and cellular immunity. These results support the continued development of anti-GITR for indications as solid tumors.
Anti-CD25 monoclonal antibody and denileukin diftitox (DD) It has been demonstrated that CD25, the alpha chain of the interleukin-2 (IL-2) receptor (IL-2Rα), was expressed on cytomembrane of Tregs at high affinity. Due to this, Tregs competitively inhibited the proliferation and excitation of effector T cells. Theoretically, CD25 blockade could enhance immune responses to tumor. Currently, two antihuman CD25 monoclonal antibodies were approved by FDA to be used only in organ transplantation. But,studies had shown that it also could be used for solid and hematologic malignancies, such as breast cancer,glioblastoma, malignant melanoma and leukemia. Rech et al. (44) suggested that daclizumab, which led to a marked and prolonged decrease in Tregs, may be an effective and available therapeutic agent for Tregs modulation in cancer patients. However, the effect was not as satisfactory as always expected, possibly because that CD25 antibodies not only deplete Tregs but also inhibit activated effector T cells, as the theoretical basis is that CD25 is not particularly expressed on Tregs, also expressed on active effector T cells transiently. Considering the above viewpoint, researches showed that dose and schedule of antibody played an irreplaceable role in Tregs depletion strategies. It had been confirmed that low-dose basiliximab could safely target CD4+CD25highTreg cells whilst relatively preserving CD4+CD25lowactivated T cells. The host conditioning with low-dose basiliximab may augment the efficacy of ACI for cancer.
DD, a fusion protein of IL-2 and diphtheria toxin, which targeted CD25 expressing cells for lysis has been utilized in human to deplete Tregs approved by FDA for cutaneous T-cell lymphoma. Several researches had demonstrated that DD could significantly reduce the number of Tregs presenting in the peripheral blood of RCC patients and abrogate Treg-mediated immunosuppressive activity in vivo. Atchison et al. (45) reported that sequential therapy of DD and high-dose IL-2 could complement each other: DD would deplete Tregs so IL-2 could more effectively stimulate proliferation and activity of CTLs. In their trials,18 patients received sequential therapy and the median decline in Tregs was 56.3% with 15 patients served as controls. However, compared to anti-CD25 monoclonal antibody, DD allows indiscriminate targeting of the lower affinity IL-2βγ receptors which are expressed on a broader subset of cells including memory T-cells. What is more,depletion of Tregs is highly transient, further aggravated by the half-life of immunotoxin being only 2 hours compared to 20 hours of daclizumab. In my opinion, their applications for antitumor treatment should be more widely discussed.
1-methyl tryptophan
As has been noted previously, IDO, acted in tryptophan metabolic pathway of MDSC, catalyzed the initial and ratelimiting step in the degradation of tryptophan and is a key enzyme in mediating tumor immune tolerance via arrest of T cells proliferation (24). Except the classical phenotype of IDO1, IDO2 is a newly discovered enzyme with 43% similarity to IDO1 protein and shares the same catalytic mechanism. A fraction of studies had shown that inhibiting its activity may break tumor immune tolerance and thus promote therapeutic effects (46). Thus, a specific inhibitor of IDO, 1-methyl-tryptophan (1-MT), is being selected more and more frequently for antitumor trials, such as RCC, ovarian cancer, breast cancer, colon cancer and multiple myeloma. Although there were two stereoisomers of 1-MT, it remains inconclusive which stereoisomer of 1-MT is the more effective inhibitor of IDO-mediated immunosuppression. Some scholars considered that the levo-isoform (L-1MT) blocks IDO1, whereas dextroisoform (D-1MT) inhibits IDO2. While other scholars indicated that IDO enzyme activity is more efficiently inhibited by L-1-MT in cell-free or in vitro settings,D-1-MT is superior to L-1-MT in the enhancement of antitumor responses in vivo. In addition, 1-MT was conjugated to a TAA, fibroblast activation protein α (FAPα),in order to enhance the antitumor immunity. Recently,Yamamoto et al. (47) found galanal as a novel, competitive inhibitor, which had stronger effect than 1-MT. Hence, how to improve the effective inhibition of IDO would become a highlighted prospect.
Regulation of arginine metabolic pathway
The common characteristic of MDSCs is their ability to suppress the proliferation of both CD4+ and CD8+ T cells through mechanisms involving arginine metabolizing enzymes, inducible NO synthase (iNOS), and/or arginase1 (ARG-1). L-arginine has a guanidine sidegroup and is a semi-essential amino acid with multiple key roles in cellular growth. It is the sole substrate for nitric oxide (NO), which is a messenger molecule with multiple functions. The exact role of NO in cancer is not fully understood but may influence tumor initiation, promotion and progression by regulating the development of neovasculature around the tumor. Analogously, ARG-1 expression has been shown to contribute to some of the critical immunosuppressive properties of MDSCs that are frequently associated with tumors, and targeting ARG-1 could result in augmented antitumor responses through the reversal of MDSC-mediated suppression (48). Ainoguanidine, a NOS inhibitor, shown its inhibitory effect of tumor cell growth in bladder cancer, RCC and breast cancer, especially in prostate cancer, due to its prevention of NO formation. Otherwise, the effect of the NOS inhibitor L-NAME was evaluated in the human prostate cancer cell line (49). Higher iNOS expression was found in prostate cancer cells compared to BPH cells. L-NAME treatment of prostate cancer cells could result in a reduction in iNOS expression. iNOS and ARG-1 inhibition is a promising approach for targeting tumor vasculature and certain iNOS and ARG-1 inhibitors could offer a potential therapeutic window for the treatment of certain refractory tumors, including urologic malignancies.
Conclusions
Along with the rapid development of molecular immunology and constantly intensive studies about immunotherapy of advanced urologic malignancies, a large amount of new ideas and methods continue to emerge. We had obtained dramatic achievements in preclinical researches, but a series of arduous difficult hurdles need to be overcome when it entered into clinical stage. Because of the multi-factors in the complex process of indetectable occurrence of urologic malignancies, a treatment attacking a single target could not reach the satisfying desired effect. Thus, combinatorial ACI strategies, even combined with other methods, would potentiate the superiority of overall outcomes. In this review, we critically scratched the surface of the current challenges and potential developments of this promising field of ACI emphatically from the cellular level. We eagerly speculated that ACI could not only provide secondary treatment options for patients poorly served by the currently available agents, but also could be taken as one of the most important measures for comprehensive treatment of advanced urologic malignancies. More works are needed to maximize the enthusiasm for ACI strategies as realized in other cancers.
Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China (No. 30901481, 81372752,81472411); Wu-Jie Ping Medical Foundation (320.6750.13261). Disclosure: The authors declare no conflict of interest.
1. Liu CZ, Zhang L, Chang XH, et al. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res 2012;24:130-7.
2. Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscleinvasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007;104:3967-72.
3. Ju SA, Park SM, Lee YS, et al. Administration of 6-gingerol greatly enhances the number of tumorinfiltrating lymphocytes in murine tumors. Int J Cancer 2012;130:2618-28.
4. Liu L, Zhang W, Qi X, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res 2012;18:1751-9.
5. Wang D, Zhang B, Gao H, et al. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer 2014;14:251.
6. Wu YL, Ding YP, Tanaka Y, et al. γδ T cells and their potential for immunotherapy. Int J Biol Sci 2014;10:119-35.
7. Siegers GM, Ribot EJ, Keating A, et al. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol Immunother 2013;62:571-83.
8. Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013;31:71-5.
9. Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management ofon-target toxicity. Mol Ther 2013;21:904-12.
10. Urbanska K, Lanitis E, Poussin M, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res 2012;72:1844-52.
11. Parmar S, Ritchie DS. Allogeneic transplantation as anticancer immunotherapy. Curr Opin Immunol 2014;27:38-45.
12. Hess Michelini R, Manzo T, Sturmheit T, et al. Vaccineinstructed intratumoral IFN-γ enables regression of autochthonous mouse prostate cancer in allogeneic T-cell transplantation. Cancer Res 2013;73:4641-52.
13. Gill S, Porter DL. Reduced-intensity hematopoietic stem cell transplants for malignancies: harnessing the graftversus-tumor effect. Annu Rev Med 2013;64:101-17.
14. Skitzki J, Craig RA, Okuyama R, et al. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Res 2004;64:2183-91.
15. Du X, Jin R, Ning N, et al. In vivo distribution and antitumor effect of infused immune cells in a gastric cancer model. Oncol Rep 2012;28:1743-9.
16. Introna M, Pievani A, Borleri G, et al. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Transplant 2010;16:1603-7.
17. Comerford I, Harata-Lee Y, Bunting MD, et al. A myriad of functions and complex regulation of the CCR7/CCL19/ CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 2013;24:269-83.
18. Shrestha B, Hashiguchi T, Ito T, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol 2010;184:4819-26.
19. Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011;470:548-53.
20. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012;23 Suppl 8:viii6-9.
21. Davidsson S, Ohlson AL, Andersson SO, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol 2013;26:448-55.
22. Kotsiou E, Davies JK. New ways to separate graftversus-host disease and graft-versus-tumour effects after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2013;160:133-45.
23. Raber PL, Thevenot P, Sierra R, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer 2014;134:2853-64.
24. Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013;190:3783-97.
25. Wang Z, Zhang Y, Liu Y, et al. Association of myeloidderived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J Immunother 2014;37:43-50.
26. Adams DL, Martin SS, Alpaugh RK, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A 2014;111:3514-9.
27. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumorinitiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128-41.
28. Santoni M, Massari F, Amantini C, et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013;62:1757-68.
29. Oldham KA, Parsonage G, Bhatt RI, et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol 2012;61:385-94.
30. Heylmann D, Bauer M, Becker H, et al. Human CD4+CD25+ regulatory T cells are sensitive to low dose cyclophosphamide: implications for the immune response. PLoS One 2013;8:e83384.
31. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after singledose cyclophosphamide associates with longer patient survival. Nat Med 2012;18:1254-61.
32. Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 2014;74:104-18.
33. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271-5.
34. Liu J, Wang J, Jiang W, et al. Effect of cytotoxic T-lymphocyte antigen-4, TNF-alpha polymorphisms on osteosarcoma: evidences from a meta-analysis. Chin J Cancer Res 2013;25:671-8.
35. Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22.
36. Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005-10.
37. Holmgaard RB, Zamarin D, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013;210:1389-402.
38. Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 2014;26:104-11.
39. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity,pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75.
40. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65.
41. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity,and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54.
42. Ponte JF, Ponath P, Gulati R, et al. Enhancement of humoral and cellular immunity with an antiglucocorticoid-induced tumour necrosis factor receptor monoclonal antibody. Immunology 2010;130:231-42.
43. Cohen AD, Schaer DA, Liu C, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intratumor accumulation. PLoS One 2010;5:e10436.
44. Rech AJ, Mick R, Martin S, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med 2012;4:134ra62.
45. Atchison E, Eklund J, Martone B, et al. A pilot study of denileukin diftitox (DD) in combination with high-dose interleukin-2 (IL-2) for patients with metastatic renal cell carcinoma (RCC). J Immunother 2010;33:716-22.
46. Ling W, Zhang J, Yuan Z, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res 2014;74:1576-87.
47. Yamamoto R, Yamamoto Y, Imai S, et al. Effects of various phytochemicals on indoleamine 2,3-dioxygenase 1 activity: galanal is a novel, competitive inhibitor of the enzyme. PLoS One 2014;9:e88789.
48. Sippel TR, White J, Nag K, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res 2011;17:6992-7002.
49. Vanella L, Di Giacomo C, Acquaviva R, et al. The DDAH/NOS pathway in human prostatic cancer cell lines: antiangiogenic effect of L-NAME. Int J Oncol 2011;39:1303-10.
Cite this article as: Shi H, Qi X, Ma B, Cao Y, Wang L, Sun L, Niu H. The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies. Chin J Cancer Res 2015;27(2):128-137. doi: 10.3978/ j.issn.1000-9604.2014.12.15
10.3978/j.issn.1000-9604.2014.12.15
Submitted Jul 20, 2014. Accepted for publication Nov 28, 2014.
View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2014.12.15
杂志排行
Chinese Journal of Cancer Research的其它文章
- Transcatheter embolization therapy in liver cancer: an update of clinical evidences
- Progression of targeted therapy in advanced cholangiocarcinoma
- Kaiso mainly locates in the nucleus in vivo and binds to methylated, but not hydroxymethylated DNA
- Variant TP53BP1 rs560191 G>C is associated with risk of gastric cardia adenocarcinoma in a Chinese Han population
- HER2 discordance between paired primary gastric cancer and metastasis: a meta-analysis
- Feasibility of cetuximab and chemoradiotherapy combination in Chinese patients with unresectable stage III non-small cell lung cancer: a preliminary report
