Potential Distribution Modeling and Morphology of Pelias barani (Böhme and Joger, 1983) in Turkey
2015-10-31Serkan
Serkan GÜL
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize 53100, Turkey
Potential Distribution Modeling and Morphology of Pelias barani (Böhme and Joger, 1983) in Turkey
Serkan GÜL*
Department of Biology, Faculty of Arts and Sciences, Recep Tayyip Erdoğan University, Rize 53100, Turkey
The climatic p
of Pelias barani, a rare Pontic endemic viper, are analysed and a new locality record is reported. According to species distribution modeling, with the average test AUC was was 0.904 ± 0.068, bioclimatic variables such as Precipitation of driest month (45.5%), Temperature seasonality (18.9%), Precipitation seasonality (17.9%) and Maximum temperature of warmest period (14.7%) appear to have the most useful information on geographic distribution of Pelias barani. Distribution models of Pelias barani under current climatic conditions showed better adaptation to the northwest and northeast part of Turkey.
Climate, Maxent, New locality, Pelias barani, Rize, Turkey, Vipera
1. Introduction
The family Viperidae includes three subfamilies, thirtyeight genera (Pyron et al., 2013) and 328 species (from The Reptile Database 2014). The genus Vipera (Laurenti, 1768) includes 32 species inhabiting Asia Minor, Eurasia, Northern Africa and the Middle East (Ananjeva et al., 2006; from The Reptile Database 2014). Currently, 14-15 species belonging to the family Viperidae have been recognized in Turkey (Tuniyev et al, 2012, Göçmen et al., 2014). Taxonomically, the genus Pelias was recognized first as a subgenus of genus Vipera (Venchi and Sindaco, 2006), and many authors used “Pelias” as subgenus (Ananjeva et al., 2006; Venchi and Sindaco, 2006; Afsar and Afsar, 2009; Avcı et al., 2010), but later according to a catalogue of living and extinct species in recent years (Wallach et al., 2014), Pelias was recognized as a genus in the family Viperidae that includes P. altaica, P. anatolica, P. barani, P. berus, P. darevskii, P. dinniki, P. ebneri, P. eriwanensis, P. kaznakovi, P. lotievi, P. magnifica, P. nikolskii, P. olguni, P. orlovi, P. pontica, P. renardi, P. sachalinensis, P. seoanei, and P. ursinii species (Wallach et al., 2014). Accordingly, I use Peliasas genus in this study. Pelias barani (Baran’s Adder) is one of the Anatolian vipers with little information available about its biology (Kumlutaş et al., 2012). P. barani was first described from its type locality in 60 km N of Adapazarı at 400 m a.s.l. (40°50' N, 30°25' E)in the northwestern Anatolia with a female specimen (Böhme and Joger, 1983). Later, new locality records were reported from the northwestern and the northeastern Anatolia (Baran et al., 1997; Franzen and Heckes, 2000; Baran et al., 2001; Avcı et al., 2004; Baran et al., 2005; Kumlutaş et al., 2012) (Table 1). The taxonomic status of P. barani was evaluated with several studies in respect to phylogenetic data. Firstly, Joger et al. (1997) studied the phylogenetic position of P. barani and P. nikolskii within the P. berus complex using morphological and hemipenial comparisons, and a partial sequence of the mitochondrial cyt b gene. According to mitochondrial DNA information, they found that P. barani might be a subspecies of P. berus, but they showed that it was a different species using both morphological and hemipenial data. Later, Joger et al. (2003) identified five groups including P. barani, and genus Pelias that is consisted basically of two lineages covering P. berus and P. ursinii-kaznakowi complex. Secondly, Kalyabina-Hauf et al. (2004) studied systematic phylogeny of P. berus complex, and they found that P. bosniensis and P. barani were morphologically different from P. bersus and are suspected to be adifferent species of them. In addition, they reported that if P. barani would be recognized a separate species, P. barani was supported as paraphyletic, with P. berus. Finally, Garrigues et al. (2005) indicated that molecular phylogeny of genus Vipera, which has three main branches of the European clade, were distinguished the Pelias group including P. barani, Vipera ammodytes, and Vipera aspis.
Species distribution modeling creates an expected distribution map of a species based on climatic and others environmental conditions known presence localities (Kozak et al., 2008). It is also useful for conservation strategies and selection of protected areas (Gül, 2013) as well as the relationship between divergence and speciation mechanisms, potential geographic distributions of species on speciation (Graham et al., 2004).
In the text the main goal is to define the climatic patterns and factors affecting species distribution. Therefore, I ran species distribution modeling by Maxent (Philips et al., 2006) because knowledge about the geographical distribution of P. barani is crucial for conservation and spatial planning.
2. Materials and Methods
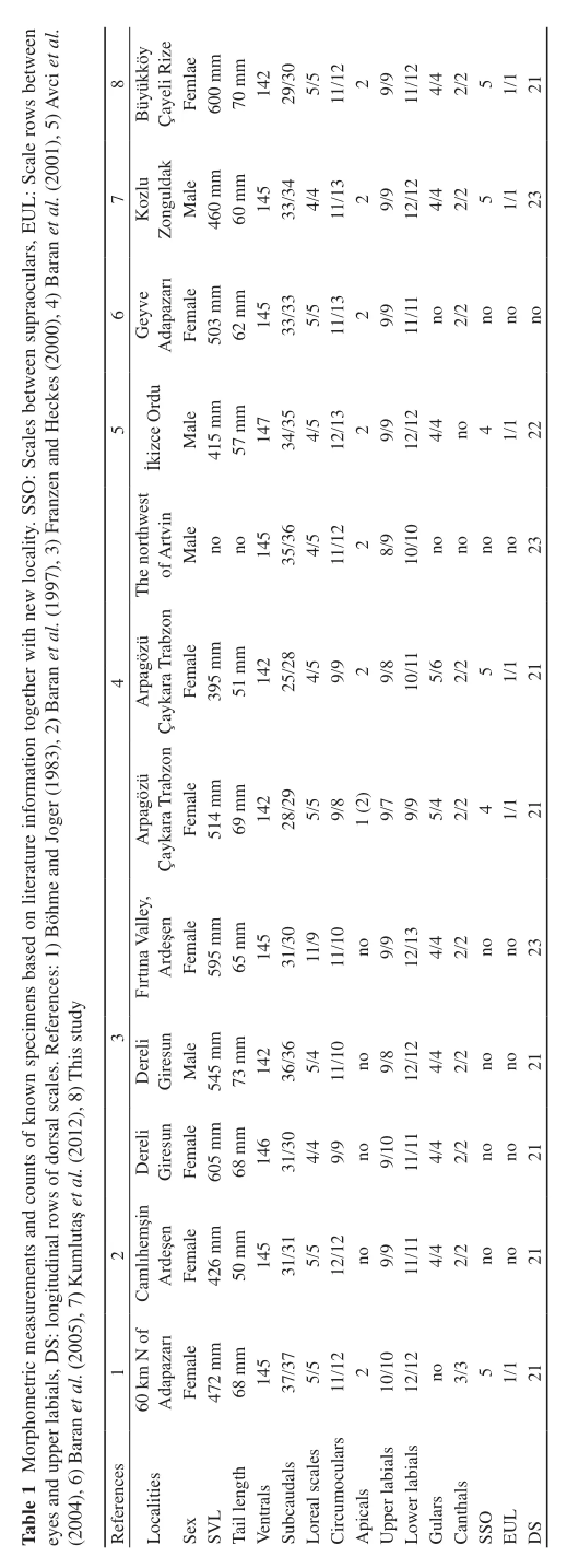
2.1 Studied Species A female Pelias barani specimen was found in village Büyükköy of Çayeli, Rize, at 529 m above sea level, on October 18, 2014 by Rahime USTABAŞ from Twelfth Region Headship in Rize under Ministry of Forest and Water Affairs (Figure 1). After analysis of morphological features of the specimen (Table 1) at the Zoology Research Laboratory, Recep Tayyip Erdoğan University, it was safely released back into its natural habitat.
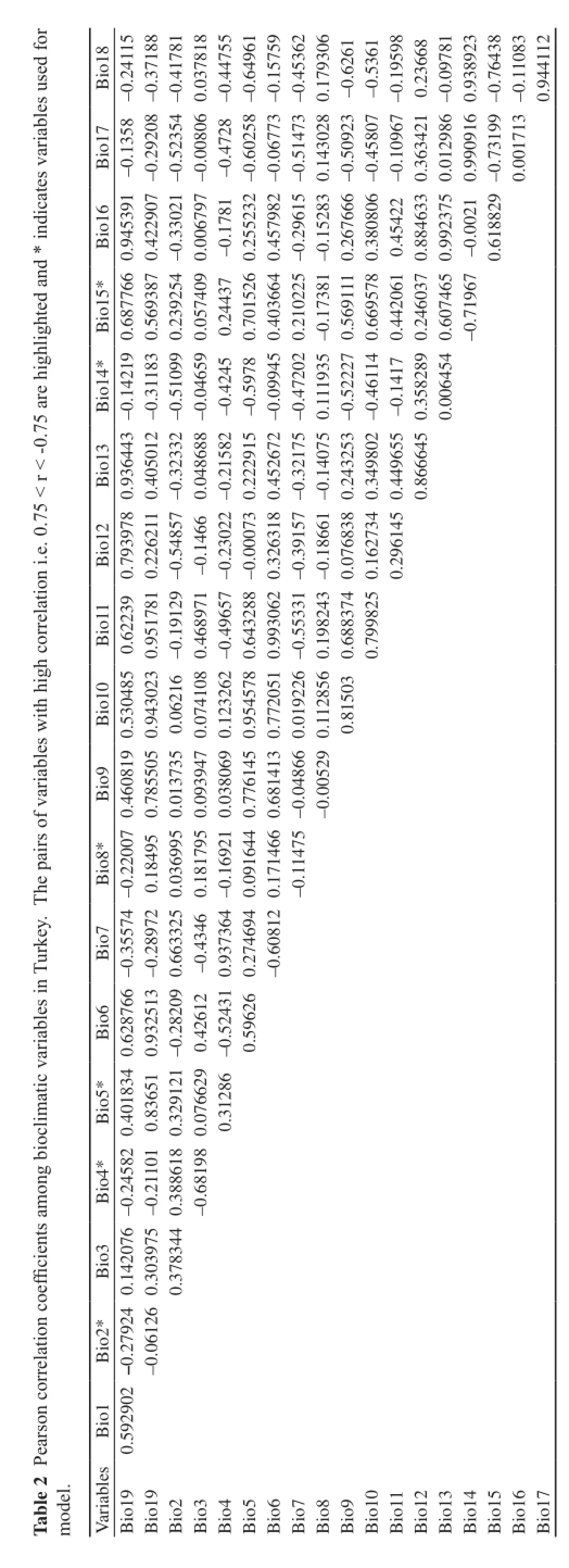
2.2 Environmental Variables 19 bioclimatic variables were downloaded from global climate layers (available at www.worldclim.org) in the highest resolution at 30 arcseconds (~1 km) under current conditions (~1950–2000) (Hijmans et al., 2005). The global climate layers were clipped to the borders of Turkey using Extract by Mask in ArcGis v. 10.1. Many of the bioclimatic variables are very similar to each other. Therefore, all bioclimatic variables were examined for Pearson correlation coefficient (0.75 < r < –0.75) using ENMTools 1.3 (Warren et al., 2010) and redundant variables were excluded. Six bioclimatic variables were eventually selected for the model (Table 2).
Species distribution modeling was performed using Maxent software v. 3.3.3e (Philips et al., 2006). In order to develop species distribution modeling, 10 presence localities were used based on the new locality record and literature data (Figure 2, Table 3). Seven test points similar to presence localities were created by using Geospatial Modeling Environment version 0.7.2.0 (Beyer, 2012). The final model was composed of the average the AUC of ten replicates.
3. Results
3.1 Morphology of the new specimen of Pelias barani The number of ventral scales of the specimen caught from Çayeli is 142 and the number of subcaudal scales is 29–30. The scales on longitudinal rows of dorsal at midbody were 21. The specimen had two apicals in contact with rostral and also had two canthals on each side of the head. Loreal scales between the preocular and the postnasal were 5–5. Circumocular scales around the eye were 11–12 in the specimen. Upper and lower labials of the specimen were 9–9 and 11–12, respectively. Gular scales that are in contact with the first ventral scales were 4–4. The specimen had a total length of 670 mm (head and body length 600 mm; tail length 70 mm) (Table 1).
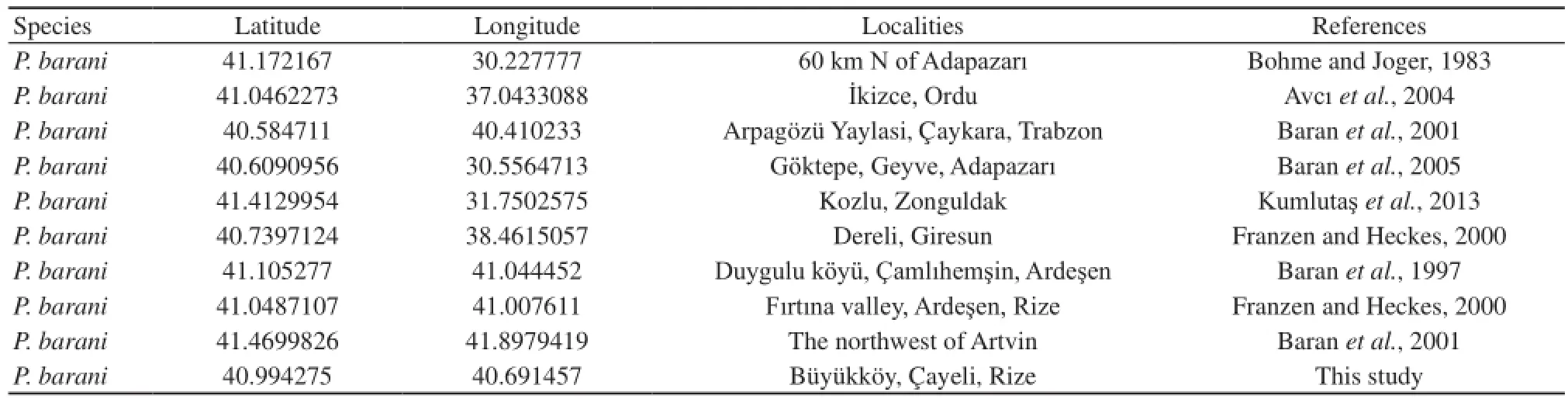
Table 3 Localities information of Pelias barani based on literatures data.
3.2 Color patterns of Pelias barani The head of the female specimen captured in Çayeli is large (Figure 3A). The dorsal color pattern is almost black in hue. The ventral color includes many different shades of brown, sometimes darkish, or whitish brown and also ground color of the ventral side is lighter than dorsal side (Figure 3B). This color variation continues across both upper labials and lower labials on each side of head. The specimen has a different color of ventral surface of the end of the tail that is orange (Figure 3C). In addition, references, which are listed in Table 1, indicated that the body color of all known specimens were totally black; however, the ground color of the second specimen of reference 4 was greyish brown with a blackish zig-zag band.
3.3 Species distribution modeling Based on current climatic conditions distribution model of Pelias barani showed that some parts of northwest and northeast of Turkey was the areas represented with better predicted conditions for Pelias barani’s habitat (Figure 2). As a result of estimates of relative contributions of the environmental variables with Maxent, the most environmental variables with highest gain that explains more than 10% of the presence of P. barani were Bio-14 (Precipitation of driest month, 45.5%), Bio-4 (Temperature seasonality, 18.9%), Bio-15 (Precipitation seasonality, 17.9%) and Bio-5 (Maximum temperature of warmest period, 14.7%). Other variables had a percent contribution less than 5%. The average test AUC (the area under the receiver operating characteristic curve) value of the distribution model of P. barani was 0.904 ± 0.068 and 10 percentile training presence logistic threshold was 0.3632. Predicted suitability was highest in northwest and northeast regions of Turkey with extreme precipitation conditions that infuence potential range of P. barani during the year, but with a lower temperature variation throughout the year, low variation in total monthly precipitation throughout the year and low warm temperature anomalies throughout the year (Figure 4).
4. Discussion
There are two-distribution patterns for P. barani in Turkey, including the Black Sea Region (Zonguldak, Ordu, Giresun, Trabzon, Rize and Artvin localities) and the Marmara Region (Adapazarı localities). The Black Sea Region has the greatest amount of precipitation in Turkey. Its summer is warm and humid and the winter is cool and damp (Borysova et al., 2005). Marmara Region consists generally of three zones (İstanbul and Yıldız Mountain to the north, İzmir and Ankara to the south, and Sakarya). Therefore, it is the region that has the greatest diversity of climate variable. It has Mediterranean climate that is warm to hot, dry summers and mild to cool, wet winters on the Agean Sea coast and the south Marmara Sea coast, and has an oceanic climate that is warm summers and cool winters on the Black Sea coast (Sertel and Ormeci, 2011). Adapazarı localities found in the Sakarya zone of P. barani are similar to the Black Sea Region with regard to climatic features (Sirdaş, 2005). The geographic distribution of P. barani that is limited with the north coastal areas of Turkey indicates that this species is not resistant to a wide variety of conditions (eurioic) (Figure 2). P. barani has actually been detected in particular regions under very rainy environmental conditions on the Anatolian range. I found that the model, which explains the current distribution pattern of P. barani, is based on the precipitation of driest period (Bio-14), Temperature seasonality (Bio-4), Precipitation seasonality (Bio-15) and Maximum temperature of warmest period (Bio-5) of northwest and northeast of Turkey. Summer temperature and precipitation are probably a limitation factor of P. barani’s dispersion (Figure 2). Therefore, P. barani inhabits towards to the Black Sea region of Turkey. In fact, the geographical structure of Anatolia plays a significant role for a rich biodiversity because the Anatolia generates from multiple glacial refugia (Bilgin, 2011; Tarkhnishvili et al., 2012). This suggests that occurrence of the genetic diversity correlates with differentiation in the ecology of local refugia within Anatolia (Gül, 2013). The important mountain chains, such as Toros Mountains, North Anatolian Mountains, the Anatolian Diagonal and Western Anatolian Mountains, are a barrier affecting the distribution of many amphibians (Gül, 2013; Özdemir et al., 2014) and reptiles (Kapli et al., 2013; Sindaco et al., 2014). Similarly, Brito et al. (2008) showed that distribution of the viperid snakes (Vipera latastei and V. monticola) were influenced by the precipitation of driest period for Eastern Iberia, Algeria, and Rif and Middle Atlas, and High Atlas except Western Iberia. In biogeographical scenario of Vipera seoanei, another study represents that the origin of genus Pelias can be expanded from the north Black Sea region toward Europe between the late Miocene and early Pliocene, and range expansions of the genus Pelias were affected by cooler temperatures of Pleistocene while warm temperatures of middle Pliocene prevailed its expansion (Martínez-Freiría et al. 2015). In fact, species of the genus Pelias can be better adapted to colder climates (Garrigues et al., 2005). In addition, several studies support that climatic factors are responsible for geographical distribution of many reptile species (Santos et al., 2006; Brito et al., 2008; Brito et al., 2011; Sow et al., 2014; Martínez-Freiría et al., 2015).
Consequently, the P. barani specimen with new locality in this study has similarities to specimens in the literature in terms of morphometric measurements (Table 1). Since P. barani is a rare species, new locality records are very important in order to understand the distributional ranges of the species. In addition, this study represents that climatic conditions restricts its potential distribution in Turkey, and also it has been suggested that local precipitation patterns was important for the distribution model of the species.
According to IUCN Red List, P. barani (Vipera barani in IUCN) is listed as near threatened (NT) and it is known as pontic endemic (Venchi and Sindaco, 2006). Population trend of P. barani has been decreased because of habitat loss, and persecution. Similarly, I observed habitat loss caused by destruction of forest through commercial harvest and construction of roads in Büyükköy locality. In addition, it is killed like many snakes when P. barani is seen by the local people. I also realized that many amphibians and reptiles were being killed on roads, and thought that P. barani might be killed like others. Therefore, habitat destruction, road deaths and deathscaused by local human activities is a major threat to survival of P. barani and significant cause of extinction of P. barani throughout Black Sea coast. As a result of all these threats, it might be candidate to enter Vulnerable (VU) category under the red list category in the near future. Literature information about its distribution and ecology are very scarce. Therefore, it’s a new locality record and climatic preferences might be useful for conservation of this pontic endemic viper.
Afsar M., Afsar B. 2009. A new locality for Vipera (Pelias) kaznakovi Nikolsky, 1909 (Reptilia, Viperidae) in the northeastern Anatolia. Russ J Herpetol,16:155–158
Ananjeva N. B., Orlov N. L., Khalikov R. G., Darevsky I. S., Ryabov I. S. Barabanov A. V. 2006. The Reptiles of Northen Eurasia: Taxonomic diversity, distribution, conservation status. Pensoft Series Faunistica, No. 47, 245pp
Avcı A., Üzüm N., Olgun K. 2004. A new record of Vipera barani Böhme and Joger, 1983 (Reptilia, viperidae) from north-eastern Anatolia, Turkey. Russ J Herpetol, 11: 77–79
Avcı A., Ilgaz Ç., Baskaya S., Baran İ., Kumlutas Y. 2010. Contribution to the distribution and morphology of Pelias darevskii (Vedmederja, Orlov et Tuniyev 1986) (Reptilia: Squamata: Viperidae) in northeastern Anatolia. Russ J Herpetol, 17: 1–7
Baran İ., Tosunoğlu M., Kaya M., Kumlutaş Y. 1997. On the herpetofauna of the vicinity of Çamlıhemşin. Turk J Zool, 21:409–416
Baran İ. Joger U., Kutrup B., Türkozan O. 2001. On new specimens of Vipera barani Böhme and Joger, 1983 from northeastern Anatolia, and implications for the validity of Vipera pontica Billing, Nilson and Sattler, 1990 (Reptilia, Viperidae). Zool Mid East, 19: 33–36
Baran İ. Kumlutaş Y., Ilgaz Ç., Iret F. 2005. Geographical distributions and taxonomical states of Telescopus fallax (Fleischman, 1831) and Vipera barani Böhme and Joger, 1983. Turk J Zool, 29: 217–224
Beyer H. L. 2012. Geospatial Modelling Environment (Version 0.7.2.0). (software). URL: 279 http://www.spatialecology.com/ gme.
Bilgin R. 2011. Back to the suture: The distribution of intraspecific genetic diversity in and around Anatolia. Int J Mol Sci, 12: 4080–4103
Borysova O., Kondakov A., Paleari S., Rautalahti-Miettinen E., Stolberg F., Daler D. 2005. Eutrophication in the Black Sea region; impact assessment and causal chain analysis. University of Kalmar, Kalmar, Sweden, 60pp
Böhme W., Joger U. 1983. Eine neue Art des Vipera berus-Komplexes aus der Turkei. Amphibia-Reptilia, 4: 265–271
Brito J. C., Santos X., Pleguezuelos J. M., Sillero N. 2008. Inferring evolutionary scenarios with geostatistics and geographical information systems for the viperid snakes Viperalatastei and Vipera monticola. Biol J Linnean Soc, 95: 790–806
Brito J.C. Fahd S., Geniez P., Martínez-Freiría F., Pleguezuelos J. M., Trape J. F. 2011. Biogeography and conservation of viperids from North-West Africa: an application of ecological niche-based models and GIS. J Arid Environ, 75: 1029–1037
Franzen M., Heckes U. 2000. Vipera barani Böhme and Joger, 1983 aus demöstlichen Pontus-Gebirge, Türkei: Differentialmerk-male, verbreitung, Habitate. Spixiana, München, 23: 61–70
Garrigues T., Ferquel C. E., Choumet V., Failloux A. B. 2005. Molecular phylogeny of Vipera Laurenti, 1768 and the related genera Macrovipera (Reuss, 1927) and Daboia (Gray, 1842), with comments about neurotoxic Vipera aspis aspis populations. Mol Phylogenet Evol, 35: 35–47
Göçmen B., Mebert K., Iğci N., Akman B., Yıldız M. Z., Oğuz M. A., Altın Ç. 2014. New locality records for four rare species of vipers (Reptilia: Viperidae) in Turkey. Zool Mid East, 60: 306–313
Graham K. H., Ron S. R., Santos J. C., Schneider C. J., Moritz C. 2004. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution, 58: 1781–1793
Gül S. 2013. Ecological divergence between two evolutionary lineages of Hyla savignyi (Audouin, 1827) in Turkey: Effects of the Anatolian Diagonal. Anim Biol, 63: 285–295
Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol, 25: 1965–1978
Iucn 2014. IUCN Red List of Threatened Species. Version 2014.1. [www.iucnredlist.org]. Downloaded on 22 October 2014.
Joger U., Lenk P., Baran I., Böhme W., Ziegler T., Heidrich P.,Wink M. 1997. The phylogenetic position of Vipera barani and of V. nikolskii within the Vipera berus complex. In Böhme W., Bischoff W., Ziegler T. (Eds), Herpetologia Bonnensis. Bonn (Zoologisches Forschungsinstitut und Museum A. koenig), 185–194
Joger U., Kalyabina-Hauf S. A., Schweiger S., Mayer W., Orlov N. L., Wink M. 2003. Phylogeny of Eurasian Vipera (Subgenus Pelias). 12th Ord Gen Meet S.E.H., St. Petersburg, Russia, 12-16 August 2003. Abstracts: 77
Kalyabina-Hauf S., Schweiger S., Joger U., Mayer W., Orlov N.,Wink M. 2004. Phylogeny and systematics of adders (Vipera berus complex). Mertensiella, 15: 7–15
Kapli P., Botoni D., Ilgaz Ç., Kumlutaş Y., Avcı A., Rastegar-Pouyani N., Fathinia B., Lymberakis P., Ahmadzadeh F.,Poulakakis N. 2013. Molecular phylogeny and historical biogeography of the Anatolian lizard Apathya (Squamata, Lacertidae). Mol Phylogenet Evol, 66: 992–1001
Kozak, K. H., Graham C. H., Wiens J. J. 2008. Integrating GIS data into evolutionary studies. Trends Ecol Evol, 23: 141–148
Kumlutaş Y., Sözen M., Ilgaz Ç. 2012. New record of the rare Vipera barani Böhme and Joger, 1983. Herpetozoa, 25: 183–188
Martínez-Freiría F., Velo-Antón G., Brito J. C. 2015. Trapped by climate: interglacial refuge and recent population expansion in the endemic Iberian adder Vipera seoanei. Divers Distrib, 21: 331–344
Özdemir N., Gül S., Poyarkov N. A., Kutrup B., Tosunoğlu M.,Doglio S. 2014. Molecular systematics and phylogeography of Bufotes variabilis (syn. Pseudepidalea variabilis) (Pallas, 1769) in Turkey. Turk J Zool, 38: 412–420
Phillips S. J., Anderson R. P., Schapire R. E. 2006. Maximum entropy modeling of species geographic distributions. Ecol Model, 190: 231–259
Pyron R. A., Burbrink F. T., Wiens J. J. 2013. A phylogeny and updated classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol, 13: 93
Santos X., Brito J. C., Sillero N., Pleguezuelos J. M., Llorente G. A., Fahd S., Parellada X. 2006. Inferring habitat-suitability areas with ecological modelling techniques and GIS: A contribution to assess the conservation status of Vipera latastei. Biol Cons, 130: 416–425
Sertel E., Ormeci C. 2011. Modelling land cover change impact on the summer climate of the Marmara Region, Turkey. Int J Glob Warm, 3: 194–202
Sindaco R., Panagiotis K., Roberto S., Petros L. 2014. Taxonomic reassessment of Blanus strauchi (Bedriaga, 1884) (Squamata: Amphisbaenia: Blanidae), with the description of a new species from south-east Anatolia (Turkey). Zootaxa, 3795: 311–326
Sirdaş S. 2005. Daily wind speed harmonic analysis for Marmara region in Turkey. Energ Convers Manage, 46: 1267–1277
Sow A. S., Martinez-Freiria F., Dieng H., Fahd S., Brito J. C. 2014. Biogeographical analysis of the Atlantic Sahara. Reptiles: Environmental correlates of species distribution and vulnerability to climate change. J Arid Environ, 109: 65–73
Tarkhnishvili D., Gavashelishvili A., Mumladze L. 2012. Palaeoclimatic models help to under- stand current distribution of Caucasian forest species. Biol J Linn Soc, 105: 231–248
Tuniyev S. B., Avcı A., Tuniyev B. S., Agasian A. L., Agasian L. A. 2012. Description of a new species of Shield-Head Vipers Pelias olguni sp. nov. from basin of upper fow of the Kura River in Turkey. Russ J Herpetol, 19: 314–332
Venchi A., Sindaco R. 2006. Annotated checklist of the reptiles of the Mediterranean countries, with keys to specific identification. Part 2—Snakes (Reptilia, Serpentes). Annali del Museo Civico di Storia Naturale "Doria G.", Genova, 98: 259–364
Wallach V., Williams K. L., Boundy J. 2014. Snakes of the World: A Catalogue of Living and Extinct Species. Boca Rattan, FL: CRC Press, 1237pp
Warren D. L., Glor R. E., Turelli M. 2010. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography, 33: 607–611
Dr. Serkan GÜL, from Recep Tayyip Erdoğan University, Rize, Turkey, with his research focusing on amphibians and reptiles of the Middle East
E-mail: serkan.gul@erdogan.edu.tr
11 November 2015 Accepted: 18 May 2015
杂志排行
Asian Herpetological Research的其它文章
- A New Record of Kaloula (Amphibia: Anura: Microhylidae) in Shanghai, China
- First Record of Male Combat in a Wild Malayan Pit Viper(Calloselasma rhodostoma)
- Reevaluation of the Taxonomic Status of a Poorly Known Gecko, Gekko liboensis (Reptilia: Squamata)
- Acoustic Characteristics of Advertisement Calls in Babina adenopleura
- Characterization of the Genetic Diversity of Trachemys dorbigni and Phrynops hilarii
- Genetic and Morphological Variations within Laudakia microlepis (Blanford, 1874) (Sauria: Agamidae) Populations in Southeastern Iran with Description of a New Subspecies
