Effect of SecinH3 on lung injury induced by sepsis of rats
2015-10-31FengGuoChunYanYan
Feng Guo, Chun-Yan Yan
1Intensive Care Unit, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
2Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
Effect of SecinH3 on lung injury induced by sepsis of rats
Feng Guo1, Chun-Yan Yan2*
1Intensive Care Unit, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
2Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
ARTICLE INFO
Article history:
in revised form 20 October 2015
Accepted 3 November 2015
Available online 20 December 2015
Sepsis
LPS
SecinH3
Acute lung injury
Objective: To study effect of SecinH3 on lung injury induced by the sepsis of rats. Methods:A total of 30 SPF Wistar rats were randomly divided into two groups, including 5 rats in the control group and 25 in the model group. The intraperitoneal injection of endotoxinlipopolysaccharide (LPS) was performed to build the animal model of sepsis. The blood gas analysis was carried out. Afterwards, change in the expression of pro-inflammatory factors of IL-1, IL-6 and TNF-α in the serum were detected. To study the mechanism of SecinH3 in the process of lung injury induced by the sepsis, the rats with the successful modeling of sepsis were randomly divided into two groups. Rats in the SecinH3 group were given the intraperitoneal injection of 100 μg/12 h SecinH3 for 24 h; while rats in the control group were given the injection of same solvent by the same dosage. The blood was drawn from the heart by 500 μL for the blood gas analysis to detect the change in the expression of proinflammatory factors of IL-1, IL-6 and TNF-α in the treatment group and control group. After separating the lung tissue, the Real-time PCR and western blotting were performed to analyze the effect of SecinH3 on the expression of cytohesins and also discuss the change of epidermal growth factor receptor (EGFR) and p-EGFR related to the signaling pathway of EGFR-p38 mitogen-activated protein kinase that is regulated by cytohesins. Results: Three rats died within 4 h after the injection of LPS, while other 22 ones had the successful modeling, with the success rate of 88%. After being stimulated by LPS, compared with the control group,the arterial partial pressure of oxygen of rats in the treatment group was significantly reduced(P<0.05), while the partial pressure of CO2was significantly increased (P<0.01). After being treated by SecinH3, Pa/O2was increased with the sepsis, while Pa/CO2was decreased with the action of SecinH3, which indicated that SecinH3 had the certain ‘repairing' ability for the lung injury. SecinH3 might inhibit the cytohesins and then inhibit the phosphorylation of EGFR. Conclusions: SecinH3 can significantly inhibit the cytohesins and then relieve the lung injury induced by the sepsis of rats.
Document heading doi:10.1016/j.apjtm.2015.11.004
1. Introduction
The sepsis is the systemic inflammatory response syndrome caused by the infection[1]. Its clinical manifestations include the infection and many common complications in the situation of stress such asthe major operation. The nature of sepsis is the release of abundant cytokines and inflammatory mediators to cause the autoimmune injury of host and then the multiple organ dysfunction syndrome. Because of many epidemic factors, the sepsis is related to the abnormal activation of many immune-related signaling pathways. A research reported that the sepsis with the main pathogenic bacteria of Gram-negative bacteria occupied over 60% of clinical cases[2],while the endotoxin-lipopolysaccharide (LPS) of Gram-negative bacteria is the important component to start the immune response. LPS is bound with the receptor on the surface of cells to activate a series of congenital immune signaling pathways, secrete a greatdeal of inflammatory factors and thus cause the uncontrollable inflammatory response of organism[3,4]. But the specific molecular mechanism for the pathogenesis of sepsis has not been clear yet.
The main organ complication of sepsis is the acute lung injury(ALI), which causes the injury of alveolar epithelial cells and capillary endothelial cells, and result in the diffuse pulmonary interstitial and alveolar edema and acute hypoxemic respiratory failure. The clinical manifestations of ALI usually included the osmotic edema and alveolar collapse[5-7]. The pathogenesis of ALI has not been clear yet, but its nature is still the inflammatory response. Because of the immunologic disorder caused by the sepsis,many inflammatory cells such as the macrophages and neutrophil granulocyte are in the extremely active state. The inflammatory mediators and cytokines released by these cells will aggravate the further injury of lung tissue and pulmonary capillary endothelium. Therefore, the study on the sepsis-induced lung injury will be of critical significance for the clinical treatment of ALI.
Cytohesins refer to some kind of small GTPases of Ras family,as the Guanine- nucleotide exchange factor of ADP-ribosylation factor[8]. Cytohesins were found by Kolanus et al in the immune system in 1996. According to a previous research, Cytohesin had the high expression in the colorectal cancer and could regulate the EGFR and IGF-IR signaling pathways. The inhibition against the expression of Cytohesin would inhibit the downstream molecules of epidermal growth factor receptor (EGFR) and IGF-IR signaling pathways[9]. EGFR is the member of human epidermal growth factor receptor family, which has the activity of tyrosine kinase and is some kind of important transmembrane receptor. The EGFR signaling pathway joins in the process of migration, adhesion, proliferation and differentiation of cells. The previous researchers found that EGFR was involved in the inflammation[10] and it played a key role of regulation in the lung injury[11,12]. The chemical antagonist of cytohesin SecinH3 that was synthesized by Waldemar et al could be bound with SEC7 to block the Cytohesins[13]. Therefore, after building the rat model of sepsis in this study, it was to study the pathogenesis of ALI induced by sepsis of rats. The model rats were given the treatment of cytohesin inhibitor-SecinH3. It was to study the mechanism of SecinH3 in the lung injury induced by the sepsis of rats, in order to provide the certain experimental reference for the clinical diagnosis and treatment.
2. Materials and methods
A total of 30 specific pathogen free (SPF) male Wistar rats with the weight of (250±50) g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. All rats were fed with the standard pellets and in the standard cage, with 5 rats in each cage. Rats were given the diet and water freely during the experiment. The ventilation was good in the feeding room, with the natural lighting day and night. The culture temperature was maintained at 18-25 ℃.
LPS was purchased from Sigma (America, item No. L2630); IL-1-β, IL-6 and TNF-α ELISA kit from USCN Business Co., Ltd.(item No. SEA563Mu, SEA079Mu and SEA033Mu); SecinH3 from Merck (Germany, item No. 565725); RNA extraction kit from Ambion (America, item No. 12183-555); the reverse transcription kit from Applied Biosystems (America, item No. 4366597); Realtime PCR fluorescent quantitative kit from Bio-Rad (America,item No. 172-5264); the monoclonal antibodies of Cytohesin-2,EGFR, p-EGFR and GAPDH from Santa Cruz Biotechnology(America, item No. sc-374640, 377229, 377547 and 365062);horseradish peroxidase (HRP) labeled secondary antibody from Beijing Zhongshan Jinqiao Biotechnology; ECL Chemiluminescent Substrate Reagent Kit from Life Technologies (America, item No. WP20005);
ABL800 FLEX blood gas analyzer was Radiometer; DNA/RNA analyzer was Qubit Fluorometer; the Full wavelength microplate reader was Molecular Devices; and the fluorescent quantitative PCR system was Bio-rad-CFX96 Touch.
2.1. Methods
2.1.1. Preparation of reagents
The SecinH3 (C24H20N4O4S) powder was dissolved in DMSO. Then it was diluted with the sterile normal saline into 1mM mother liquid and stored at 4 ℃. LPS was dissolved in the normal saline to achieve the concentration of 10 μg/mL and then stored at 4 ℃.
2.1.2. Modeling of sepsis rats
After 7 d of adaptive feeding, 30 SPF Wistar rats were randomly divided into two groups, with 5 rats in the control group and 25 in the model group. Rats in the model group were given the intraperitoneal injection of 10 mg/kg LPS; while rats in the control group were given the injection of normal saline by the same dosage. Twenty-four hours later, rats were anesthetized using the chloral hydrate method. After drawing the blood from the heart, the blood gas analysis was performed. After separating the serum, ELISA was employed to detect the related inflammatory factors.
2.1.3. Detection of related inflammatory factors
After the blood sampling, it was kept still at the room temperature for 2 h. It was then centrifuged to separate the serum. IL-1,IL-6 and TNF-α ELISA kits were adopted for the quantitative detection of IL-1, IL-6 and TNF-α in the serum samples. It set the standard wells, samples wells to be tested and blank wells. There were 7 standard wells. The standard samples with the different concentrations were added, as well as the samples to be tested, which was incubated at 37 ℃ for 2 h. A total of 100 μL test solution (biotinylated primary antibodies) were added in each well. The ELISA plate was covered with the film and then incubated at 37 ℃ for 1 h. The solution in the well was removed and each well was washed with 350 μL cleaning solution for 1-2 min. The plate was washed repeatedly 3 times. A total of 100 μL HRP-labeled secondary antibodies were added in each well. The ELISA plate was covered with the film and then incubated at 37 ℃ for 30 min. A total of 90 μL TMB substrates were added in each well. The ELISA platewas covered with the film and then colored at 37 ℃ and in a dark place for 15-25 min. When the first 3-4 of standard wells showed the obvious gradient blue, the reaction stopped. A total of 50 μL 2M H2SO4was then added. The plate reader was employed to measure the OD value of each well at 450 nm.
2.1.4. Real-time PCR detection
The lung tissues were separated to extract the total RNA (according to the instruction manual of RNA extraction kit). Qubit Fluorometer system was employed to detect the concentration and purity of RNA. The total RNA was reversely transcripted to cDNA following the instruction manual of reverse transcription kit. The real-time PCR was employed to detect the expression of related genes (Table 1,2). The mRNA sequence of MMP-13 and β-actin genes could be referred to NCBI database and then the Real-time PCR primers could be designed. All primers were synthesized by Shanghai Sangon Biotech, with the specific sequence shown in Table 3. Ct value of each gene amplification was detected, where Ct value was negatively correlated to the starting copy number. The double △Ct method was adopted to calculate the relative expression of target gene: the mean of three parallel repeated experiments was treated as the CT value of each sample, △Ct =Ct (target gene) - Ct (reference), △△CT=△Ct(sample) -△Ct (contro1). Therefore, the relative expression of target gene =2-△△Ctand the relative expression of control group was 20=1[14].
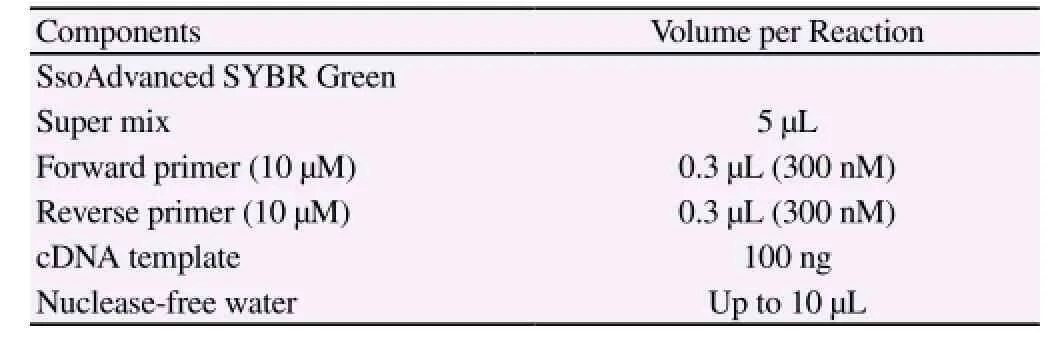
Table 2 Synthetic system of PCR.
2.1.5. Western blotting
The collected lung tissues of rats were lysed with the RIPA lysis buffer (adding the protease inhibitor cocktail for blowing and mixing). After being put on the ice for 30 min, the probe-type ultrasound was used to produce the short impact with the appropriate frequency on the ice. The lysis mixture was centrifuged at 4 ℃ and 13 000 r/min for 20 min. The supernatant was transferred to the new centrifuge tube. Protein Assay Kit was employed to detect the protein concentration.
SDS-PAGE electrophoresis was performed on the protein samples. The gel was soaked in the transfer buffer for 10 min of equilibrium. It was installed with the transfer ‘sandwich' with 100 V and 45-60 min. After the transfer, PVDF film was washed with TBS for 10-15 min. The film was placed in TBS/T blocking buffer containing 5%(w/v) skimmed milk powder and shaken at the room temperature for 1 h. Then the primary antibody with the appropriate degree of dilution was added [diluted with TBST containing 1% (w/v)skimmed milk powder]. It was incubated at the room temperature for 2 h and then the film was washed with TBST for 3 times, 5-10 min every time. The film was incubated with the secondary antibody(1:10 000, HRP-labeled) that was diluted with TBST containing 0.05% (w/v) skimmed milk powder. It was incubated at the room temperature for 1h and then the film was washed with TBST for 3 times, 5-10 min every time. It was exposed and then photographed to save the experimental results. Quantity one v4.62 was used to measure the gray value of molecular band (trace tracking). The semiquantitative value of target protein / reference protein was chosen as the quantitative basis and the statistical analysis was performed.
2.2. Statistical analysis
The experimental data was treated with SPSS11.5. The results were expressed by `mean±SD. The t test was employed for the comparison between groups, where P<0.05 indicated the significant difference.
3. Results
3.1. Modeling of sepsis rats
During the experiment, 3 rats died within 4 h after the injection of LPS, while other 22 showed successful modeling. The results showed that (Table 4), compared with the control group, the arterial partial pressure of oxygen of rats in the sepsis model group wassignificantly reduced (P<0.05), while the partial pressure of CO2was significantly increased (P<0.01).
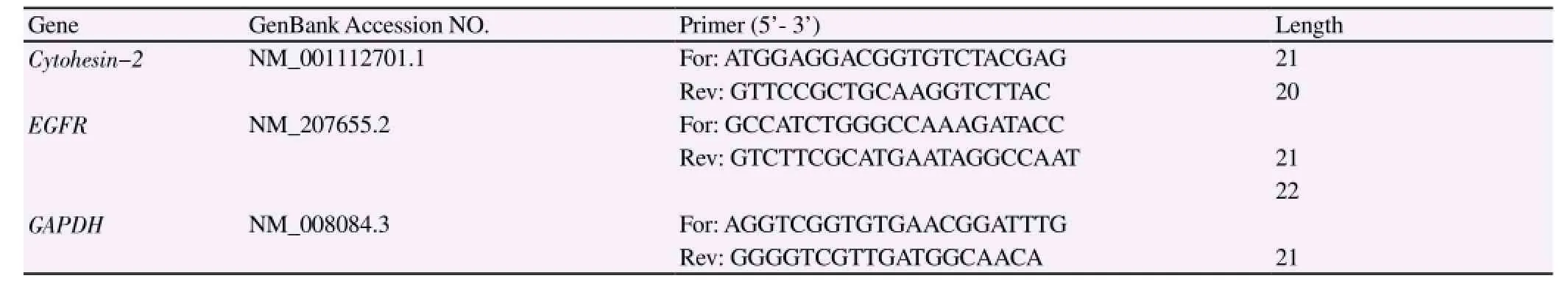
Table 3 Primers used in real-time PCR.
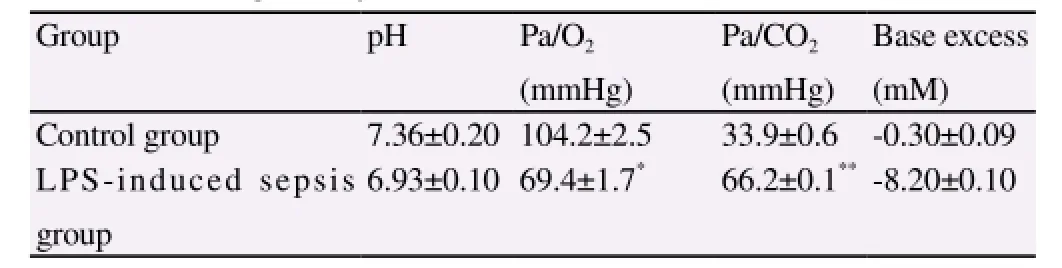
Table 4 Results of blood gas analysis 24 h after treatment of LPS.
After separating the serum of rats, ELISA was employed to detect the change in the expression of pro-inflammatory factors of IL-1,IL-6 and TNF-α in the serum. According to the results, 24 h after the treatment of LPS, the content of IL-1, IL-6 and TNF-α was significantly increased in the serum (P<0.05)(Table 5).

Table 5 Expression of IL-1, IL-6 and TNF-α.
3.2. Protection of SecinH3 for sepsis-induced lung injury
According to the results in Table 6, compared with the control group, the content of IL-1 and TNF-α in the serum of rats in the SecinH3 group was significantly decreased, while the change in the content of IL-6 showed no significant difference (P>0.05).

Table 6 Expression of IL-1, IL-6 and TNF-α.
After being treated by SecinH3, Pa/O2was increased with the sepsis, while Pa/CO2was decreased with the action of SecinH3,which indicated that SecinH3 had the certain ‘repairing' ability for the lung injury (Figure 1).
3.3. Inhibitive effect of SecinH3 on activation of cytohesins and EGFR
According to Figure 2, SecinH3 could significantly inhibit the expression of cytohesin, but had limited effect on the expression of downstream signaling molecule EGFR. The further detection on the change of protein using the Western blotting showed that, though the change in the total amount of EGFR was not significant, its activation was significantly inhibited (P<0.05). It indicated that SecinH3 might inhibit the cytohesins and then inhibit the phosphorylation of EGFR.
4. Discussion
The sepsis is the systemic inflammatory response syndrome caused by the infection. With the in-depth researches on the sepsis,the progression of sepsis has been gradually clear. The clinical pathological process of sepsis mainly includes the cytokine storm,inflammatory mediator falls, enteral bacterial translocation and intestinal endotoxemia, and the interaction between the coagulation system and inflammation[15,16]. But the specific pathogenesis of sepsis has not been clear yet. The sepsis is always accompanied with the injury of tissues and organs, which is also one of reasons for the high mortality of sepsis. Where, the ALI is the typical complication of sepsis and its clinical manifestations mainly include the osmotic edema, alveolar collapse and hypoxemia[17,18]. As its nature is still the inflammatory response, many inflammatory cells such as the macrophages and neutrophil granulocyte are in the extremely active state. The inflammatory mediators and cytokines released by these cells will aggravate the further injury of lung tissue and pulmonary capillary endothelium. Therefore, the study on the sepsis-induced lung injury will be of critical significance for the clinical treatment of ALI.
The endotoxin-LPS of Gram-negative bacteria is the important component to start the immune response. LPS is bound with the receptor on the surface of cells to activate a series of congenital immune signaling pathways, secrete a great deal of inflammatory factors and thus cause the uncontrollable inflammatory response of organism[3,4]. Meanwhile, the intraperitoneal injection of LPS to cause the excessive inflammatory response and then build the model of sepsis is also in accordance with the form of endogenous infection, which can thus simulate the progression of human sepsis. Accordingly, the intraperitoneal injection of LPS was performed to build the animal model of sepsis. During the experiment, rats in the model group were given the intraperitoneal injection of 10 mg/ kg LPS; while rats in the control group were given the injection ofnormal saline by the same dosage. Two hours after the injection of LPS, there were the disease symptoms, namely rats in the model group moved slowly and had the upright fur. Three rats died within 4 h, while other 22 showed the successful modeling. The blood gas analysis was performed to study the progression of sepsis-induced lung injury. The results showed that, compared with the control group, the arterial partial pressure of oxygen of rats in the treatment group was significantly reduced (P<0.05), while the partial pressure of CO2was significantly increased (P<0.01), which indicated the certain injury to the lung of rats in the model group.
The small G protein has the activity of GTPase. When being bound with GTP, it becomes the active form to stimulate the downstream molecules and activate them. When GTP is hydrolyzed into GDP(the GTPase), it becomes the inactive form[19]. The small G protein is some kind of ‘molecular switch' to regulate many signaling pathways of organism. Cytohesins refer to some kind of small GTPases of Ras family, as the Guanine- nucleotide exchange factor of ADP-ribosylation factor[8].
SecinH3 (C24H20N4O4S) is a synthesized cytohesins inhibitor, which can be bound with SEC7 (Sec7-domain) to block the cytohesins. In this study, after the treatment of SecinH3, the expression of cytohesins in the lung tissue of rats was decreased, but the molecular mechanism of such process had not been clear and it might be related to the feedback regulation. The previous researches showed that the blockage of cytohesins could block the proliferation signaling pathway of downstream EGFR of ErbB receptor family to inhibit the proliferation of tumor cells and promote the apoptosis[20]; the EGFR-p38 MAPK signaling pathway had been proved to be involved in the mechanical ventilation-induced lung injury of rats[21]. Therefore, the effect of SecinH3 in the process of sepsis-induced lung injury of rats was also discussed in this study. According to the results, SecinH3 could relieve the sepsis-induced lung injury. After being treated by SecinH3, Pa/O2was increased with the sepsis, while Pa/CO2was decreased with the action of SecinH3, which indicated that SecinH3 had the certain ‘repairing' ability for the lung injury. The further research found that SecinH3 could inhibit the phosphorylation of EGFR, in which SecinH3 might inhibit the cytohesins to block the signaling pathway and then inhibit the activation of downstream signaling molecules.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003; 101(10): 3765-3777.
[2] Wang Q. A study on relationship between the activation of JAK/STAT pathway and the expression of tumor necrosis factor α (TNFα) in rats with sepsis. Master degree thesis. The Fourth Military Medical University, 2004.
[3] Kwak S, Ku SK, Kang H, Baek MC, Bae JS. Methylthiouracil, a new treatment option for sepsis. Vascul Pharmacol 2015. doi:10.1016/ j.vph.2015.07.013
[4] Durosier LD, Herry CL, Cortes M, Cao M, Burns P, Desrochers A, et al. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol Meas 2015; 36(10): 2089-2102.
[5] Lin WC, Chen CW, Huang YW, Chao L, Chao J, Lin YS, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep 2015; 5:12463.
[6] Filgueiras LR Jr, Martins JO, Serezani CH, Capelozzi VL, Montes MB,Jancar S. Sepsis-induced acute lung injury (ALI) is milder in diabetic rats and correlates with impaired NFkB activation. PLoS One 2012; 7(9):e44987.
[7] Tang ZH, Hu JT, Lu ZC, Ji XF, Chen XF, Jiang LY, et al. Effect of mild hypothermia on the expression of toll-like receptor 2 in lung tissues with experimental acute lung injury. Heart Lung Circ 2014; 23(12): 1202-1207.
[8] Pan T, Sun J, Zhou J, Fu Z, Hu Y, Zheng S, et al. Function and mode of action of cytohesins in the epidermal growth factor pathway in colorectal cancer cells. Oncol Lett 2013; 5(2): 521-526.
[9] Sun JF. Study on activation of Cytohesin in EGFR and IGF-IR pathway in colorectal cancer. PhD. dissertation, Zhejiang University, 2013.
[10] Campbell P, Morton PE, Takeichi T, Salam A, Roberts N, Proudfoot LE,et al. Epithelial inflammation resulting from an inherited loss-of-function mutation in EGFR. J Invest Dermatol 2014; 134(10): 2570-2578.
[11] Goldkorn T, Filosto S, Chung S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid Redox Signal 2014;21(15): 2149-2174.
[12] Giordani VM, DeBenedictus CM, Wang Y, Sanchez-Esteban J. Epidermal growth factor receptor (EGFR) contributes to fetal lung fibroblast injury induced by mechanical stretch. J Recept Signal Transduct Res 2014;34(1): 58-63.
[13] Hayallah AM. Design and synthesis of new (SecinH3) derivatives as potential cytohesin inhibitors. Indian J Pharm Sci 2014; 76(5): 387-400.
[14] K.J. Livak, T.D. Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001; 25(4): 402-408.
[15] Salluh JI, Bozza PT, Bozza FA. Surviving sepsis campaign: a critical reappraisal. Shock 2008; 30(Suppl 1):70-72.
[16] Zeng QY. Research of mitochondrial injury and mutiple organ dysfunction in early stage of sepsis in rats. PhD. Dissertation, Southern Medical University, 2009.
[17] Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, et al. Consensus conference definitions for sepsis, septic shock,acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med 2000; 28(1): 232-235.
[18] Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin 2000; 16(2): 289-317.
[19] Yang S, Rosenwald AG. The roles of monomeric GTP-binding proteins in macroautophagy in Saccharomyces cerevisiae. Int J Mol Sci 2014;15(10): 18084-18101.
[20] Bill A, Schmitz A, Albertoni B, Song JN, Heukamp LC, Walrafen D,et al. Cytohesins are cytoplasmic ErbB receptor activators. Cell 2010;143(2): 201-211.
[21] Tamg CL, Ding N, Cheng AB. EGFR-p38 MAPK signaling pathway is involved in expression of HMGB1 in pulmonary tissues of rats with ventilator-induced lung injury. Chin J Pathophysiol 2013; 29(6): 1029-1033.
15 September 2015
Chun-Yan Yan, PhD., Attending Physician; Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Tel: 13588706724
E-mail: yanchun-yan@163.com
Foundation project: It was supported by Zhejiang Provincial Natural Science Foundation (LY12H02005).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice
- Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae)
- Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka
- Childhood brucellosis: Review of 317 cases
- Effect of cyclophosphamide on fungal infection in SLE mice detected by fluorescent quantitative PCR
- Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin
