Therapeutic effect and mechanism of breviscapine on cisplatin-induced nephrotoxicity in mice
2015-10-31XiaoYuLouJingLiangChengBoZhang
Xiao-Yu Lou, Jing-Liang Cheng, Bo Zhang
1Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450003, Henan, China
2Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Luohe Medical College, Luohe 462002, Henan, China
3Department of Ultrasound in Medicine, East Hospital, Tongji University School of Medicine, Shanghai 200120, China
Therapeutic effect and mechanism of breviscapine on cisplatin-induced nephrotoxicity in mice
Xiao-Yu Lou1,2, Jing-Liang Cheng1*, Bo Zhang3
1Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450003, Henan, China
2Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Luohe Medical College, Luohe 462002, Henan, China
3Department of Ultrasound in Medicine, East Hospital, Tongji University School of Medicine, Shanghai 200120, China
ARTICLE INFO
Article history:
in revised form 20 August 2015
Accepted 10 September 2015
Available online 20 October 2015
Cisplatin
Renal injury
Breviscapine
Protective effect
Objective: To observe the protective effect of breviscapineon mice with cisplatin-induced nephrotoxicity. Methods: Mice were given a single injection of cisplatin (8 mg/kg, i.p.); then,breviscapine was given to mice at 25 mg/kg and 50 mg/kg doses, respectively, once a day for seven days. Renal tissue structure was observed after animals were sacrificed. Blood urea nitrogen (BUN), serum creatinine (Scr), lipid peroxide (MDA) and superoxide dismutase(SOD) serum levels were detected; and MDA, glutathione peroxidase, and SOD levels in the renal cortex were detected. Results: Compared with the blank control group (BCG), the kidney pathological damage of mice in the model control group (MCG) was more severe. After applying different doses of breviscapine, different degrees of renal injury improvement appeared. Compared with the BCG, the serum levels of Scr and BUN in the MCG increased to(89.92±6.78) μmoL/L and (15.32±4.53) mmoL/L. The differences were statistical significant(P<0.01). Compared with the MCG, the serum levels of Scr and BUN in the Bre low-dose groups and Bre high-dose groups decreased significantly (P<0.05). Compared with the BCG,the MDA levels in serum and in the renal cortex in the MCG significantly increased, while the SOD levels significantly decreased. Both the differences were statistically significant (P<0.01). In the Bre low-dose groups and Bre high-dose groups, MDA levels in serum and in the renal cortex significantly decreased, while SOD and glutathione peroxidase levels in the renal cortex significantly increased, compared with the MCG; and the differences were statistically significant (P<0.05). Conclusions: Breviscapine can reduce cisplatin induced renal toxicity in mice and it's possible through inhibition of renal tubule cell lipid peroxidation and reduces the nephrotoxicity of cisplatin.
Document heading doi: 10.1016/j.apjtm.2015.09.017
1. Introduction
Cisplatin is a common cycle nonspecific clinical platinum anticancer drug that has achieved a satisfactory antineoplastic effectin the treatment of a variety of solid tumors[1,2]. Cisplatin is involved in approximately 80% of antineoplastic combined chemotherapy protocols. Since the antineoplastic effect of cisplatin is dosedependent, high doses would lead to renal injury, gastrointestinal reactions, ototoxicity and peripheral neuropathy[3,4]. The high aggregation, hypermetabolism and discharge characteristics of cisplatin in kidneys may cause acute or chronic renal injury; and although hydration treatment can prevent the nephrotoxicity of cisplatin to some extent, renal dysfunction appears in approximately 25%-30% of patients after reaching a dosage of 80 mg/cm2[5]. Renal toxicity is a main obstacle in the clinical usage of cisplatin,and determining how to control renal toxicity while retaining the antineoplastic effect of cisplatin is the main focus in clinical researches. Breviscapine (Bre) is a kind of flavonoid extracted from the compositae plant, erigeron breviscapus; which has shown anti-microbial, anti-inflammatory, free radical-eliminating and antineoplastic effects[6,7]. A study has reported its curative effect in the treatment of diabetic and hypertensive nephropathy[8], but whether it has a protective effect on cisplatin-induced renal nephropathy remains unclear. In this study, a cisplatin-induced Kunming mice nephropathy model was established; then, pathological sections and blood biochemistry were analyzed to investigate the effect of Bre on renal toxicity, and explore its mechanism.
2. Materials and methods
2.1. Drugs and reagents
Bre lyophilized powder (50 mg/bottle) was purchased from Hunan Hangseng Pharmaceutical Co., Ltd. (20100403). Cisplatin lyophilized powder (20 mg/bottle) was purchased from Shandong Qilu Pharmaceutical Product (908027 CF). Creatinine (Scr) test kit, blood urea nitrogen (BUN) test kit, malondialdehyde (MDA)detection kit, and superoxide dismutase (SOD) detection kit were supplied by Nanjing Jiancheng Biological Engineering Co., Ltd. Other reagents were all pure analysis.
2.2. Animals and equipment
Equal numbers of male and female Kunming mice, weighting 18-22 g, were purchased from the Medical Experimental Animal Center of Henan Province; and were used in the experiment. The 752N ultraviolet visible spectrophotometer was provided by Shanghai Precision and Scientific Instrument Co., Ltd. The TL80-1 medical centrifuge was obtained from Jiangsu JiangyanTianli Equipment Co., Ltd. The AU2700 automatic biochemical analyzer and BX40 optical microscope were obtained from Olympus, Japan.
2.3. Groups and drugs
Kunming mice were randomly divided into four groups according to weight with 12 mice in each group: (1) blank control group(BCG), mice were routinely fed and lavaged with 0.5% CMCNa solution; (2) model control group (MCG), a cisplatin-induced renal injury in mice model was established by intraperitoneally injecting cisplatin lyophilized powder diluted with 8 mg/kg of saline water, and mice were lavaged with 0.5% CMC-Na solution;(3) Bre low-dose group (BLDG), after a nephropathy model was established, mice were lavaged with a Bre mixed suspension (0.5% CMC-Na) at 25 mg/kg; (4) Bre high-dose group (BHDG), after a nephropathy model was established, mice were lavaged by a Bre mixed suspension (0.5% CMC-Na) at 50 mg/kg. After establishing a nephropathy model, Bre was administered to all animals once a day for seven days, with a drug delivery volume of 0.1 mL/10 g.
2.4. Observation of kidney morphology
On the eighth day, two mice were randomly selected from each group, and sacrificed by dislocation. Kidneys were taken out by caesarean section. Kidney tissues were fixed with 10% formalin,embedded in paraffin, sliced in a paraffin slicing machine, and H&E staining was performed. Shape and structure of kidney tissues were observed under a light microscope.
2.5. Preparation and determination of samples
After administering ether anesthesia, blood was collected from the angular vein of mice and placed into a tube with heparin anticoagulant, centrifuged at 3 000 r/min for 10 min, and stored in a refrigerator at -4 ℃. Mice were sacrificed, kidneys were quickly removed and placed in a refrigerator at -40 ℃, frozen kidneys were taken out, small renal cortical tissues were chipped in the ice bath,0.1 moL/L of PBS solution (pH=7.4, 4 ℃) was added, 10% of the renal cortical tissue homogenate was made by grinding, centrifuged at 3 000 r/min for 10 min, and the liquid supernatant was placed in a refrigerator at -4 ℃. Scr and BUN were determined using an automatic analyzer. MDA, SOD and glutathione peroxidase (GSHPx) were analyzed according to the kit's instructions.
2.6. Statistical analysis
Results were expressed as mean±SEM. SPSS 11.0 was used for statistical analysis. One-way analysis of variance and t-test comparison were applied in the statistical analysis. P<0.05 was considered significant.
3. Results
3.1. Cisplatin-induced kidney structural changes in mice
H&E staining results revealed that kidney structure was normal and renal tubular and glomerular structures were clear in the BCG. Compared with the BCG, mice in the MCG had severe renal pathological injury, renal tubular epithelial cell swelling occurred,cell vacuolization, necrosis, and shedding appeared, fibrous tissue proliferated in the renal interstitium, infiltration of a large number of inflammatory cells occurred, and parts of the brush-borderwere damaged. After applying different doses of Bre, renal injury improved in different degrees. In the BLDG, renal tubules were slightly swollen, and necrosis occurred in some renal tubules. The fibrous hyperplasia in renal interstitium was not obvious, with a small amount of inflammatory cell infiltration, and some parts of the brush-border fell off. In the BHDG, renal tissue structure was basically normal, and only the renal tubules were slightly swollen. The renal interstitium was normal (As shown by the arrows in Figure 1).
3.2. Effect of Breon GSH-Px serum activity in cisplatininduced renal injury mice
GSH-Px activity decreased in the MCG, compared with the BCG;and the difference was statistically significant (P<0.05). GSH-Px activity in the BLD and BHDGs remained much lower than the BCG, but was significantly improved in the MCG; and the difference was statistically significant (P<0.05) (Figure 2).
3.3. Effect of Breon Scr and BUN serum levels in cisplatininduced renal injury in mice
Scr and BUN serum levels significantly improved in the MCG(P<0.01), compared with the BCG; while Scr and BUN serum levels in the BLD and BHDGs significantly increased, compared with the MCG (P<0.05). These results indicate that cisplatin can induce renal injury, while Bre can alleviate renal injury in a dose dependent manner (Table 1).

Table 1 Effect of breviscapine on Scr and BUN serum levels in cisplatin-induced renal injury in mice (mean±SD, n=10).
3.4. Effect of Bre on MDA and SOD serum levels in cisplatininduced renal injury in mice
MDA serum levels significantly improved and SOD levels significantly decreased in the MCG, compared with the BCG;and the difference was statistically significant (P<0.01). MDA serum levels significantly decreased and SOD levels significantly increased in the BLD and BHDGs, compared with the MCG; and the difference was statistically significant (P<0.01) (Table 2).
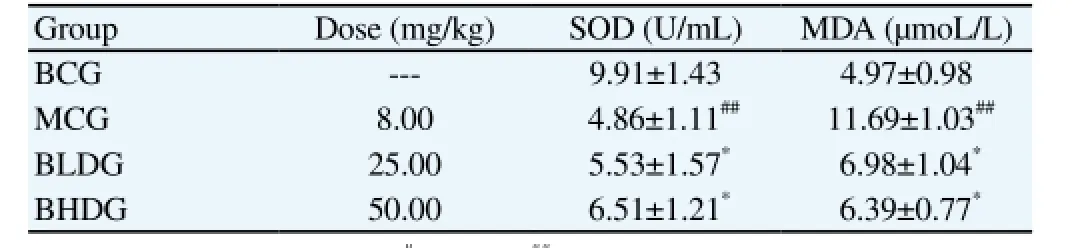
Table 2 Effect of Bre on MDA and SOD serum levels in cisplatin-induced renal injury in mice (mean±SD, n=10).
3.5. Effect of Breon MDA and SOD levels in cisplatininduced renal cortex injury in mice
MDA levels significantly increased and SOD levels significantly decreased in the renal cortex of mice in the MCG, compared with the BCG; and the difference was statistically significant (P<0.01). MDA levels decreased and SOD levels significantly increased in the renal cortex of mice in the BLD and BHDGs, compared with the MCG(P<0.05); and level changes in the BHDG was more significant than in the BLDG (Table 3).

Table 3 Effect of Bre on MDA and SOD levels in cisplatin-induced renal cortex injury in mice (mean±SD, n=10).
4. Discussion
Cisplatin is one of the most effective antineoplastic drugs used in clinical practice, and its treatment effect has been demonstrated in a dose dependent manner. Cisplatin is mainly excreted in the kidney,and its concentration is much higher in the epithelium of renal tubules than in blood; thereby causing serious renal toxicity[9,10]. Injury to renal tubules under low doses is reversible. However, the accumulation of this drug under high doses would cause irreversible injury, kidney failure, or even death[11,12]. Thus, determining how to improve the quality of chemotherapy, the alleviation of kidney injury, and the application of the antitumor efficiency of cisplatin has become the focus of research.
Combined therapy is an effective method to improve chemotherapy efficiency in tumor patients. Two kinds of drugs are combined to improve chemotherapy efficiency; however, toxic reactions have also been increased through this method[13-15]. In recent years, several flavonoids received extensive attention for their ability to improve chemotherapy efficiency without causing any toxic reaction[16,17]. Bre is a kind of flavonoid extracted from lamps of flowers[18,19]. In our previous study, we found that Bre has a synergistic antitumor effect with Maryland[20]. Bre can be used to treat diabetic kidney disease and hypertensive kidney disease[18,21]. Therefore, we speculate that combining Bre with cisplatin may lower the toxic reactions of cisplatin while exerting its antitumor effects. In this study, a cisplatin-induced renal injury model was established. Different doses of Bre were given to mice by gavage, and renal pathological injury and blood biochemistry were observed to analyze the function and mechanism of Bre on cisplatin-induced renal toxicity.
Cisplatin may generate oxygen free radicals in the body and induce mitochondrial damage and vacuolization, resulting in renal injury[22,23]. Free radicals may cause changes in cell structure and function, thereby affecting tissue and organ function. GSHPx can specifically catalyze the reduction reaction of glutathione to hyperoxide to protect the structure and function of the cell membrane. In this study, excessive hydrogen peroxide was generated in the renal cortex after the injection of cisplatin; which lead to a decrease in GSH-Px activity in the renal cortex, a decrease in SOD levels and an increase MDA levels in serum and in the renal cortex,and an increase in BUN and Scr levels in serum. Normally, there is a dynamic balance of active oxygen metabolism in the body; and in the pathological state, a large amount of active oxygen is generated. When generation rate exceeds the removal limits of antioxidase,lipid peroxide is accumulated, renal tubules are injured, excretory function of the kidney is reduced, and finally resulting in BUN and Scr retention in vivo. These indicate that renal toxicity and lipid peroxidation are closely related.
In our study, pathological changes in renal injury induced by doses of cisplatin and the treatment of diabetic nephropathy with Bre were used as reference. Mice in the BLD and BHDGs were given 25 mg/kg and 50 mg/kg doses of Bre, respectively, for seven days. Results indicate that renal tubular and glomerular injury in these two treatment groups were mitigated compared with the MCG; wherein,only a small amount of inflammatory cells was seen, no tissue fibrosis was observed, and cisplatin-induced brush border injury was alleviated. These results suggest that Bre could increase cell membrane stability and improve renal condition. Meanwhile, Bre can decrease Scr and BUN serum levels, as well as decrease MDA levels and increase SOD levels in serum and in the renal cortex. These suggest that Bre can improve the body's antioxidant capacity and reduce cell membrane damage caused by oxidative stress. Thus,Bre may reduce cisplatin-induced renal toxicity by inhibiting lipid peroxidation.
In summary, Bre can reduce cisplatin-induced renal toxicity by inhibiting lipid peroxidation; and this method has good clinical feasibility. This means that Bre combined with cisplatin may be applied to tumor patients to improve chemotherapy efficiency in the near future. This study has some limitations. For example, the experiment in this study only lasted for seven days; and the extent of recovery after cisplatin-induced renal injury with a prolonged treatment time remains unclear. Further studies and long-term experiments are needed.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Park J, Morley TS, Scherer PE. Inhibition of endotrophin, a cleavage product of collagen, confers cisplatin sensitivity to tumours. EMBO Mol Med 2013; 5(6): 935-948.
[2] Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A,et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014; 5(5): e1257.
[3] Oliver TG, Mercer KL, Sayles LC, Burke JR, Mendus D, Lovejoy KS,et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev 2010;24(8): 837-852.
[4] Nor C, Zhang Z, Wamer KA, Bernardi L, Visioli F, Helman JI, et al. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014; 16(2): 137-146.
[5] Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M,Rachamalla SS, et al. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF- B activation and antioxidant defence. PLoS One 2014; 9(9): e105070.
[6] He M, Xue ZM, Li J, Zhou BQ. Breviscapine inhibits high glucoseinduced proliferation and migration of cultured vascular smooth muscle cells of rats via suppressing the ERK1/2 MAPK signaling pathway. Acta Pharmacol Sin 2012; 33(5): 606-614.
[7] Liu Z, Okeke CI, Zhang L, Zhao H, Li J, Aggrey MO, et al. Mixed polyethylene glycol-modified breviscapine-loaded solid lipid nanoparticles for improved brain bioavailability: preparation,characterization, and in vivo cerebral microdialysis evaluation in adult sprague dawley rats. AAPS Pharm Sci Tech 2014; 15(2): 483-496.
[8] Zheng C, Ou W, Shen H, Zhou Z, Wang J. Combined therapy of diabetic peripheral neuropathy with breviscapine and mecobalamin: asystematic review and a meta-analysis of Chinese studies. Biomed Res Int 2015;2015: 680756.
[9] Mukhopadhyay P, Horváth B, Zsengellér Z, Zielonka J, Tanchian G, Holovac E, et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med 2012; 52(2): 497-506.
[10] Mohamad AM, Mohamad RA, Fatemeh A, Mohamad RS. Histological study of toxic effects of cisplatin single dose injection on rat kidney. Gene Cell Tissue 2014; 1(2): e21536.
[11] Pani SR, Mishra S, Sahoo S, Panda PK. Nephroprotective effect of Bauhinia variegata (Linn.) whole stem extract against cisplatin-induced nephropathy in rats. Indian J Pharmacol 2011; 43(2): 200-202.
[12] Domitrovi R, Poto njak I, Crn evi -Orli, Škoda M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem Toxicol 2014; 66: 321-328.
[13] Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the first-line advanced gastric cancer study. Eur J Cancer 2013; 49(17):3616-3624.
[14] Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res 2013;67(1): 94-109.
[15] Kobayashi S, Ueno M, Ohkawa S, Irie K, Goda Y, Morimoto M. Renal toxicity associated with weekly cisplatin and gemcitabine combination therapy for treatment of advanced biliary tract cancer. Oncology 2014;87(1): 30-39.
[16] Johnson JL, Gonzalez de Mejia E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol 2013; 60: 83-91.
[17] Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann NY Acad Sci 2012; 1259(1): 95-103.
[18] Xu XX, Zhang W, Zhang P, Qi XM, Wu YG, Shen JJ. Superior renoprotective effects of the combination of breviscapine with enalapril and its mechanism in diabetic rats. Phytomedicine 2013; 20(10): 820-827.
[19] Jiang T, Gao Y, Xiong ZY. Effects of breviscapine on protein expression of c-fos, c-jun in glomerular mesangial cells cultured under high glucose conditions. Chin Pharmacol Bulletin 2001; 17(5): 503-505.
[20] Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett 2006; 231(1): 113-122.
[21] Du J, Lin H, Xu T. Breviscapine promotes K562 cell apop-tosis induced by doxorubicin. Chin Pharmacol Bulletin 2007; 23(8): 1043-1047.
[22] Jaiman S, Sharma AK, Singh K, Khanna D. Signalling mechanisms involved in renal pathological changes during cisplatin-induced nephropathy. Eur J Clin Pharmacol 2013; 69(11): 1863-1874.
[23] Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 2013; 8(11): e81162.
10 July 2015
Jing-Liang Cheng, Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450003,Henan, China.
Tel: 0371-67966331
Mobile: 13603863860
E-mail: zhy6290@163.com
Foundation project: It was supported by the National Natural Science Foundation of China (No. 81401428).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Is there a way out for the 2014 Ebola outbreak in Western Africa?
- Bithynia siamensis goniomphalos, the first intermediate host of Opisthorchis viverrini in Thailand
- Is toxoplasmosis a potential risk factor for liver cirrhosis?
- Effects of Gastrodiae rhizoma on proliferation and differentiation of human embryonic neural stem cells
- Potential of four marine-derived fungi extracts as anti-proliferative and cell death-inducing agents in seven human cancer cell lines
- Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats
