Effect of microRNA-155 on angiogenesis after cerebral infarction of rats through AT1R/VEGFR2 pathway
2015-10-31YingChunMengZhenYingDingHaiQuanWangLiPingNingChaoWang
Ying-Chun Meng, Zhen-Ying Ding, Hai-Quan Wang, Li-Ping Ning, Chao Wang
1Department of Rehabilitation, Affiliated Shandong Provincial Hospital of Shandong University, Ji'nan 250021, Shandong, China
2Department of Neurology, Shandong Qixia People's Hospital, Qixia 370686, Shandong, China
Effect of microRNA-155 on angiogenesis after cerebral infarction of rats through AT1R/VEGFR2 pathway
Ying-Chun Meng1*, Zhen-Ying Ding2, Hai-Quan Wang1, Li-Ping Ning1, Chao Wang1
1Department of Rehabilitation, Affiliated Shandong Provincial Hospital of Shandong University, Ji'nan 250021, Shandong, China
2Department of Neurology, Shandong Qixia People's Hospital, Qixia 370686, Shandong, China
ARTICLE INFO
Article history:
in revised form 20 August 2015
Accepted 10 September 2015
Available online 20 October 2015
microRNA-155
Cerebral infarction
Angiogenesis
AT1R/VEGFR2
Pathway
Objective: To explore the function and mechanism of microRNA-155 to regulate the angiogenesis after the cerebral infarction of rats through the angiotensin Ⅱ receptor 1 (AT1R)/ vascular endothelial growth factor (VEGF) signaling pathway. Methods: Female SD rats were chosen for the construction of cerebral infarction model of rats using the modified right middle cerebral artery occlusion. The real-time PCR (RT-PCR) method was employed to detect the expression of microRNA-155 in each group at different time points after the cerebral infarction (1 h, l d, 3 d and 7 d). SD rats were randomly divided into four groups (n=20 rats):sham operation group (Sham group), MACO group, MACO+microRNA-155 mimic group,and MACO+microRNA-155 inhibitor group. Sham group was given the free graft, while MACO+microRNA-155 mimic group and MACO+microRNA-155 inhibitor group were treated with microRNA-155 mimic and microRNA-155 inhibitor respectively. The Zea Longa 5-point scale was used to score the neurologic impairment of rats in each group; 2, 3, 5-triphenyl tetrazolium chloride staining to evaluate the volume of cerebral infarction of rats in each group;the immunohistochemistry to detect the expression of CD31; Western blot and RT-PCR to detect the expression of AT1R and VEGF receptor 2 (VEGFR2). Results: The expression of microRNA-155 was increased in the cerebral ischemia tissue after the cerebral infarction. It was significantly increased at 1 d of ischemia and maintained at the high level for a long time. Rats in the Sham group had no symptom of neurologic impairment, while rats in the MACO group had the obvious neurologic impairment. After being treated with microRNA-155 inhibitor,the neural function of MACO rats had been improved, with the decreased area of cerebral infarction. But after being treated with microRNA-155 mimic, the neural function was further worsened, with the increased area of cerebral infarction. Results of immunohistochemical assay indicated that microRNA-155 inhibitor could up-regulate the expression of CD31, while microRNA-155 mimic could down-regulate the expression of CD31. The RT-PCR found that,after being treated with microRNA-155 inhibitor, MACO rats had the increased expression of AT1R and VEGFR2 messenger RNA (mRNA); but after being treated with microRNA-155 mimic, the expression of AT1R and VEGFR2 mRNA was decreased. Results of Western blot showed that, after being treated with microRNA-155 inhibitor, MACO rats had the increased expression of AT1R and VEGFR2 mRNA; but after being treated with microRNA-155 mimic,the expression of AT1R and VEGFR2 mRNA was decreased. Conclusions: The inhibition of microRNA-155 can improve the neurologic impairment of rats with the cerebral infarction,reduce the volume of cerebral infarction and effectively promote the angiogenesis in the region of ischemia, which may be mediated through AT1R/VEGFR2 pathway.
Document heading doi: 10.1016/j.apjtm.2015.09.009
1. Introduction
The epidemiological survey shows the upward trend of incidenceof cerebral infarction in China, which has attracted a wide attention because of its high disability rate and mortality rate. The cerebral infarction can cause the irreversible degeneration and necrosis of brain neurons and thus lead to a series of neurological dysfunction. Therefore, the pursuit of effective means to early promote the local angiogenesis in the region of cerebral infarction to recover the tissue perfusion will be of critical significance for the prognosis of patients with cerebral infarction. But there are still no effective therapeutic programs.
The angiogenesis could improve the perfusion in the region of cerebral infarction and thus promote the regeneration of central nerve, as the basis for the recovery of brain neurons[1,2]. The vascular endothelial growth factor (VEGF) is one of the most important vascular growth factors in the process of angiogenesis. It's reported that its specific effect on the endothelial cells could be involved in the angiogenesis[3]. VEGF plays its role through three receptors, including VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2) and VEGF receptor 3 (VEGFR3), where VEGFR2 is of most important for the early angiogenesis. Angiotensin Ⅱ(Ang Ⅱ) is a multifunctional bioactive peptide, which can promote the angiogenesis and plays the key role in the angiogenesis. Angiotensin Ⅱ receptor 1 (AT1R)/VEGFR2 signaling pathway can promote the proliferation and migration of vascular endothelial cells and play a role in regulating the angiogenesis both in the physiological and pathological condition. According to researches,the angiogenesis began at the time of cerebral infarction, and the regulation of AT1R/VEGFR2 pathway on the angiogenesis has been widely recognized[4]. However, when the cerebral infarction occurred, such reaction of the body still could not improve the revascularization in the region of cerebral infarction and the perfusion of brain tissue. Therefore, it would be necessary to seek the effective measures to enhance the role of AT1R/VEGFR2 pathway in such process.
microRNA is some kind of small non-coding RNA molecule with about 22 nucleotides, which can be widely found in the plants, animals and some viruses[5]. Based on the complementary pairing with messenger RNA (mRNA) of the target gene, the microRNA can play a critical role in the gene regulation after the silencing and transcription of RNA. Some researchers found that microRNA could be widely involved in the biological processes of proliferation, apoptosis and differentiation of cells and play a key role in the regulation of onset and development of many diseases(cardiovascular diseases and tumors)[6,7]. It's reported that there was the close relationship between microRNA-155 and angiogenesis and the target gene of microRNA-155 was AT1R, which could target at reducing the expression of AT1R[8] to inhibit Ang Ⅱ/AT1R signaling pathway and thus result in the inhibition of angiogenesis. A research found that the expression of microRNA-155 was significantly increased in the condition of cerebral ischemia, while the inhibition of microRNA-155 could protect the nerves and thus improve the damage of cerebral ischemia[9].
Consequently, this study was to build the cerebral infarction model of rats. According to the in vivo over-expression and the inhibition against the expression of microRNA-155, it was to observe the effect of microRNA-155 on the angiogenesis of rats with cerebral infarction and further explore the role of AT1R/VEGFR2 pathway in such process.
2. Materials and methods
2.1. Materials
Adult female SD rats with the weight of about 200-250 g were chosen as the subjects in this study (which were purchased from Shanghai SLAC Laboratory Animal Co. Ltd.); other materials included RNA extraction kit (Invitrogen, USA); reverse transcription kit (Takara, Japan); Real-time fluorescent quantitative PCR kit(TaKaRa, Japan); microRNA-155 mimic (Life Technologies, USA);microRNA-155 inhibitor (Life Technologies, USA); triphenyl tetrazolium chloride (TTC) (Sigma, USA); Rat CD31, AT1R,VEGFR2, β-actin antibody (Santa Cruz, USA); horseradish peroxidase-labeled goat anti-rabbit IgG (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.).
2.2. Methods
2.2.1. Construction of cerebral infarction model of rats
Adult female SD rats (200-250 g) were fed in the single cage at the room temperature of about 25 ℃ and humidity of about 50%,with the light/dark cycle of 12 h. They were given with diet and water freely. After one week of adaptive feeding, rats were given the intraperitoneal injection of pentobarbital sodium (0.3 mL/100 g)for the anesthesia. The right middle cerebral artery occlusion was performed on rats under sterile conditions. Referring to the modified Zea-Longa method[10], the specific procedures were as follows:the incision was taken in the middle of neck and the tissues were separated layer by layer to expose and free the right common carotid artery, internal carotid artery and external carotid artery. A small incision was taken in the external carotid artery close to the heart. The suture was inserted in the common carotid artery through the incision and then taken back to pass by the bifurcation of common carotid artery and enter in the internal carotid artery. Afterwards,the suture was slowly delivered to the starting point of middle cerebral artery, with the insert length of about (8.0±0.5) cm from the bifurcation of common carotid artery. The suture was fixed and the neck skin was stitched. Rats in the sham operation group (sham group) were only freed the vessels. According to Zea-Longa 5-point scale (Table 1), the neurologic impairment of rats in each group was scored[11].
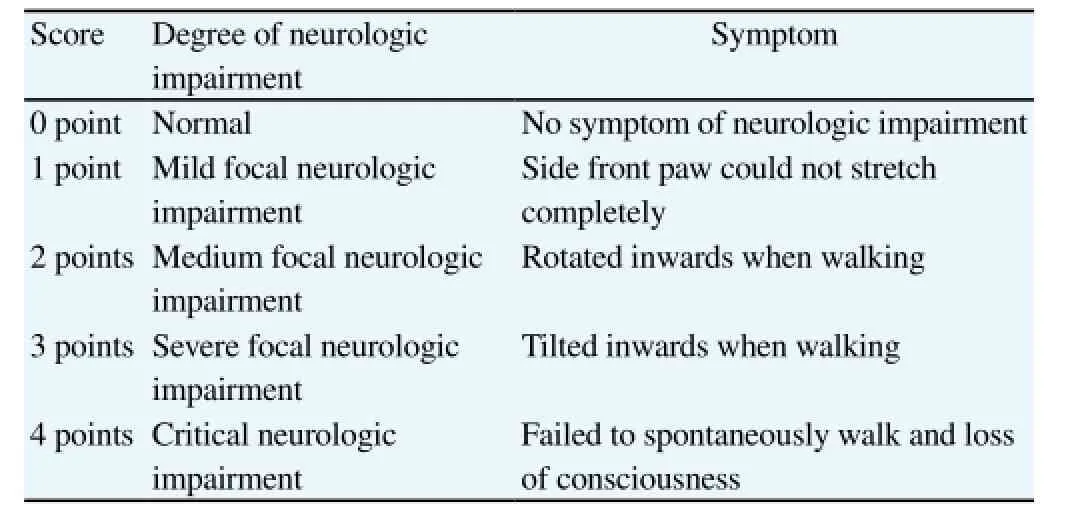
Table 1 Score of neurologic impairment of rats with cerebral infarction.
2.2.2. Grouping
Laboratory rats were randomly divided into four groups (n=20 rats): Sham group, MACO group, MACO+microRNA-155 inhibitor group and MACO+microRNA-155 mimic group. At 5 min of sham operation for rats in the sham group, they were injected with the equal normal saline through the lateral ventricle; at 5 min of cerebral ischemia, rats in the MACO group were injected with the equal normal saline through the lateral ventricle; at 5 min of cerebral ischemia, rats in the MACO+microRNA-155 mimic group were injected with 10 μg miRNA-155 mimic through the lateral ventricle;at 5 min of cerebral ischemia, rats in the MACO+microRNA-155 inhibitor group were injected with 10 μg microRNA-155 inhibitor through the lateral ventricle.
2.2.3. Detection of expression of miR-155 by RT-PCR
Referring to the instruction manual of trizol kit (Invitrogen),the total RNA was extracted in the condition without RNAase. The primer design was as follows, miR-155 upstream primer: 5'-GTCGTATCCAGTGCAGGGTCCGAGG-3', downstream primer:TATTCGCACTGGATACGACCCCCTA. RNA was reversetranscripted into cDNA through one-step real-time PCR (RTPCR) kit. After PCR amplification, 5 μL amplification products were collected and further tested using 2% agarose gel. The UV spectrophotometer was used to detect the electrophoretic band and photograph it.
2.2.4. Evaluation of volume of cerebral infarction by TTC staining
Rats in each group were given the intraperitoneal injection of 0.3 mL/100 g pentobarbital sodium for the anesthesia. The chest was cut off to expose the heart and the heart was perfused with the normal saline. The brain tissue was then completely taken out and cut into slices with the thickness of about 2 mm along the coronal plane. They were sunk into 2% TTC normal saline and incubated in a dark place and at 37 ℃. After 30 min, they were photographed. The normal brain tissue appeared to be red, while the region of cerebral infarction looked pale white. Image Pro was employed to calculate the volume of cerebral infarction.
2.2.5. Detection of expression of CD31 by immunohistochemical assay
After the intervention, the brain tissues were taken from the ischemia area from rats in each group and they were fixed with 4% paraformaldehyde. After being fully washed, they were given the ethanol for the gradient dehydration. They were transparent with xylene and imbedded and cut with the paraffin. After the dewaxing and hydration of slices, the sodium citrate was used to repair the antigen under the high pressure and 3% H2O2to block the endogenous peroxidase. The serum was closed and the primary antibody (CD31) was added and incubated overnight at 4 ℃. After being washed, the biotin-labeled IgG was added for 10 min of incubation. When they were washed, the streptin avidin-peroxidase was added for 10 min of incubation at the room temperature. They were colored with DAB for 3 min and re-stained with hematoxylin for 10 s. Afterwards, they were differentiated with 1% hydrochloric acid-alcohol for 2 s. They were mounted with the neutral resin and then observed under the microscope.
2.2.6. Detection of expression of AT1R and VEGFR2 mRNA by RT-PCR
The total RNA was extracted from the brain tissue. The UV spectrophotometer was employed to detect the concentration and purity of RNA. According to the instruction of reverse transcription kit, cDNA was synthesized and stored at -80 ℃ for further operation. SYBR method was employed for Real-time fluorescent quantitative PCR,with the reaction system of: SYBR PremixEx TaqTMⅡ (2×) 12.5 μL, 1.0 μL upstream and downstream primer respectively, 2.0 μL cDNA template and 8.0 μL sterilized water;the reaction conditions included: Initial denaturation (1 cycle) at 95 ℃ for 30 s; PCR reaction (40 cycles) at 95 ℃ for 5 s and at 60 ℃for 30 s (collection of fluorescence signal); melting (1 cycle) at 95 ℃ for 15 s; at 60 ℃ for 30 s; and at 95 ℃ for 15 s (collection of fluorescence signal). After the amplification, it was followed by the results analysis. Taking β-actin as the reference and according to Ct value of RT-PCR results, the relative expression of mRNA was calculated. Each group had three parallel wells and the experiment had three repeats.
2.2.7. Detection of expression of CD31, AT1R and VEGFR2 by Western blot
The protein was extracted from the brain tissue. The protein assay was employed to detect the concentration of protein. Protein samples were given SDS-PAGE electrophoresis. They were transferred to polyvinylidene fluoride membrane using the semi-dry transfer buffer. After being closed with 5% skimmed milk powder for 1 h,the primary antibody that was diluted with TBST (CD31, AT1R,VEGFR2, β-actin) was added and then incubated at 4 ℃ overnight. The membrane was washed with TBST for three times and then the horseradish peroxidase-labeled secondary antibody was added and incubated at the room temperature for 1 h. Afterwards, it waswashed with TBST for three times. The gel imaging analysis system was used to scan the density of bands and determine the gray-level integration.

Table 2 Sequence of RT-PCR primer.
2.3. Statistical analysis
All data was treated with SPSS 19.0. The measurement data was expressed by mean±SD. Levene test was used for the homogeneity of variance test (reference standard of 0.10). Means of each group that met the homogeneity of variance were treated with one-way ANOVA and LDS test, means that not met the homogeneity of variance were treated with Kruskal-Wallis H test. The pairing t test was used for the comparison before and after the experiment in the same group. P<0.05 indicated the statistical difference.
3. Results
3.1. Change in expression of microRNA-155 of cerebral ischemia tissue after the cerebral infarction
The expression of microRNA-155 of cerebral ischemia tissue after the cerebral infarction was detected at 1 h, 1 d, 3 d and 7 d of cerebral infarction. Results showed that the expression of microRNA-155 at each time point after the ischemia was significantly higher than the one in the sham group (P<0.05). The expression of microRNA-155 was increased even at 1 h of ischemia, while it was significantly increased at 1 d of ischemia (P<0.01) and maintained at the high level for a long time (till 7 d) (Figure 1).
3.2. In vivo effect on expression of microRNA-155
The microRNA mimic can simulate the endogenous microRNA and thus up-regulate the expression of microRNA and enhance its function. But microRNA inhibitor can specifically target at knocking down the microRNA and thus down-regulate the expression of microRNA. In this study, MACO rats were given the microRNA-155 mimic and microRNA-155 inhibitor and then RT-PCR was employed to detect the expression of microRNA-155 in the brain tissue of rats in each group. Results showed that, compared with the MACO group, the expression of microRNA-155 in the microRNA-155 mimic group was significantly increased (P<0.01), while it was significantly decreased in the microRNA-155 inhibitor group(P<0.01) (Figure 2).
3.3. Effect of microRNA-155 on neural function and area of cerebral infarction of MACO rats
As shown in Table 3, rats in the sham group had no symptom of neurologic impairment, while the score of neurologic impairment for rats in the MACO group was 2.77±0.64. After being treated with the microRNA-155 inhibitor, the score of neurologic impairment was decreased than the one in the MACO group (P<0.05), but after being treated with the microRNA-155 mimic, the score of neurologic impairment was increased (P<0.05). Results of TTC staining showed that, after being treated with the microRNA-155 inhibitor, its area of cerebral infarction was decreased (P<0.05), while after being treated with the microRNA-155 mimic, its area of cerebral infarction was increased. Above results indicated that the inhibition of microRNA-155 could improve the neurologic function and reduce the volume of cerebral infarction of MACO (Table 3).

Table 3 Score of neurologic impairment and volume of cerebral infarction of rats in each group (mean±SD, n=8).
3.4. Effect of microRNA-155 on expression of CD31 in cerebral ischemia tissue after cerebral infarction
CD31 is mainly distributed in vascular endothelial cells and it is usually used to evaluate the vessel density in the tissue. Thus in this study, the immunohistochemistry was employed to further detect the expression of CD31 in the cerebral ischemia tissue after cerebral infarction, as shown in Figure 3A. Results indicated that,compared with the sham group, there was no significant change in the expression of CD31 in the brain tissue after ischemia (P>0.05),as shown in Figure 3B. After being treated with microRNA-155 inhibitor, the expression of CD31 was significantly increased(P<0.05), as shown in Figure 3C; while after being treated with microRNA-155 mimic, the expression of CD31 was significantly decreased (P<0.05), which indicated that the inhibition of microRNA-155 could promote the angiogenesis after cerebral infarction (Figure 3B).
3.5. Effect of microRNA-155 on expression of CD31 in cerebral ischemia tissue after cerebral infarction
As shown in Figure 4, results of Western blot indicated that,compared with the sham group, there was no significant change in the expression of CD31 in the brain tissue after ischemia (P>0.05). After being treated with microRNA-155 inhibitor, the expression of CD31 was significantly increased (P<0.05); while after being treated with microRNA-155 mimic, the expression of CD31 was significantly decreased (P<0.05), which indicated that the inhibition of microRNA-155 could promote the angiogenesis after cerebral infarction.
3.6. Effect of microRNA-155 on expression of AT1R and VEGFR2 protein in cerebral ischemia tissue after cerebral infarction
In this study, Western blot was employed to further detect the expression of AT1R and VEGFR2 protein in the cerebral ischemia tissue after cerebral infarction, which was consistent with the change in the expression of mRNA. To be specific, after being treated with microRNA-155 inhibitor, the expression of AT1R and VEGFR2 protein was significantly increased (P<0.05), while being treated with microRNA-155 mimic, the expression of AT1R and VEGFR2 protein was significantly decreased (P<0.05, Figure 5), which indicated that the inhibition of microRNA-155 could promote the angiogenesis after cerebral infarction and it might be mediated through AT1R/VEGFR2 pathway.
3.7. Effect of microRNA-155 on expression of AT1R and VEGFR2 mRNA in cerebral ischemia tissue after cerebral infarction
The detection on the expression of AT1R and VEGFR2 mRNA in the cerebral ischemia tissue after cerebral infarction showed that,compared with the sham group, there was no significant change in the expression of AT1R and VEGFR2 mRNA in the brain tissue after ischemia (P>0.05). After being treated with microRNA-155 inhibitor, the expression of AT1R and VEGFR2 mRNA was significantly increased (P<0.01); while after being treated with microRNA-155 mimic, the expression of AT1R and VEGFR2 mRNA was significantly decreased (P<0.01, Figure 6).
4. Discussion
The cerebrovascular diseases have become one of important cause for human death, where most of them are the ischemic cerebrovascular diseases, accounting for about 75%-85% of the total. The cerebral infarction can cause the irreversible degeneration and necrosis of brain neurons and thus lead to a series of neurological dysfunction, bringing along the huge burden to patients, their families and society[12]. For patients with cerebral infarction, the main treatments included the symptomatic treatment,neuroprotection and anti-thrombin thrombolysis[13]. With the indepth studies in this field, researchers proposed the concept of penumbra zone of cerebral ischemia in recent years, namely the relative ischemic zone around the central zone of ischemia of cerebral infarction. Because of the support of collateral circulation in such zone, lots of neurons only had the reversible degeneration and the early recovery of blood supply could reverse the degeneration and necrosis. According to clinical researches, the higher density of neovascularization in the zone of cerebral ischemia of stroke patients would lead to the better clinical prognosis[14]. Therefore, the pursuit of effective means to early promote the local angiogenesis in the region of cerebral infarction to recover the tissue perfusion will be of critical significance for the prognosis of patients with cerebral infarction. But there are still no effective therapeutic programs.
microRNA is some kind of small non-coding RNA molecule, which can degrade the target gene mRNA and play a critical role in the gene regulation[15]. Some researchers found that microRNA could be widely involved in the biological processes of proliferation, apoptosis and differentiation of cells and play a key role in the regulation of onset and development of many diseases (cardiovascular diseases and tumors). According to the regulation of related target genes, microRNA could be involved in many pathological and physiological processes after the cerebral ischemia, which could inhibit some microRNA and protect the nerve of patients with the brain injury[16,17]. It's reported that, in the situation of many brain injuries, the expression of microRNA-155 was increased in the brain tissue, which indicated that microRNA-155 might be related to the brain injury[18]. In this study, the expression of microRNA-155 in the cerebral ischemia tissue was increased even at 1 h of cerebral infarction, while it was significantly increased at 1 d of ischemia and maintained at the high level for a long time, which indicated that the overexpression of microRNA-155 after cerebral ischemia was involved in the injury of brain tissue. To specify the function of microRNA-155 in the injury of brain tissue after cerebral infarction,the microRNA mimic was used to up-regulate the expression of microRNA and microRNA inhibitor to down-regulate the expression of microRNA. Results showed that microRNA-155 inhibitor could improve the neurological function of MACO rats and reduce the volume of cerebral infarction; while microRNA-155 mimic would further worsen the neurological function of MACO rats and increase the volume of cerebral infarction.
According to previous researches, it's reported that there was the close relationship between microRNA-155 and angiogenesis and the brain angiogenesis was the foundation for the tissue repair in the ischemic zone. The new blood vessels can not only improve the micro circulation in the zone of cerebral ischemia and inhibit the apoptosis of nerve cells, but also induce the release of nutritional factors to promote the recovery of neurological function. It can thus be presumed that the reason for the inhibition of microRNA-155 to improve the neurological function of rats with cerebral infarction may be the action to promote the angiogenesis. CD31 is the endothelial cell adhesion molecule, which can be expressed in the vascular endothelium and has become the common indicator of angiogenesis in the clinical practice. According the further detection of expression of CD31 in the cerebral ischemia tissue after, it's found that microRNA-155 mimic could inhibit the expression of CD31. After being treated with microRNA-155 inhibitor, the expression of CD31 was significantly increased,indicated that the inhibition of microRNA-155 could promote the angiogenesis after cerebral infarction. Ang Ⅱ is a multifunctional bioactive peptide, which can promote the angiogenesis and plays the key role in the angiogenesis[19]. It's found that the target gene of microRNA-155 is the receptor of Ang Ⅱ, AT1R. The microRNA-155 can target at decreasing the expression of AT1R, inhibit the Ang Ⅱ/AT1R signaling pathway and thus inhibit the angiogenesis. In this study, results showed that microRNA-155 mimic could inhibit the expression of AT1R in the cerebral ischemia tissue, while microRNA-155 inhibitor could promote the expression of AT1R in the cerebral ischemia tissue. In the process of angiogenesis, it requires the synergistic action of many factors, where the most important one is VEGF. VEGF is some kind of specific mitogen of vascular endothelial cells, which can not only stimulate the proliferation and migration of endothelial cells and promote the fusion of capillary, but also mobilize the endothelial cells to enter in the avascular zone and promote the angiogenesis. VEGF plays its role through three receptors of VEGFR1, VEGFR2 and VEGFR3,where VEGFR2 is of most critical for the early angiogenesis. The knockdown of VEGFR2 in the embryonic stage might result in the deletion of vascular cells[20]. Ang Ⅱ could promote the expression of VEGFR2 through the dose-dependent AT1R and enhance the VEGFR2-mediated proliferation and angiogenesis of cells[21]. In this study, it's found that microRNA-155 mimic could inhibit the expression of VEGFR2 in the cerebral ischemia tissue after cerebral infarction, while microRNA-155 inhibitor could promote the expression of VEGFR2 in the cerebral ischemia tissue after cerebral infarction, which indicated that microRNA-155 might regulate the angiogenesis through AT1R/VEGFR2 pathway.
In conclusion, according to the overexpression and inhibited expression of microRNA-155 in the cerebral infarction model of rats, it shows that the inhibition of microRNA-155 can improve the neurologic impairment of rats with the cerebral infarction,reduce the volume of cerebral infarction and effectively promote the angiogenesis in the region of ischemia, which may be mediated through AT1R/VEGFR2 pathway.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J Neuroimmune Pharmacol 2007; 2(3): 284-289.
[2] Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 2012; 43(8): 2270-2274.
[3] Silpanisong J, Pearce WJ. Vasotrophic regulation of age-dependent hypoxic cerebrovascular remodeling. Curr Vasc Pharmacol 2013; 11(5):544-563.
[4] Watanabe T, Suzuki J, Yamawaki H, Sharma VK, Sheu SS, Berk BC. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin Ⅱ type 1 receptor-independent effects of EXP3179. Circulation 2005; 112(12): 1798-1805.
[5] Szafranski K, Abraham KJ, Mekhail K. Non-coding RNA in neural function, disease, and aging. Front Genet 2015; 6: 87.
[6] Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal 2015; 8(368): re3.
[7] Purvis N, Bahn A, Katare R. The role of MicroRNAs in cardiac stem cells. Stem Cells Int 2015; 2015: 194894.
[8] Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin Ⅱ type 1 receptor expression in fibroblasts. J Biol Chem 2013; 288(6): 4226.
[9] Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, et al. microRNAs in stroke pathogenesis. Curr Mol Med 2011; 11(2): 76-92.
[10] Liu G, Wang T, Song J, Zhou Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 2013;1(6): 861-867.
[11] Chen J, Tang YX, Liu YM, Hu XQ, Liu N, Wang SX, et al. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther 2012; 18(10): 847-854.
[12] Rodriguez-Gonzalez R, Hurtado O, Sobrino T, Castillo J. Neuroplasticity and cellular therapy in cerebral infarction. Cerebrovasc Dis 2007; 24(Suppl 1): 167-180.
[13] Robinson AA, Ikuta K, Soverow J. Anticoagulation for the acute management of ischemic stroke. Yale J Biol Med 2014; 87(2): 199-206.
[14] Chen YC, Wu JS, Yang ST, Huang CY, Chang C, Sun GY, et al. Stroke,angiogenesis and phytochemicals. Front Biosci (Schol Ed) 2012; 4: 599-610.
[15] Yan H, Fang M, Liu XY. Role of microRNAs in stroke and poststroke depression. Sci World J 2013; 2013: 459692.
[16] Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 2010; 30(1):92-101.
[17] Jaenisch N, Witte OW, Frahm C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 2010;41(3): e151-159.
[18] Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 2009; 1792(6):497-505.
[19] Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ,Chotani M, et al. The human angiotensin Ⅱ type 1 receptor +1166 A/ C polymorphism attenuates microRNA-155 binding. J Biol Chem 2013;288(6): 4227.
[20] Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13(1): 9-22.
[21] Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin Ⅱ potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 1998; 82(5): 619-628.
10 July 2015
Ying-Chun Meng, Department of Rehabilitation, Affiliated Shandong Provincial Hospital of Shandong University, No. 324 Nanjingwu Road,Ji'nan 250021, Shandong, China.
Tel: 15168889866
E-mail: myc9866@163.com
Foundation project: It was supported by Shandong Key Scientific and Technological Project Fund (No. 2013YD 18021).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Is there a way out for the 2014 Ebola outbreak in Western Africa?
- Bithynia siamensis goniomphalos, the first intermediate host of Opisthorchis viverrini in Thailand
- Is toxoplasmosis a potential risk factor for liver cirrhosis?
- Effects of Gastrodiae rhizoma on proliferation and differentiation of human embryonic neural stem cells
- Potential of four marine-derived fungi extracts as anti-proliferative and cell death-inducing agents in seven human cancer cell lines
- Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats
