Clinicopathological features and survival analysis of gastroenteropancreatic neuroendocrine neoplasms: a retrospective study in a single center of China
2015-10-27XuelongJiaoYujunLiHongyanWangShanglongLiuDongfengZhangYanbingZhou
Xuelong Jiao*, Yujun Li*, Hongyan Wang, Shanglong Liu, Dongfeng Zhang, Yanbing Zhou
1General Surgery Department,2Pathology Department, The Affiliated Hospital of Qingdao University, Qingdao 266003, China;3Department of Epidemiology and Health Statistics, The Medical College of Qingdao University, Qingdao 266003, China
*These authors contributed equally to this work.
Correspondence to: Yanbing Zhou, MD. General Surgery Department, The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Qingdao 266003, China. Email: zhouyanbing999@aliyun.com.
Clinicopathological features and survival analysis of gastroenteropancreatic neuroendocrine neoplasms: a retrospective study in a single center of China
Xuelong Jiao1*, Yujun Li2*, Hongyan Wang1, Shanglong Liu1, Dongfeng Zhang3, Yanbing Zhou1
1General Surgery Department,2Pathology Department, The Affiliated Hospital of Qingdao University, Qingdao 266003, China;3Department of Epidemiology and Health Statistics, The Medical College of Qingdao University, Qingdao 266003, China
*These authors contributed equally to this work.
Correspondence to: Yanbing Zhou, MD. General Surgery Department, The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Qingdao 266003, China. Email: zhouyanbing999@aliyun.com.
Objective: To investigate the clinicopathological features, survival and prognostic factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) in a Chinese population.
Methods: We investigated 154 consecutive patients (88 males, 66 females; median age 56 years, age range 9-86 years) diagnosed with GEP-NENs between 2001 and 2013 at The Affiliated Hospital of Qingdao University. Demographic, clinical and pathological variables and survival data were retrieved.
Results: The pancreas was the most common site of involvement (63/154, 40.9%). Tumor size varied from 0.3 to 16.0 cm (median, 1.2 cm). The patients were followed up for a median period of 22 months (range,1-157 months). The estimated 3- and 5-year overall survival (OS) rates for all patients were 84.0% and 81.9%, respectively. Multivariate analysis showed that larger tumor size, lymphatic metastases and distant metastases were significant predictors for poor survival outcome.
Conclusions: Our data provide further information on the clinicopathological features of GEP-NENs in China. Additionally, we identified tumor size, lymphatic metastases and distant metastases as independent prognostic factors for long-term survival.
Gastroenteropancreatic neuroendocrine neoplasm (GEP-NEN); clinicopathological characteristics;survival analysis; Ki-67; retrospective study
Introduction
Neuroendocrine neoplasms (NENs) are a diverse group of tumors that derive from epithelial cells with neuroendocrine differentiation (1). According to an analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database, which is currently the largest epidemiological series, the incidence of NENs has risen substantially in the past 30 years (2). Gastroenteropancreatic(GEP)-NENs are a subset of NENs that arise from tissues throughout the gastrointestinal tract, which is the most commonly affected site (2,3). To date, many studies on GEP-NENs refer to western countries (2-5). However, for Asian populations (6), especially China, there is a paucity of large epidemiological studies of patients with this condition(7-10). For this reason, detailed data are needed for comprehensive knowledge of GEP-NENs in China. Based on the 13-year data of The Affiliated Hospital of Qingdao University, a retrospective study was carried out to explore the clinicopathological features and survival of GEP-NENs.
Patients and methods
A review of all the cases with histologically confirmed GEP-NENs was carried out in the medical record libraryof The Affiliated Hospital of Qingdao University over a period of 13 years [2001-2013]. Clinical features, including age, gender, location, clinical symptoms, histopathological characteristics and outcomes, were collected. The cases were reclassified according to the WHO 2010 criteria (11). Follow-up data were obtained both via query system of social health insurance and by phone call, if available.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up in living patients. Data were entered and cleaned by using Epi-Info version.3.5.3 and then were exported to SPSS 17.0 (SPSS Inc., Chicago,IL, USA). Descriptive analyses were applied to determine the patients’ baseline of clinicopathological characteristics. Kaplan-Meier survival curve was used to estimate the mean survival time or median survival time (if available). In univariate analysis, survival time was compared by Logrank test. Multivariate Cox-proportional hazard model was employed to identify predictors of survival outcomes. Hazard ratio (HR) with 95% confidence interval (95% CI) was computed, and P<0.05 (two-side) was considered statistically significant.
The study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University {with a reference number: 308 [2013]} and complied with the Declaration of Helsinki (6th revision, 2008). Patient records were anonymized and de-identified prior to analysis.
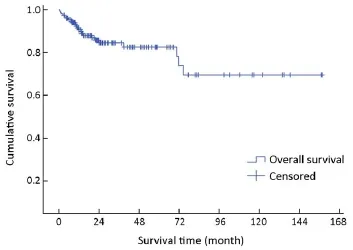
Figure 1 Overall survival curve of 154 cases.
Results
Survival
The median follow-up time was 22 months (range, 1-157 months). Complete data accounted for 15.1% of the total. During the observation period, 28 deaths occurred. The median survival time was not reached during the observation period. The mean survival time was 121±8 months(95% CI: 106-136). The estimated 3- and 5-year survival rates were 84.0% and 81.9%, respectively (Figure 1).
Gender and age at diagnosis
Of 154 patients with GEP-NENs, 88 (57.1%) were men and 66 (42.9%) were women. The male to female ratio was 1.33. Univariate analysis showed no significant difference in survival according to gender (χ2=2.692, P=0.101) (Table 1,Figure 2). The median age at diagnosis was 56 years (range,9-86 years). For 54 patients aged <50 years, the survival was significantly higher compared with that of 100 patients aged ≥50 years (χ2=9.647, P=0.002) (Table 1, Figure 3).
Primary tumor sites
The pancreas was the primary tumor site in 63 patients(40.9%), the large intestine (rectum plus colon) in 43(27.9%), and the stomach in 34 (22.1%). Among the nonpancreatic tumors, the rectum (35/91, 38.5%) and stomach (34/91, 37.4%) were the most frequent sites of origin. Details about tumor primary locations are listed in Table 2.
A trend toward longer survival for pancreatic NENs as compared to nonpancreatic NENs was found (χ2=16.309,P<0.001) (Table 1, Figure 4).
Clinical presentation
Nonfunctional tumors comprised the majority of GEPNENs (102/154, 66.2%), whereas functional tumors accounted for the remainder (52/154, 33.8%). Of the 102 nonfunctional cases, abdominal pain was the most frequent symptom (49/102, 48.0%), which was not specific for the diagnosis. Other nonspecific symptoms were abdominal distension (16/102, 15.7%), gastrointestinal bleeding (12/102, 11.8%), jaundice (5/102, 4.9%), nausea or vomiting (4/102, 3.9%), and diarrhea (3/102, 2.9%). Incidental diagnosis occurred in 9/102 cases (8.8%), which were usually asymptomatic.
Of the 52 functional cases, the most frequent functionality was fasting hyperinsulinemic-hypoglycemianeuroglycopenia syndrome (46/52, 88.5%) induced by insulinoma primarily located in the pancreas. Carcinoid syndrome was the second most frequent functionalityfound in four cases, including three gastric NENs with liver metastases and one colonic NEN with pulmonary metastasis. No other hypersecretion-induced syndromes such as Zollinger-Ellison syndrome, Verner-Morrison syndrome, or glucagonoma syndrome were found.
Mean survival time was significantly different between functional and nonfunctional patients (χ2=14.159, P<0.001)(Table 1, Figure 5).
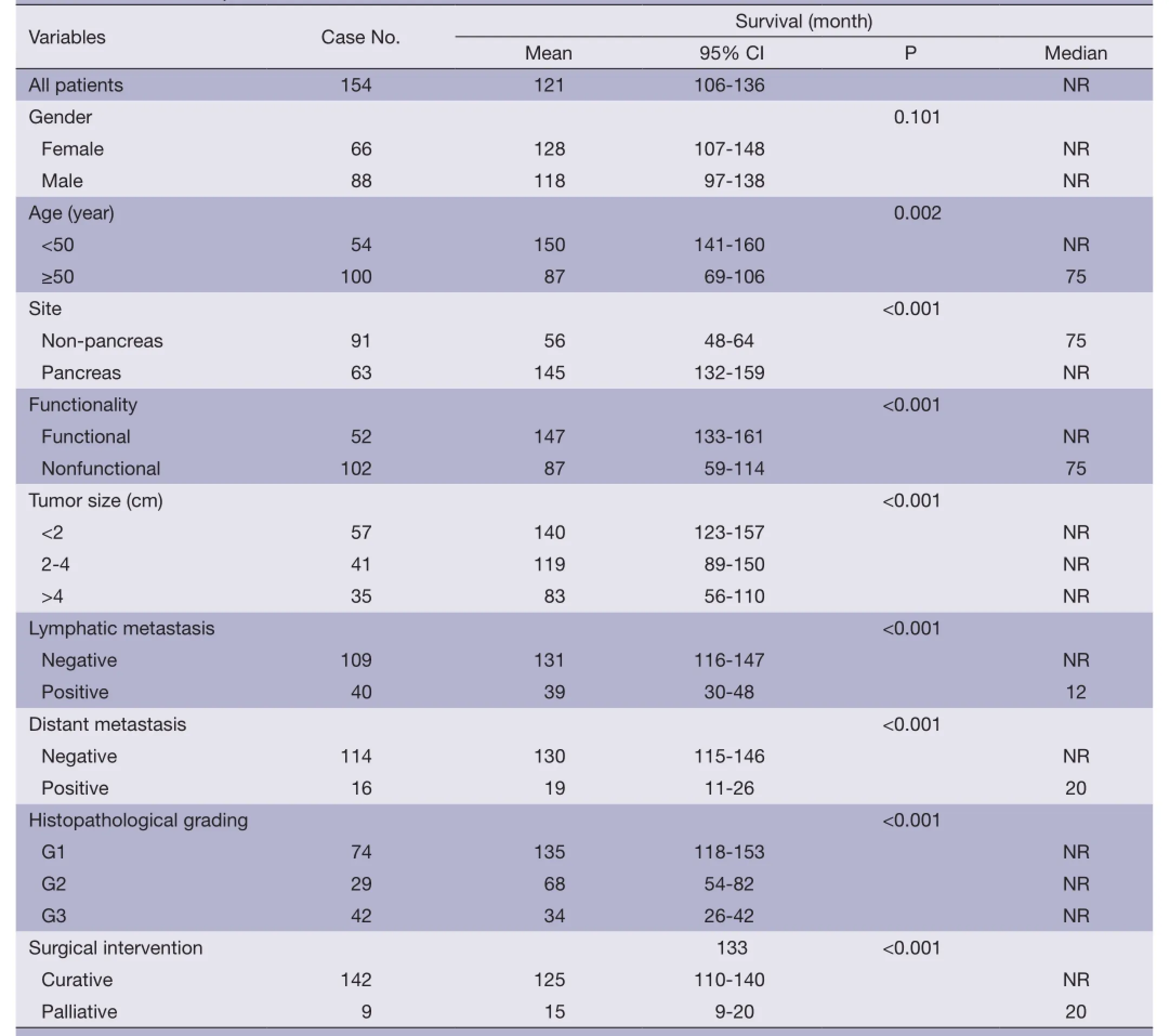
Table 1 Univariate analysis of each survival factor
Tumor-node-metastasis characteristics
One hundred and thirty-three of 154 (86.4%) cases were available for analysis of tumor size. The median tumor diameter was 1.2 cm (range, 0.3-16.0 cm): 57 (42.9%) were<2 cm in diameter, 41 (30.8%) were 2-4 cm, and 35 (26.3%)were >4 cm.
T-staging of the primary tumor (81.2% cases available for analysis, 125/154) was classified according to the Unionfor International Cancer Control (UICC) scheme (12). Tumor in situ accounted for 0.8% (1/125) of the cases, while T1, T2, T3and T4accounted for 44.8% (56/125), 18.4%(23/125), 12.8% (16/125) and 23.2% (29/125), respectively. As a result of the nonuniformity of the T-staging scheme (12) for different organs, tumor size (diameter)instead was applied to univariate analysis. Difference in mean survival time was significant between subgroups when divided by diameter (χ2=22.135, P<0.001) (Table 1, Figure 6).
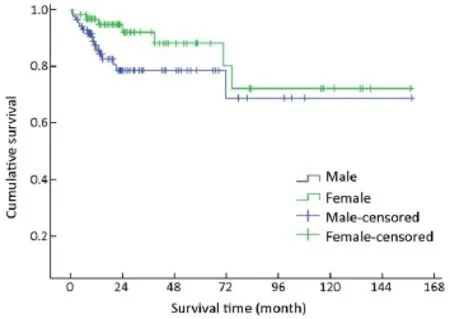
Figure 2 Overall survival curves by gender.
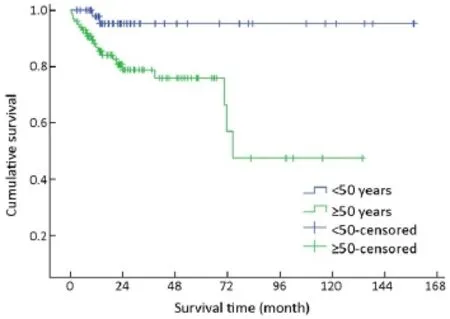
Figure 3 Overall survival curves by age at diagnosis.
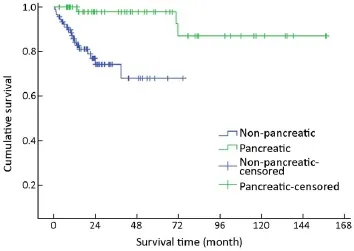
Figure 4 Overall survival curves by primary tumor site.
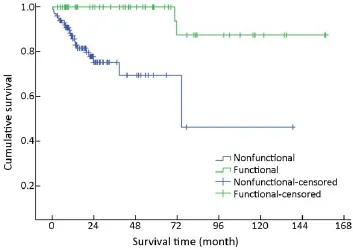
Figure 5 Overall survival curves by functionality.
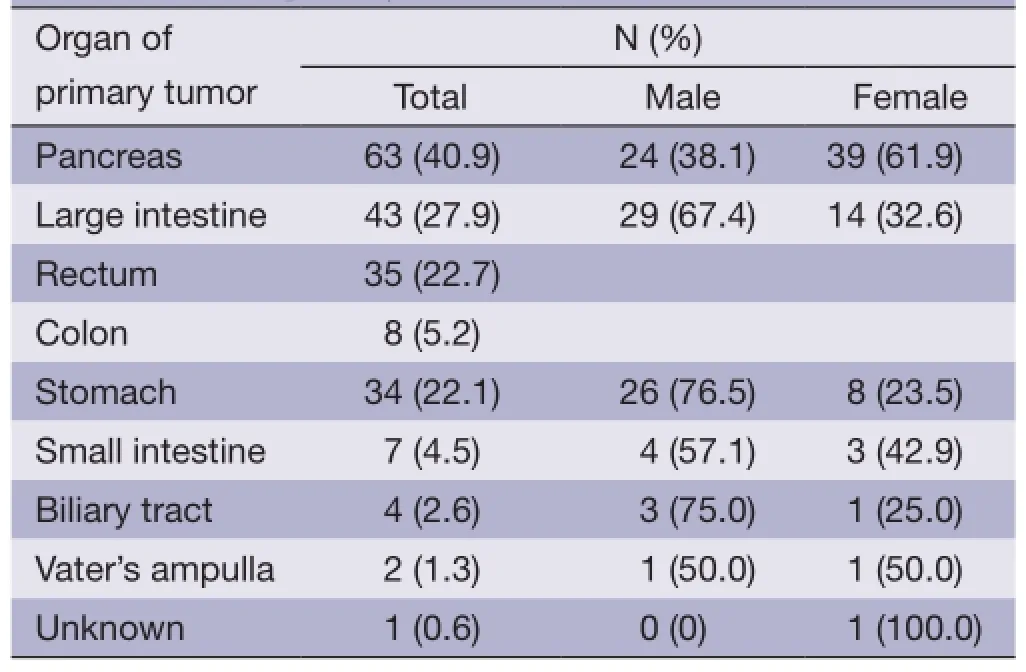
Table 2 Sites of primary tumor
One hundred and forty-nine of 154 (96.8%) cases were available for analysis of the regional lymph nodes. As for the N-staging according to the UICC scheme (12), N0accounted for 73.2% (109/149) of the cases, while N1, N2and N3accounted for 22.1% (33/149), 3.4% (5/149) and 1.3% (2/149), respectively. The number of metastatic nodes ranged from 1 to 8. The absence of lymph node metastases improved survival compared to those patients with lymph node metastasis with a mean survival time of 131 vs. 39 months(χ2=17.633, P<0.001) (Table 1, Figure 7).
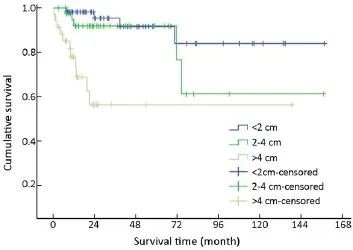
Figure 6 Overall survival curves by tumor size.
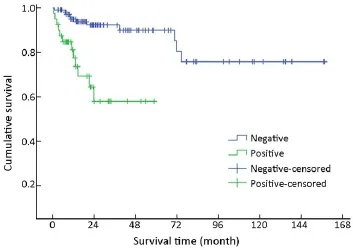
Figure 7 Overall survival curves by status of lymphatic metastasis.

Figure 8 Overall survival curves by status of distant metastasis.
Metastases were found in 16/154 (10.4%) patients at initial diagnosis. During follow-up, the number increased to 23 (14.9%). The most common site of distant metastases was liver (14/23, 60.9%), followed by lung (4/23, 17.4%),bone (3/23, 13.0%), and retroperitoneal lymph node (2/23,8.7%). Metastases in other organs were also observed in spermary, duodenum, pancreas, spleen, kidney and Virchow lymph node. Among the patients mentioned above,metastasis in multiple organs was a frequent event (12/23,52.2%). Furthermore, multiple metastases affecting the liver accounted for half of the multiple metastatic cases (6/12). The absence of metastases improved survival compared to those patients with metastases with a mean survival time of 130 vs. 19 months (χ2=30.826, P<0.001) (Table 1, Figure 8).
Immunohistochemistry and histopathology grading
Immunohistochemical staining showed an 83.0% positive rate for chromogranin A and an 89.0% positive rate for synaptophysin. The Ki-67 index and mitotic rate were assessed in all of the patients to estimate their proliferative activities. According to the current WHO 2010 criteria (11),145/154 (94.2%) cases were available for analysis of grading. Among these, 51.0% were reclassified as NEN grade 1 (G1),while 20.0% were NEN grade 2 (G2), and 29.0% were NEC grade 3 (G3). The difference in survival among G1,G2 and G3 was significant (χ2=36.266, P<0.001) (Table 1,Figure 9).
Surgical intervention
After initial diagnosis, tumor removal was performed in 151/154 (98.1%) patients, of which 142 (94.0%) were with curative intent and 9 (6.0%) were for palliative purpose. Radical resection was performed via laparotomy,laparoscopy or endoscopy, including endoscopic mucosal resection, endoscopic submucosal dissection, and endoscopic electroexcision. Overall, endoscopic excision was performed in 36/151 (23.8%) patients. As an alternativeto radical resection, palliative procedures were applied to patients with advanced malignant disease in order to obtain relief of obstruction, or debulking of tumor load. The mean survival time for these 142 patients with curative resection was higher compared with those with palliative treatment(χ2=14,441, P<0.001) (Table 1, Figure 10).
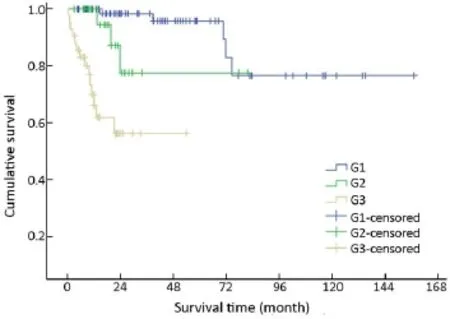
Figure 9 Overall survival curves by tumor grades.
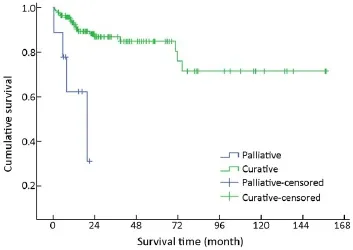
Figure 10 Overall survival curves by types of surgical intervention.
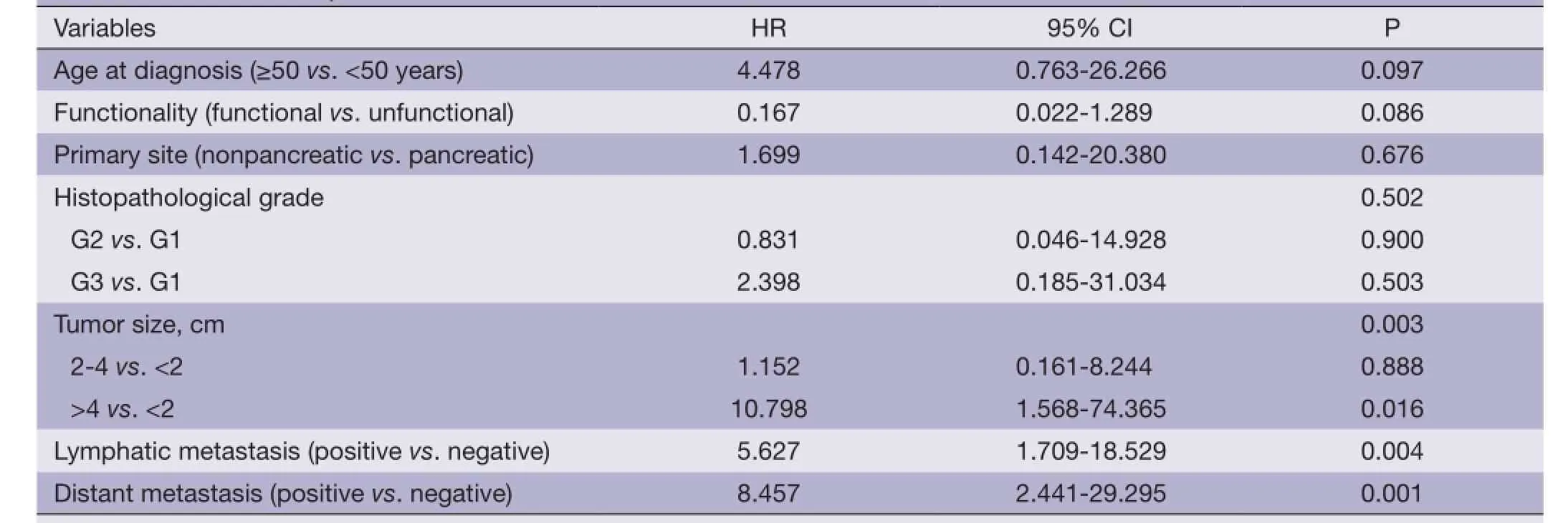
Table 3 Multivariate analysis of survival factors of GEP-NENs
Multivariate analysis modality. Multivariate analysis of the above factors showed that tumor size, status of lymphatic metastasis, and distant organ metastasis were the most significant predictors of survival (Table 3).
Discussion
GEP-NENs account for the largest group of NENs (13). We confirmed that GEP-NENs comprise a heterogeneous group in relation to their primary locations. With a higher percentage of pancreatic NENs (40.9%), the distribution of tumor primary site in our study was not comparable to that in another Chinese referral center (14) or to studies from
Univariate analysis mentioned above showed significance for age at diagnosis, primary tumor site, status of functionality,tumor size, regional lymphatic metastasis, distant organ metastasis, histopathological grading, and treatmentother countries (6,15). Coincidentally, the pancreas was also found to be the most frequent primary organ in a study in Turkey (16). These disparities may be due to the referral bias, and suggest racial variation in carcinogenesis of GEPNEN. Therefore, a larger patient population is required for further investigation. In our cohort, the survival of patients with tumors localized in the pancreas was better than those with nonpancreatic tumors. In a previous study (17),pancreatic localization was significant in univariate analysis(P=0.0024). This is consistent with our data.
NENs can be classified into functional and nonfunctional tumors according to the presence or absence of symptoms associated with hormone overproduction (18). In accordance with earlier experience (19), the current study showed that nonspecific symptoms were evident in most of the nonfunctional cases, which may give rise to misdiagnosis. Abdominal pain was the most common presenting symptom in our cohort, which was consistent with previous reports(19,20). Our study demonstrated that insulinomas were the most common functional tumors in the pancreas,accounting for 73.0 % (46/63) of pancreatic NENs.
Patients with positive nodes (N1-3) accounted for 25.9% of the cases in our cohort. This differs from a previous study (21),in which up to 51.7% of all patients had lymph node metastasis. Despite all this, a radical approach with lymph node dissection seems mandatory in surgical treatment.
In our cohort, there was a lower rate of distant metastases at initial diagnosis (10.4%) compared with other studies(52.0-77.0%) (10,13,19). Coincidentally, the most common metastatic site was the liver, which is in agreement with other studies (10,13). It is accepted that full inspection of the liver, lungs and other organs with suspicious metastases should be recommended at the time of initial diagnosis,because multiple metastases may be present even though the primary site may be clinically silent.
The classification of NENs is still under debate. The WHO revised the nomenclature and classification of GEP-NENs in 2010 (11). Thereafter, China established its own classification system for GEP-NENs in 2011 (22). In our study, the cases were reclassified according to the above consensus. Overall, G1 tumors accounted for 51.0% of cases, followed by G3 (29.0%) and G2 (20.0%). This is comparable to another Chinese study (10). In a recently publicized series of patients with GEP-NENs in Italy (23),54.7% had G1 NENs, 31.5% G2 NENs and 19.1% G3 NENs.
In the majority (94.0%) of patients, surgery was conducted with curative intent. The high rate of curative resection was attributed to the number of NENs localized in the pancreas and classified as benign. An increasing number of NENs in the gastrointestinal tract are treated successfully by a variety of endoscopic techniques. Candidate NENs for endoscopic procedure are localized and well-differentiated tumors with a low risk of metastasis (24). As an alternative to radical resection, palliative procedures are applied to patients with advanced malignant disease in order to obtain relief of obstruction or debulking of tumor load.
In prior studies, the 3- and 5-year survival rates in patients with GEP-NETs were 71.0% (16) and 45.0-60.0% (2,5,25), respectively. In our study, prognosis was more favorable, with 3- and 5-year survival rates of 84.0% and 81.9%, respectively. Our results, however, may be overestimated due to insufficient follow-up in the context of a slow-growing disease with a high rate of late events.
In univariate analysis, a significant difference was observed for nearly all the variables except for gender. As observed by others, younger age, pancreatic location of primary tumor, presence of hormonal syndrome, smaller tumor, absence of lymphatic or distant metastases, lower histopathological grading, and curative treatment were determined as positive factors affecting the prognosis in our univariate analysis. However, in multivariate analysis, tumor size, and lymphatic and distant metastases became the independent prognostic factors, whereas age at diagnosis, primary tumor site, functionality,and histopathological grading became nonsignificant prognostic factors. This is different from other studies which demonstrated that difference in survival prognosis was significant among different grades. Other studies have shown that possible improvement in the prognostic capability of the G grading system may be achieved by modifying the Ki-67 cut-off value (26,27). Consequently,the tumor grade has a long way to become an independent prognostic factor and represents a useful tool to evaluate prognosis.
Conclusions
Our data provide further information on the clinicopathological features of GEP-NENs in China. This lays the groundwork for further characterization of GEP-NENs in the Asian population and worldwide. Additionally, this analysis identified some variables as independent prognostic factors for long-term survival, while raising discussion of modification to the G grading system. Further follow-up of this patient population will be conducted in the future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
1. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72.
2. Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72.
3. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59.
4. Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 2008;113:2655-64.
5. Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 2007;132:899-904.
6. Lim T, Lee J, Kim JJ, et al. Gastroenteropancreatic neuroendocrine tumors: incidence and treatment outcome in a single institution in Korea. Asia Pac J Clin Oncol 2011;7:293-9.
7. Wang DS, Zhang DS, Qiu MZ, et al. Prognostic factors and survival in patients with neuroendocrine tumors of the pancreas. Tumour Biol 2011;32:697-705.
8. Zhang MH, Liu YH, Luo XL, et al. Neuroendocrine neoplasm of digestive system with different grades: a clinicopathologic and prognostic study. Zhonghua Bing Li Xue Za Zhi 2012;41:448-51.
9. Zeng YJ, Liu L, Wu H, et al. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine tumors: analysis from a single-institution. Asian Pac J Cancer Prev 2013;14:5775-81.
10. Wang YH, Lin Y, Xue L, et al. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord 2012;12:30.
11. Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press, 2010.
12. Sobin LH, Gospodarowicz MK, Wittekind C. editors. TNM classification of malignant tumours. 7th ed. Hoboken: John Wiley & Sons, 2009.
13. Pape UF, Böhmig M, Berndt U, et al. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci 2004;1014:222-33.
14. Wang X, Song ZF, Yao WX, et al. Clinicopathological features and multivariate analysis of prognostic factors for patients with gastroenteropancreatic neuroendocrine tumors. Zhonghua Yi Xue Za Zhi 2013;93:1411-4.
15. Levi F, Te VC, Randimbison L, et al. Epidemiology of carcinoid neoplasms in Vaud, Switzerland, 1974-97. Br J Cancer 2000;83:952-5.
16. Yucel B, Babacan NA, Kacan T, et al. Survival analysis and prognostic factors for neuroendocrine tumors in Turkey. Asian Pac J Cancer Prev 2014;14:6687-92.
17. Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083-92.
18. Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol 2010;34:300-13.
19. Ter-Minassian M, Chan JA, Hooshmand SM, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer 2013;20:187-96.
20. Zhang X, Ma L, Bao H, et al. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord 2014;14:54.
21. Niederle MB, Niederle B. Diagnosis and treatment of gastroenteropancreatic neuroendocrine tumors: current data on a prospectively collected, retrospectively analyzed clinical multicenter investigation. Oncologist 2011;16:602-13.
22. Chinese Pathologic Consensus Group for Gastrointestinal and Pancreatic Neuroendocrine Neoplasm. Chinese pathologic consensus for standard diagnosis of gastrointestinal and pancreatic neuroendocrine neoplasm. Zhonghua Bing Li Xue Za Zhi 2011;40:257-62.
23. Massironi S, Rossi RE, Casazza G, et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology 2014;100:240-9.
24. Klöppel G, Couvelard A, Perren A, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology 2009;90:162-6.
25. Lepage C, Bouvier AM, Faivre J. Endocrine tumours: epidemiology of malignant digestive neuroendocrine tumours. Eur J Endocrinol 2013;168:R77-83.
26. Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372-7.
27. Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23:824-33.
Cite this article as: Jiao X, Li Y, Wang H, Liu S, Zhang D,Zhou Y. Clinicopathological features and survival analysis of gastroenteropancreatic neuroendocrine neoplasms: a retrospective study in a single center of China. Chin J Cancer Res 2015;27(3):258-266. doi: 10.3978/j.issn.1000-9604.2015.06.04
10.3978/j.issn.1000-9604.2015.06.04
Submitted Sep 25, 2014. Accepted for publication May 18, 2015.
View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2015.06.04
杂志排行
Chinese Journal of Cancer Research的其它文章
- Usefulness of human epididymis protein 4 in predicting cytoreductive surgical outcomes for advanced ovarian tubal and peritoneal carcinoma
- Single nucleotide polymorphisms of MAGE-A3 gene and its clinical implications in Chinese patients with non-small cell lung cancer (NSCLC)
- Epidermal growth factor receptor mutation analysis in cytological specimens and responsiveness to gefitinib in advanced non-small cell lung cancer patients
- Presentation delay in breast cancer patients and its association with sociodemographic factors in North Pakistan
- Comparison of the clinical characteristics and survival between Uyghur patients with hepatitis virus-related and non-B, non-C hepatocellular carcinoma in Xinjiang, China
- microRNA-218 promotes gemcitabine sensitivity in human pancreatic cancer cells by regulating HMGB1 expression
