Surgery for oligometastasis of pancreatic cancer
2015-10-27FengchunLuKatherinePorukMatthewWeiss
Fengchun Lu, Katherine E. Poruk, Matthew J. Weiss
1Department of General Surgery, Union Hospital, Fujian Medical University, Fuzhou 350001, China;2Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Correspondence to: Matthew J. Weiss, MD. Department of Surgery and Medical Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA. Email: mweiss5@jhmi.edu.
Surgery for oligometastasis of pancreatic cancer
Fengchun Lu1,2, Katherine E. Poruk2, Matthew J. Weiss2
1Department of General Surgery, Union Hospital, Fujian Medical University, Fuzhou 350001, China;2Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Correspondence to: Matthew J. Weiss, MD. Department of Surgery and Medical Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA. Email: mweiss5@jhmi.edu.
Dr. Matthew J. Weiss is an Assistant Professor of Surgery and Oncology at the Johns Hopkins University School of Medicine. He is board-certified in general surgery and dual fellowship-trained in both complex surgical oncology and hepatopancreatobiliary. He trained in general surgery at the Johns Hopkins Hospital and completed a research fellowship at the Massachusetts General Hospital in immunology. He completed clinical fellowships at Memorial Sloan-Kettering Cancer Center in both surgical oncology and hepatopancreatobiliary surgery. His clinical interests include both benign and malignant tumors of the pancreas, liver, bile ducts, and gallbladder. He is a co-director of the Johns Hopkins Pancreas Cancer Multidisciplinary Clinic and Associate Program Director of the Johns Hopkins Surgical Oncology Fellowship Program.

Fengchun Lu

Matthew J. Weiss
The incidence of pancreatic adenocarcinoma (PDAC) has steadily increased over the past several decades. The majority of PDAC patients will present with distant metastases, limiting surgical management in this population. Hepatectomy and pulmonary metastasectomy (PM) has been well established for colorectal cancer patients with isolated, resectable hepatic or pulmonary metastatic disease. Recent advancements in effective systemic therapy for PDAC have led to the selection of certain patients where metastectomy may be potentially indicated. However, the indication for resection of oligometastases in PDAC is not well defined. This review will discuss the current literature on the surgical management of metastatic disease for PDAC with a specific focus on surgical resection for isolated hepatic and pulmonary metastases.
Pancreatic cancer (PC); oligometastasis; hepatic metastasis; pulmonary metastasis; surgical management
Introduction
Pancreatic adenocarcinoma (PDAC) is a highly aggressive malignancy with one of the worst prognoses among gastrointestinal tumors. The American Cancer Society estimates that approximately 48,960 patients will be diagnosed with PDAC in 2015 with more than 40,560 deaths due to the disease (1). The median 5-year survival is only 6%,likely a result of the tumor's invasiveness and propensity towards metastases (2). The majority of patients will have distant metastases discovered by imaging at the time of diagnosis or in the operating room during attempted pancreatic resection (3). The liver is the most common location of distant metastasis in PDAC based on autopsy studies, followed by the peritoneum, lung and pleura, bones,and adrenal glands (4-8). However, distant metastases of PDAC has reported in almost every organ, including the brain and leptomeninges, diaphragm, gallbladder, heart and pericardium, small and large intestines, kidneys, ovaries and uterus, seminal vesicles, skin, stomach, spleen, testis, thyroid gland, urinary bladder, and orbit (5,7-17). Once PDAC has metastasized to distant organs, prognosis is dismal with an overall 5-year survival of only 1% (18,19).
The most effective treatment for PDAC is surgical resection, but patients with distant metastases are considered unresectable based upon National Comprehensive Cancer Network (NCCN) and National Cancer Institute (NCI)treatment guidelines (20,21). Therefore, unlike other malignancies, synchronous metastasectomy of PDAC is rarely performed in current clinical practice when distant disease is found. However, in some patients, distant metastases are not discovered until surgery despite a thorough pre-operative workup with negative imaging. In these situations, an extended resection could be advocated in select patients despite the fact that oligometastases are already present. The goal in this situation is to achieve total resection of all tumor with a microscopically negative(R0) resection margin, one of the most important factors contributing to increased long-term survival for patients with PDAC (22,23). However, little information exists about the value of synchronous metastasectomy together with pancreatectomy in patients with PDAC, particularly with regards to survival.
Hepatic metastasis of pancreatic cancer (PC)
The liver is the most common initial location of distant recurrence (24) in part because it is the first major organ reached by portal venous blood draining from the pancreas or lymphatic spread. With improvements in computed tomography (CT) imaging and three-dimensional reconstruction techniques, the ability of preoperative imaging to identify metastatic PDAC has increased dramatically over the last few decades. Approximately 50% of new PDAC cases are found to have distant metastases at diagnosis (3), and only 10-20% are surgical candidates at presentation (20,25-28). Currently, PDAC patients with stage IV disease on diagnostic imaging are referred for systemic adjuvant therapy and pancreatic resection is not routinely considered. Several large randomized control trials have demonstrated increased overall survival(OS) in patients with metastatic PDAC who undergo treatment with either gemcitabine-based chemotherapy or FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) (29,30). However, the literature suggests thatup to 12% of occult liver metastasis are only discovered at the time of explorative laparotomy, often due to limitations in the ability of pre-operative imaging to detect small(<5 mm) liver and peritoneal metastases (31,32). Treatment in such patients is met with great controversy and is a challenge for surgeons, especially when occult liver metastases are found concomitantly in a patient with a locally resectable pancreatic tumor. This creates a difficult decision about whether to perform a palliative bypass or the intended pancreatic resection. If pancreatectomy is chosen, similar controversy remains about whether to leave the liver metastases in situ or to perform the intended pancreatectomy together with a synchronous hepatectomy.
Hepatectomy for liver metastatic disease
Due to advancements in surgical technique and improvements in perioperative management, pancreatic resection can be performed with relatively low rates of morbidity and mortality. Many high volume surgical centers have reported in-hospital mortality rates of less than 5%, with a few selected centers reporting no operative mortality after pancreaticoduodenectomy (33-37). Pancreatic resection has been offered to a greater number of patients in recent years, in part due to the use of vascular reconstruction at high volume surgical centers for patients with tumor invasion into the portal vein or superior mesenteric artery. Vascular resection with reconstruction is performed in order to increase the likelihood of obtaining an R0 surgical margin, and studies have demonstrated improvement in OS with this technique (37-42). In this background, a discussion on whether to expand resection in the case of incidental synchronous liver metastases may be appropriate.
Several large randomized trials have shown the benefit of chemoradiation therapy (CRT) on OS after surgical resection of PDAC (43,44). Specifically, improved survival has been demonstrated in patients with tumor diameter <20 mm or early pT stage by TNM staging (45,46). Patients with resectable hepatic metastases that remain stable or decrease in size with neoadjuvant chemotherapy could theoretically be selected for simultaneous liver resection and pancreatectomy given favorable tumor biology and a propensity towards improved survival. In addition, resection of oligometastases could potentially benefit the patient by reducing tumor burden prior to adjuvant systemic therapy. Therefore, in selected patients, pancreatectomy combined with liver resection and systemic therapy may provide a chance for cure. Although surgery is critical to the curative therapeutic paradigm, recent improvements in survival have been largely due to more effective systemic therapy,highlighting the importance of a multidisciplinary treatment approach in these patients (47) (Figure 1).
Hepatectomy is common for resectable colorectal and neuroendocrine liver metastases. Most surgical centers have reported 5-year survival rates ranging from 40% to 58% for colorectal liver metastases (48-54) and up to 76% for neuroendocrine metastases (55-57) after resection. As a result of improved survival, the criteria for resectability of colorectal liver metastases has been significantly expanded over the course of the last decade and resection, when possible, has become standard of care in these patients (58). Although various complications such as bile leak,hemorrhage, and hepatic abscess have been reported after liver resection for colorectal cancer metastases (59), these can usually be managed non-operatively and without added mortality. Numerous studies have suggested that hepatic resection for colorectal liver metastases and neuroendocrine metastases is safe and effective, and liver resection in these patients is now well established. In contrast, hepatectomy for PDAC liver metastases is extremely controversial. The data for resection of PDAC liver metastasis are not well established, and the available literature is limited to surgery in a small number of extremely selected patients. Additionally, it is unclear from these studies how many patients received neoadjuvant chemotherapy prior to metastatectomy. Establishing hepatectomy for PDAC liver metastasis will only be justified if an improvement in survival and/or quality of life without an increase in surgeryrelated morbidity and mortality can be demonstrated. Currently, there is little information on outcomes after pancreatectomy for PDAC in patients with metastatic disease, making an objective conclusion difficult to achieve and a treatment guideline difficult to formulate.
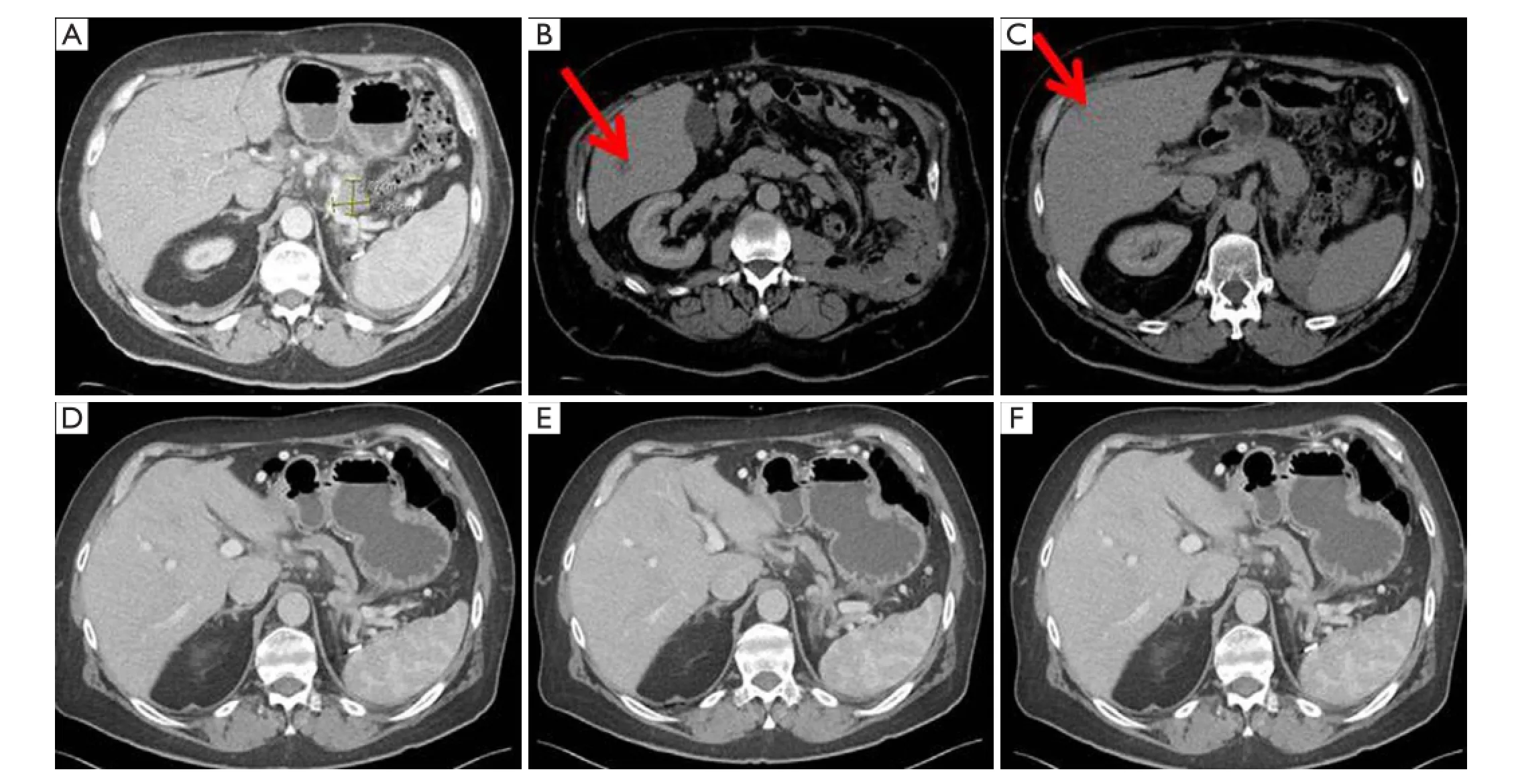
Figure 1 A 66-year-old man diagnosed with biopsy proven oligometastatic pancreas cancer in 2012. CT demonstrates (A) biopsy proven pancreas primary tumor; (B) biopsy proven liver metastasis; (C) 2nd liver metastasis. Patient was treated with gemcitabine and abraxane followed by 5-FU, leucovorin, oxaliplatin, and irinotecan. Twenty-four months after diagnosis, CT demonstrated significant radiologic response in (D) pancreas primary; (E) liver metastasis; and (F) 2nd liver metastasis. Surgical resection of pancreas and liver lesions demonstrated complete pathologic response in all three sites and patient currently has no evidence of disease 6 months post resection and 30 months after diagnosis. CT, computed tomography; 5-FU, 5-fluorouracil.
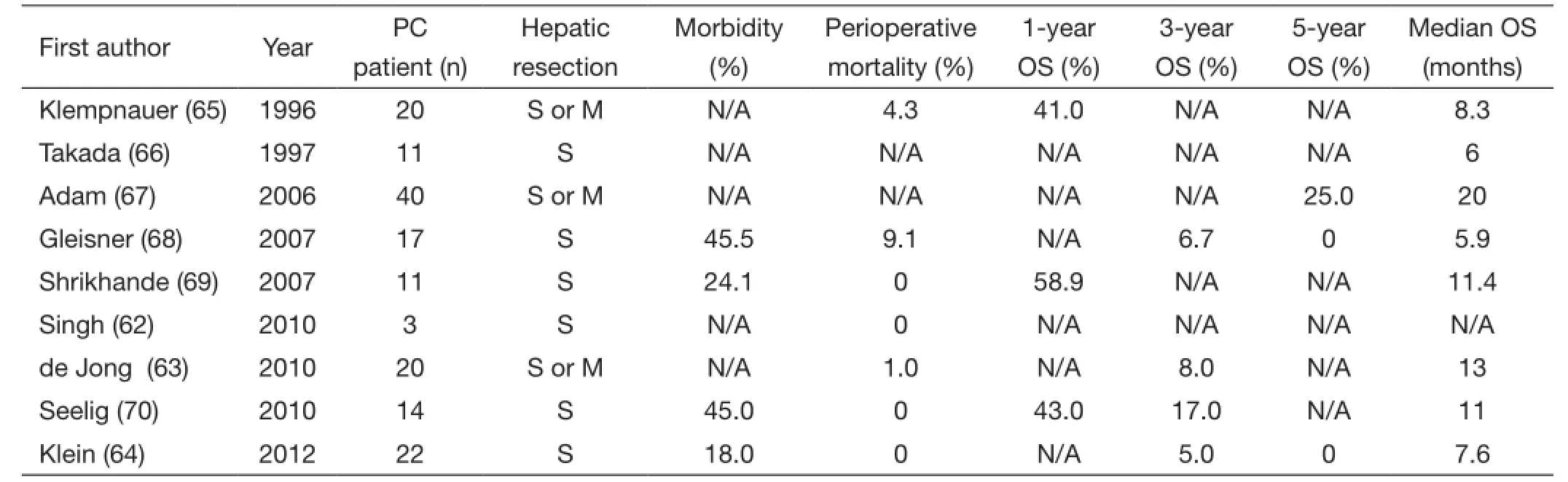
Table 1 Summary of recent studies of PC liver metastatic patients for pancreatectomy with hepatectomy
Current literature has shown that pancreatectomy with synchronous hepatic metastasectomy can be performed safely without a significant increase in perioperative morbidity and mortality (60-64) (Table 1). However, the potential benefit on long-term survival is less clear (62,68). Singh et al. (62) demonstrated that the resection of a solitary liver metastasis can be safely performed together with pancreaticoduodenectomy. However, whether OS improved was not definitively proven. In this study, three PDAC patients underwent synchronous metastasectomy and pancreaticoduodenectomy and died at 7, 14 and 18 months post-operatively. de Jong et al. (63) examined 40 patients who underwent surgery with curative intent [resection and/or radiofrequency ablation (RFA)] for periampullary liver metastases. Among the 40 patients in the study, 20 patients had a tumor that originated in the pancreas, only four of which underwent neoadjuvant chemotherapy. Additionally,27 of the 40 patients presented with synchronous metastatic disease while the other 13 had metachronous metastatic disease, although metastatic disease at presentation did not affect median survival (synchronous vs. metachronous,16 vs. 19 months; P=0.55). Surgery consisted of resection only (n=31; 78%), RFA only (n=8; 20%) or resection plus RFA (n=1; 2%). In the 32 patients, the extent of hepatic resection was a wedge resection (n=22; 69%),segmentectomy (n=6; 25%), and hemihepatectomy(n=4, 10%). The median survival of patients with a pancreaticobiliary tumor was 13 months with overall 3-year survival of 8%. Klein et al. (64) reported an overall median survival in PDAC patients with hepatic metastases of 228 days (±298.0) after resection, with a two-year survival of 5% (one patient). This included 22 PDAC patients who underwent synchronous, liver-directed therapy either with liver segmentectomy (7 patients, 32%) or enucleation of the hepatic metastases (15 patients, 68%). No patient achieved five-year survival after hepatic resection. All patients received adjuvant therapy with gemcitabine, but it is unclear which patients may have also received neoadjuvant chemotherapy. Gleisner et al. (68) reported that the median OS of periampullary or PDAC patients who underwent hepatic resection of synchronous metastasis was not different from the OS of matched patients who underwent palliative bypass (5.9 vs. 5.6 months; P=0.46). This study included 17(77.3%) PDAC patients and the majority of patients (86.4%)had a solitary hepatic metastasis, with a median size of 0.6 cm. Hepatic resection included a wedge resection in 20 patients (90%), a segmentectomy in one patient (4.5%), and a hemi-hepatectomy in one patient (4.5%). Only six of the PDAC patients received adjuvant chemotherapy. Finally,Takada et al. (66) noted no improvement in OS in addition to higher surgical morbidity and mortality in patients undergoing pancreatoduodenectomy with synchronous partial liver resection.
Alternatively, several publications have demonstrated improved long-term survival after the successful resection of a pancreatic lesion and hepatic metastases (64,71-73). Adam et al. (67) reported 5-year survival rates upwards of 25% and a median survival of 20 months for patients who underwent hepatic resection of metastatic lesions from pancreatic primary tumors. The subset of patients with PDAC had a 5-year survival of 20%, which is comparable to patients with resectable PDAC without metastases. Klempnauer et al. (65) reported a median survival of 8.3 months after synchronous liver and pancreatic resection and 5.8 months after metachronous hepatic resection. Oneyear survival rates were 41% after synchronous resection and 40% after metachronous resection of hepatic metastases of pancreatic (n=20) or ampullary (n=2) carcinomas. Shrikhande et al. (69) suggested that pancreatic resections with simultaneous liver resection for metastatic disease can be performed with acceptable safety in highly selected patients. Of the 11 PDAC patients with liver metastasis,those who underwent pancreatectomy with synchronous hepatectomy had significantly longer median survival than the patients who underwent exploratory laparotomy without any resection (11.4 vs. 5.9 months, P=0.038). Of note, the patients included in this study were considered to be in good overall health with an ASA grade of III or better,had only one or two isolated liver metastases, and a high probability of an R0 resection. Only one patient in the study received neoadjuvant therapy, while the majority received adjuvant chemotherapy. Given the strict inclusion criteria,the authors suggested that resection of liver metastases in PDAC patients, although safe in this series, cannot be generally recommended until further controlled trials are conducted.
Although the studies on the surgical management of PDAC liver metastasis were all single institution retrospective a study involving a small number of patients without well-defined indications for resection, the data suggests that hepatic resection is safe and may be appropriate for highly selected PDAC patients. Survival data at this time is mixed and a prospective study is needed to determine the exact benefit, if any, the resection of hepatic metastasis will have on OS. Furthermore, the use of neoadjuvant and adjuvant CRT should be standardized in these patients to prolong survival and avoid confounding results. At this time, resection of PDAC liver metastases should only be considered in patients who are in overall good general health without significant comorbidities. It should be recommended that patients undergo neoadjuvant chemotherapy with an assessment by imaging for stability or decrease in the size and number of metastases prior to hepatic resection. In order to preserve vascular inflow and outflow as well as biliary drainage and preserve an adequate future liver remnant (45), wedge resection, segmentectomy or hemihepatectomy may be considered for these selected patients. Until it is determined which patient population will achieve the greatest benefits with metastastectomy,pancreatic resection with hepatectomy should be cautiously considered only in selected PDAC patients with limited liver metastases in whom surgery is considered.
Ablation techniques for PDAC liver metastasis
Ablation techniques have become widely used in the treatment of hepatic metastases, including RFA, microwave,laser, cryoablation, and irreversible electroporation. Ablation can be performed using an open, laparoscopic,or percutaneous image-guided approach. Within the past several decades, numerous publications on ablation therapy techniques for liver metastases have demonstrated the effectiveness and safety of this therapy (74-79). These techniques are currently used to treat colorectal cancer liver metastasis in selected patients (33,80-84), and have been proposed as an alternative to hepatectomy in patients with limited hepatic involvement or with solitary liver metastasis(79,83,84). Simo et al. (79) reported that laparoscopic RFA of resectable colorectal liver metastases is associated with low perioperative morbidity and mortality with comparable long-term survival to hepatic resection in carefully selected patients. This was especially true in patients where the hepatic metastases were smaller than 3 cm and no tumors were within 1 cm of central biliary structures.
RFA has also been shown to be beneficial in the treatment of liver metastases from pancreatic neuroendocrine tumors,especially to control symptoms and optimize quality of life(85,86). However, there are differing opinions about the utility of RFA for unresectable PDAC. Girelli et al. (87)reported that RFA of a locally advanced PC is feasible and relatively well tolerated. Moreover, RFA in parallel to palliative therapy may provide a survival benefit, especially for stage III patients with unresectable PDAC (88). Alternatively, Pezzilli et al. (89) concluded that although RFA is a feasible technique, its safety and long-term results are disappointing for unresectable PDAC. Few studies have specifically analyzed the outcomes of ablation techniques for PDAC liver metastasis. Therefore, further research is needed to determine the benefit of ablation techniques as therapeutic options for the isolated liver metastases in PDAC patients.
Pulmonary metastasis of pancreatic cancer (PC)
The lung is another common site for distant metastasis in PDAC patients (6,8). Most notably, recurrence to the lung after initial primary tumor resection is associated with the most long-term survivors of at least 5 years for any patient with metastatic PDAC (24). Although pulmonary metastasectomy (PM) has been shown to provide a survival benefit for colorectal cancer patients with lung metastases(90-94), an evaluation of PM for PDAC is limited. At our institution, a retrospective study of PM for isolated PC metastases by Arnaoutakis et al. (95) reported that PM for isolated PDAC lung metastases is safe and effective. Compared to the non-PM patients, the median OS of PM patients was significantly improved (52 vs. 22 months,P=0.04). Additionally, there was a trend in favor of PM for post-relapse survival. Patients undergoing PM had a median survival after relapse of 18.6 months, compared with only 7.5 months for non-PM patients. It is important to note that the patients in this study were highly selected and had a good biologic tumor character identified by a favorable response to systemic therapy. In addition, patients undergoing PM had a relatively long interval between initial pancreatectomy and pulmonary relapses. No studies to date have been published with regards to simultaneous PM and pancreatic resection, and further analysis of treatment in patients with synchronous lung metastases is needed for PDAC.
The successful outcomes of patients undergoing PM after pancreatectomy indicate that the complete resection of the primary tumor and lung metastases is possible with favorable outcomes. PM should be performed for isolated lung metastases after resection for PDAC in patients with an acceptable performance status with tumors exhibiting a favorable response to systemic therapy. Furthermore, RFA can be considered for the treatment of PDAC pulmonary metastases in patients that have contraindications for surgery, although further analysis is needed (74).
Conclusions
Recent improvements in operative management of PDAC have reduced perioperative morbidity and mortality in patients undergoing pancreatectomy, and subsequently have led to increased 5-year survival. While the majority of PDAC patients will present with metastatic disease and will not be operative candidates, in certain situations,metastasectomy may be beneficial and warrants further investigation. However, resection of metastatic pancreas cancer should be approached with extreme caution, knowing that the data is extremely limited. As systemic therapy for PDAC improves, appropriate selection of patients may lead to more aggressive surgical approaches, similarto the current paradigm for metastatic colorectal cancer. In current practice, metastasectomy for solitary hepatic or pulmonary metastases of PDAC should be considered only when (I) a negative surgical (R0) resection can be achieved by pancreatectomy; (II) the PDAC has responded to neoadjuvant systemic therapy; (III) the oligometastases are resectable; (IV) the patient is in overall good general health with limited comorbidities. When applied in these situations, surgery may be considered for these selected patients with the primary goal of improving long-term survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29.
2. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71.
3. Louvet C, Philip PA. Accomplishments in 2007 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res 2008;2:S37-41.
4. Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med 2009;133:413-22.
5. Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 1995;11:345-9.
6. Embuscado EE, Laheru D, Ricci F, et al. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther 2005;4:548-54.
7. Disibio G, French SW. Metastatic patterns of cancers:results from a large autopsy study. Arch Pathol Lab Med 2008;132:931-9.
8. Mao C, Domenico DR, Kim K, et al. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch Surg 1995;130:125-34.
9. Lemke J, Scheele J, Kapapa T, et al. Brain metastasis in pancreatic cancer. Int J Mol Sci 2013;14:4163-73.
10. Rao R, Sadashiv SK, Goday S, et al. An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res 2013;6:90-2.
11. Mirrakhimov AE, Khan FN. Epidural brain metastases in a patient with early onset pancreatic cancer: a case report and literature review. Case Rep Oncol Med 2012;2012:962305.
12. Kolokythas A, Miloro MB, Olsson AB, et al. Metastatic pancreatic adenocarcinoma to the mandibular condyle:a rare clinical presentation. J Oral Maxillofac Surg 2014;72:83-8.
13. Monson BK, Patel BC, Kim CH. Metastatic mucinous adenocarcinoma of the orbit. Orbit 2011;30:18-20.
14. Webber NP, Sharma S, Grossmann AH, et al. Metastatic pancreatic adenocarcinoma presenting as a large pelvic mass mimicking primary osteogenic sarcoma: a series of two patient cases. J Clin Oncol 2010;28:e545-9.
15. Bellows C, Gage T, Stark M, et al. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J 2009;102:748-50.
16. Vähätalo K, Ekfors T, Syrjänen S. Adenocarcinoma of the pancreas metastatic to the mandible. J Oral Maxillofac Surg 2000;58:110-4.
17. Rosser CJ, Gerrard E. Metastatic adenocarcinoma of the pancreas to the testicle: a case report. Am J Clin Oncol 1999;22:619-20.
18. Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-8; discussion 698-700.
19. Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma:postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621-33; discussion 633-6.
20. Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012;118:2674-81.
21. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results,outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79.
22. Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94.
23. Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s:pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60.
24. Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009;16:836-47.
25. Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer:a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267.
26. Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions,management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46.
27. Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33.
28. Werner J, Combs SE, Springfeld C, et al. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol 2013;10:323-33.
29. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25.
30. Poruk KE, Firpo MA, Mulvihill SJ. Screening for pancreatic cancer. Adv Surg 2014;48:115-36.
31. Kneuertz PJ, Cunningham SC, Cameron JL, et al. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: trends and lessons learned from a large, single institution experience. J Gastrointest Surg 2011;15:1917-27.
32. Toomey P, Childs C, Luberice K, et al. Nontherapeutic celiotomy incidence is not affected by volume of pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2013;79:781-5.
33. Sharaiha RZ, Natov N, Glockenberg KS, et al. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci 2014;59:3099-102.
34. Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217:430-5; discussion 435-8.
35. Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58.
36. Büchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 2003;138:1310-4;discussion 1315.
37. Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, "unresectable" pancreatic cancer. Surgery 2012;152:S33-42.
38. Hartwig W, Hackert T, Hinz U, et al. Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg 2009;250:81-7.
39. Burdelski CM, Reeh M, Bogoevski D, et al. Multivisceral resections in pancreatic cancer: identification of risk factors. World J Surg 2011;35:2756-63.
40. Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systematic review. J Gastrointest Surg 2010;14:1442-52.
41. Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882-93.
42. Hackert T, Büchler MW. Pancreatic cancer: advances in treatment, results and limitations. Dig Dis 2013;31:51-6.
43. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81.
44. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10.
45. Morganti AG, Falconi M, van Stiphout RG, et al. Multiinstitutional pooled analysis on adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol Biol Phys 2014;90:911-7.
46. Merchant NB, Rymer J, Koehler EA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma:who really benefits? J Am Coll Surg 2009;208:829-38;discussion 838-41.
47. Page AJ, Weiss MJ, Pawlik TM. Surgical management of noncolorectal cancer liver metastases. Cancer 2014;120:3111-21.
48. Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7.
49. Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11:1057-77.
50. Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481-91.
51. Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509-20;discussion 520-2.
52. Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21.
53. Rees M, Tekkis PP, Welsh FK, et al. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35.
54. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66.
55. Bonney GK, Gomez D, Rahman SH, et al. Results following surgical resection for malignant pancreatic neuroendocrine tumours. A single institutional experience. JOP 2008;9:19-25.
56. Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45.
57. Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37.
58. Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64.
59. Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer--competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 1990;16:360-5.
60. Nikfarjam M, Sehmbey M, Kimchi ET, et al. Additional organ resection combined with pancreaticoduodenectomy does not increase postoperative morbidity and mortality. J Gastrointest Surg 2009;13:915-21.
61. McKay A, Sutherland FR, Bathe OF, et al. Morbidity and mortality following multivisceral resections in complex hepatic and pancreatic surgery. J Gastrointest Surg 2008;12:86-90.
62. Singh A, Singh T, Chaudhary A. Synchronous resection of solitary liver metastases with pancreaticoduodenectomy. JOP 2010;11:434-8.
63. de Jong MC, Tsai S, Cameron JL, et al. Safety and efficacy of curative intent surgery for peri-ampullary liver metastasis. J Surg Oncol 2010;102:256-63.
64. Klein F, Puhl G, Guckelberger O, et al. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract 2012;2012:939350.
65. Klempnauer J, Ridder GJ, Piso P, et al. Is liver resection in metastases of exocrine pancreatic carcinoma justified?Chirurg 1996;67:366-70.
66. Takada T, Yasuda H, Amano H, et al. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival?Hepatogastroenterology 1997;44:567-73.
67. Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35.
68. Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007;110:2484-92.
69. Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118-27.
70. Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma:the value of synchronous metastasectomy. HPB Surg 2010;2010:579672.
71. Ibusuki M, Hiraoka T, Kanemitsu K, et al. Complete remission of pancreatic cancer after multiple resections of locally pancreatic recurrent sites and liver metastasis:report of a case. Surg Today 2008;38:563-6.
72. Spinelli GP, Zullo A, Romiti A, et al. Long-term survival in metastatic pancreatic cancer. A case report and review of the literature. JOP 2006;7:486-91.
73. Shimada K, Kosuge T, Yamamoto J, et al. Successful outcome after resection of pancreatic cancer with a solitary hepatic metastasis. Hepatogastroenterology 2004;51:603-5.
74. de Baere T, Deschamps F. Treatment of hepatic and pulmonary metastases with radiofrequency. Diagn Interv Imaging 2014;95:683-8.
75. Chen J, Tang Z, Dong X, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol 2013;39:701-6.
76. de Baere T, Deschamps F. New tumor ablation techniques for cancer treatment (microwave, electroporation). Diagn Interv Imaging 2014;95:677-82.
77. Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors,prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 2014;21:1276-86.
78. Sofocleous CT, Sideras P, Petre EN. "How we do it" - a practical approach to hepatic metastases ablation techniques. Tech Vasc Interv Radiol 2013;16:219-29.
79. Simo KA, Sereika SE, Newton KN, et al. Laparoscopicassisted microwave ablation for hepatocellular carcinoma:safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011;104:822-9.
80. Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 2013;7:1-6.
81. Kwan BY, Kielar AZ, El-Maraghi RH, et al. Retrospective review of efficacy of radiofrequency ablation for treatment of colorectal cancer liver metastases from a Canadian perspective. Can Assoc Radiol J 2014;65:77-85.
82. Ungureanu BS, Sandulescu L, Şurlin V, et al. Surgical hepatic resection vs. ultrasonographic guided radiofrequency ablation in colorectal liver metastases: what should we choose? Med Ultrason 2014;16:145-51.
83. Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol 2011;18:1947-54.
84. Ripley RT, Kemp CD, Davis JL, et al. Liver resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol 2011;18:1972-9.
85. O'Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. Eur J Surg Oncol 2008;34:324-32.
86. Moug SJ, Leen E, Horgan PG, et al. Radiofrequency ablation has a valuable therapeutic role in metastatic VIPoma. Pancreatology 2006;6:155-9.
87. Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010;97:220-5.
88. Spiliotis JD, Datsis AC, Michalopoulos NV, et al. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg 2007;392:55-60.
89. Pezzilli R, Ricci C, Serra C, et al. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers (Basel)2010;2:1419-31.
90. Goya T, Miyazawa N, Kondo H, et al. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer 1989;64:1418-21.
91. Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer:a systematic review of published series. Ann Thorac Surg 2007;84:324-38.
92. Limmer S, Oevermann E, Killaitis C, et al. Sequential surgical resection of hepatic and pulmonary metastases from colorectal cancer. Langenbecks Arch Surg 2010;395:1129-38.
93. Sakamoto Y, Sakaguchi Y, Oki E, et al. Surgical outcomes after resection of both hepatic and pulmonary metastases from colorectal cancer. World J Surg 2012;36:2708-13.
94. Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435-40.
95. Arnaoutakis GJ, Rangachari D, Laheru DA, et al. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg 2011;15:1611-7.
Cite this article as: Lu F, Poruk KE, Weiss MJ. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res 2015;27(4):358-367. doi: 10.3978/j.issn.1000-9604.2015.05.02
10.3978/j.issn.1000-9604.2015.05.02
s’ introduction: Dr. Fengchun Lu is an associate professor of surgery of the Union Hospital, Fujian Medical University. He obtained a doctoral degree at the Department of Surgery, Fujian Medical University. He pursued his 1-year post-doctoral study at the Department of Surgery, the Johns Hopkins University School of Medicine. He is active in basic and clinical research, especially in pancreas diseases. At present, he is responsible for one national projects and two provincial projects in his specialties. He is a winner of three provincial prizes for science and technology advancement. He holds many organizational positions, including vice chairman of Youth Committee of the Fujian province branch of the Chinese Medical Association, member of the Youth Committee of Chinese Hernia College of Surgeons.
Submitted Mar 19, 2015. Accepted for publication Apr 08, 2015.
View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2015.05.02
杂志排行
Chinese Journal of Cancer Research的其它文章
- Immunotherapy for pancreatic ductal adenocarcinoma: an overview of clinical trials
- Pancreatic cancer surgery: past, present, and future
- Surgical treatment of pancreatic head cancer: concept revolutions and arguments
- Early detection of pancreatic cancer
- The crown jewelry of the surgeries for pancreatic cancer
- Prognostic effect analysis of molecular subtype on young breast cancer patients
