Recent Progress on Application of"Click"Chemistry in Labeling of Biomolecules
2015-10-22WUJieYUYanhua
WU Jie,YU Yanhua
(Institute for Interdisciplinary Research;Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education,Jianghan University,Wuhan 430056,Hubei,China)
0 Introduction
The concept of"click"chemistry was first introduced by Barry Sharpless in 1999 at the 21thAmerican Chemical Society Annual Conference.Since then,it has gained considerable attention as evidenced by a nearly exponential growth in the amount of related publications.In his landmark review in 2001[1],Sharpless defined"click"chemistry as a group of reactions that must be modular,wide in scope,give very high yields,generate only inoffensive byproducts that can be removed by nonchromatographic methods,and be stereospecific(but not necessarily enantioselective).
Based on the above mentioned requirements,five major types of"click"reactions have been identified[1].Among these five major classes,cycloadditions,particularly the Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddi⁃tion of azides and terminal alkynes(CuAAC)to form 1,2,3-triazoles[2-5],are by far the most widely used,not only in organic chemistry but also in material sciences[6],biological conjugation[7-9]and drug discovery[10],especially in labeling of biomolecules.
Labeling and imaging of biomolecules by means of fluorescent tags is an important tool for the study of complex biological processes both in vitro and in vivo[11-12].Among bioorthogonal tagging reactions,the"click"reaction,involving CuAAC is one of the most valuable.This method is superior to other labeling techniques because of the inertness of the chemical reporters and the exogenously delivered probes,and the selective and efficient reaction between the reporter and the probe.The extreme rareness of azide and alkyne functions in biological systems additionally increases the importance of tagging by the means of an azide/alkyne cycloaddition.This reaction has been shown to be quite versatile in terms of biological applications[13-15].
1 Labeling of Peptides and Proteins
1.1 Labeling of peptides
M.Z.Kamaruddin,et al.have reported the synthesis of a fluorophore-labeled peptide by"click"chemistry as a substrate of Src kinases(Scheme 1)[16].The Src optimal peptide(SOP),with the sequence AEEEIYGEFEA,was previously found to be a selective and efficient peptide substrate of Src-family protein tyrosine kinases(SFKs).The phenylalanine at the Y+3 position was determined to be an essential determinant dictating ef fi cient phosphorylation of SOP by SFKs.Based on these finding,a peptide with the sequence AEEEIYGE(Pra)EAKKKK was synthesized by using standard Fmoc-based solid phase peptide synthesis,in which the Pra represents a propargylglycine residue(containing an alkyne side chain).In order to synthesize a library of fluorophore-peptide conjugates,the screening of various reaction conditions revealed that a mixture of 10 equiv each of[Cu(CH3CN)4]PF6,sodium ascorbate,tris-(benzyltriazolylmethyl)amine(TBTA)and DIPEA in DMF could drive the solid phase click reaction to completion,when used in conjunction with microwave heating at a temperature of 80°C for 30 min.
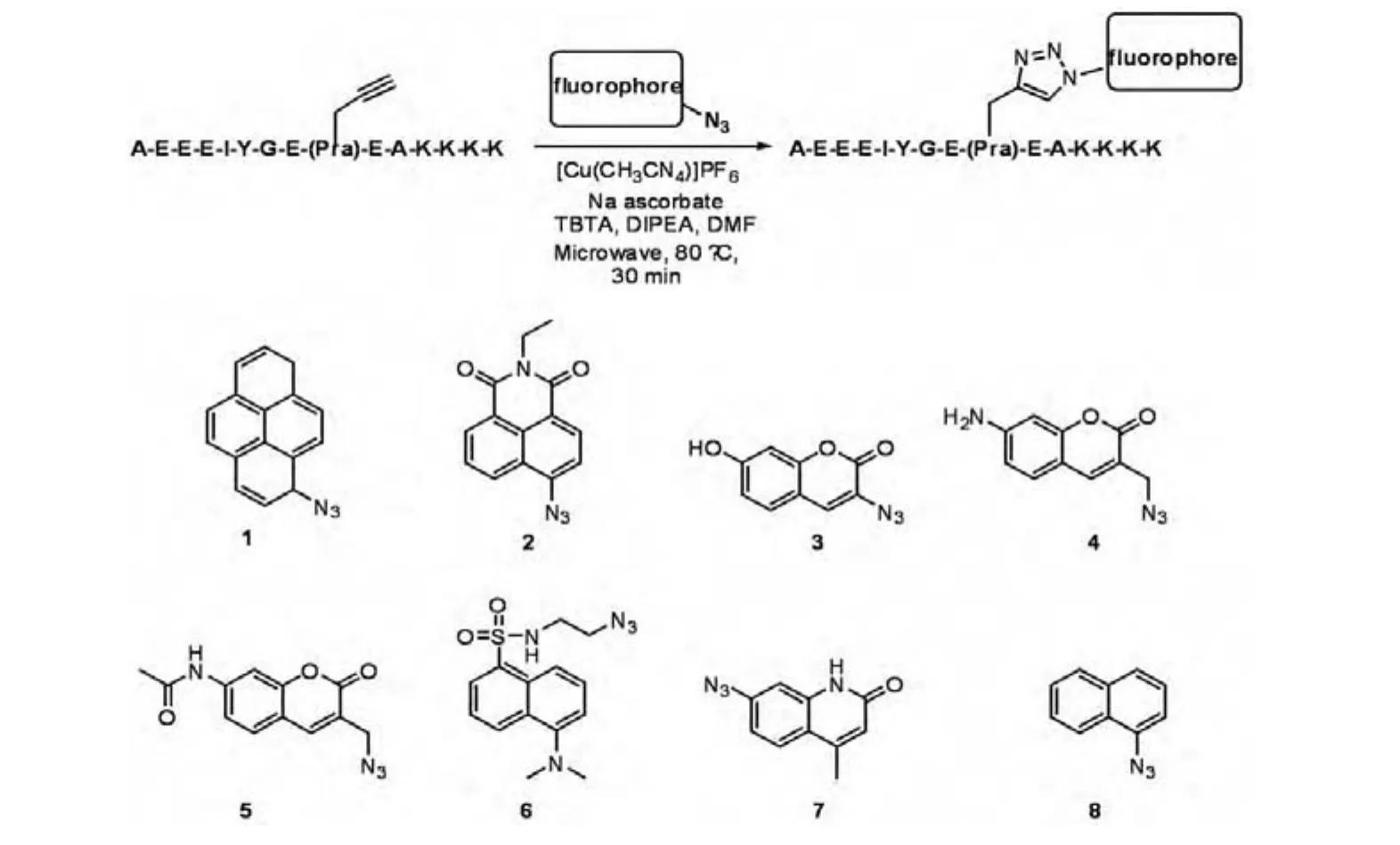
Scheme 1 Assembly of fluorophore-labeled SFK peptide substrates via"click"reaction between propargylglycine-modified Src optimal peptide and a small library of azide-derivatized fluorophores
Phosphorylation of the tyrosine residue within the fluorophore-labeled peptides by the SFK family,Lyn resulted in enhanced fluorescence in five out of eight cases(peptides with fluorophores 1,2,3,4 and 5).The peptide constructed using the pyrene derivative(peptide-1)gave the greatest fluorescence enhancement.The fluorescence of the pyrene moiety is initially quenched by the phenol group of the tyrosine via a dynamic H abstraction mechanism(Scheme 2).Upon phosphorylation,this quenching ability is eliminated,leading to restoration of fluorescence.One of the major advantages of employing fluorescent peptides for assaying SFKs over other methods is the capability to monitor kinase activity in real-time.The author also investigated the utility of fl uorophore 1-labeled SOP for speci fically monitoring SFK activity in the crude lysate of cancer cells(K562 CML cell line).Incubation of the biosensor peptide with the crude lysate furnished a time-dependent increase in fluorescence intensity.This increase was abolished when 10 mM of a selective SFK inhibitor was introduced,suggesting that the chemosensor peptide selectively monitors SFK activity in the lysate.

Scheme 2 Mechanism of fluorescence increasing by phosphorylation of tyrosine residue
1.2 Labeling of proteins
Wolfbeis and co-workers have reported a dual labeling of bovine serum albumin BSA 1 involving copper-free and copper-mediated 1,3-dipolar cycloadditions[17].Firstly,among the azido labels considered,they chose azido coumarin 9 and 10(Fig.1),Indeed,9 had the salient feature that only its click products are fluorescent,thus eliminating background fluorescence of unreacted starting material,while compound 10 emits in the red region of the visible spectrum,and exhibits excellent photostability.
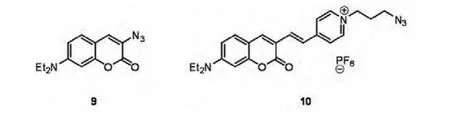
Fig.1 Fluorophore-containing azides for"click"chemistry
BSA 1 was treated first with cyclooctyne-modified maleimide linker compound 11 to create a copper-free anchor through the free sulfhydryl group of BSA 2.This step was then followed by treatment with pentynoic succinimide ester to provide numerous sites on the protein for the copper-mediated"click"reactions.The modified BSA 3 was then separated from excess reagents by gel filtration and then compound 10 was introduced.After removal of the unreacted dyes,the mono-labeled BSA 4 was reacted with compound 10 in the presence of CuBr(Scheme 3)and TEA.The intensity of both emission bands of BSA 4 at 460 nm and 620 nm increased as the second"click"reaction proceeded.Changes of the second band at 620 nm also indicated the presence of Förster resonance energy transfer(FRET).
2 Labeling of DNA and RNA
The labeling of both DNA and RNA using"click"chemistry has been successfully performed and exploited for various studies[18].Ju and co-workers[19]introduced an azide tag to the 5′-end of single-stranded DNA,which was then labeled with a fluorescent tag.As shown in Scheme 4,the oligonucleotide 5′-amino-GTT TTC CCA GTC ACG ACG-3′DNA 1(M13-40 universal forward sequencing primer)was reacted with succinimidyl 5-azidovalerate 12 to produce the azido-labeled DNA 2.Copper free 1,3-dipolar cycloaddition between the alkyne 13 and the azidolabeled DNA 2 was carried out at 80°C in aqueous conditions to produce a mixture of FAM-labeled DNA 3 and DNA 4.After the reaction,excess alkyne 13 was removed by size-exclusion chromatography and the resulting labeled DNA was desalted with an oligonucleotide purification cartridge and used for DNA sequencing.

Scheme 3 Schematic representation of dual labeling of alkyne modified BSA
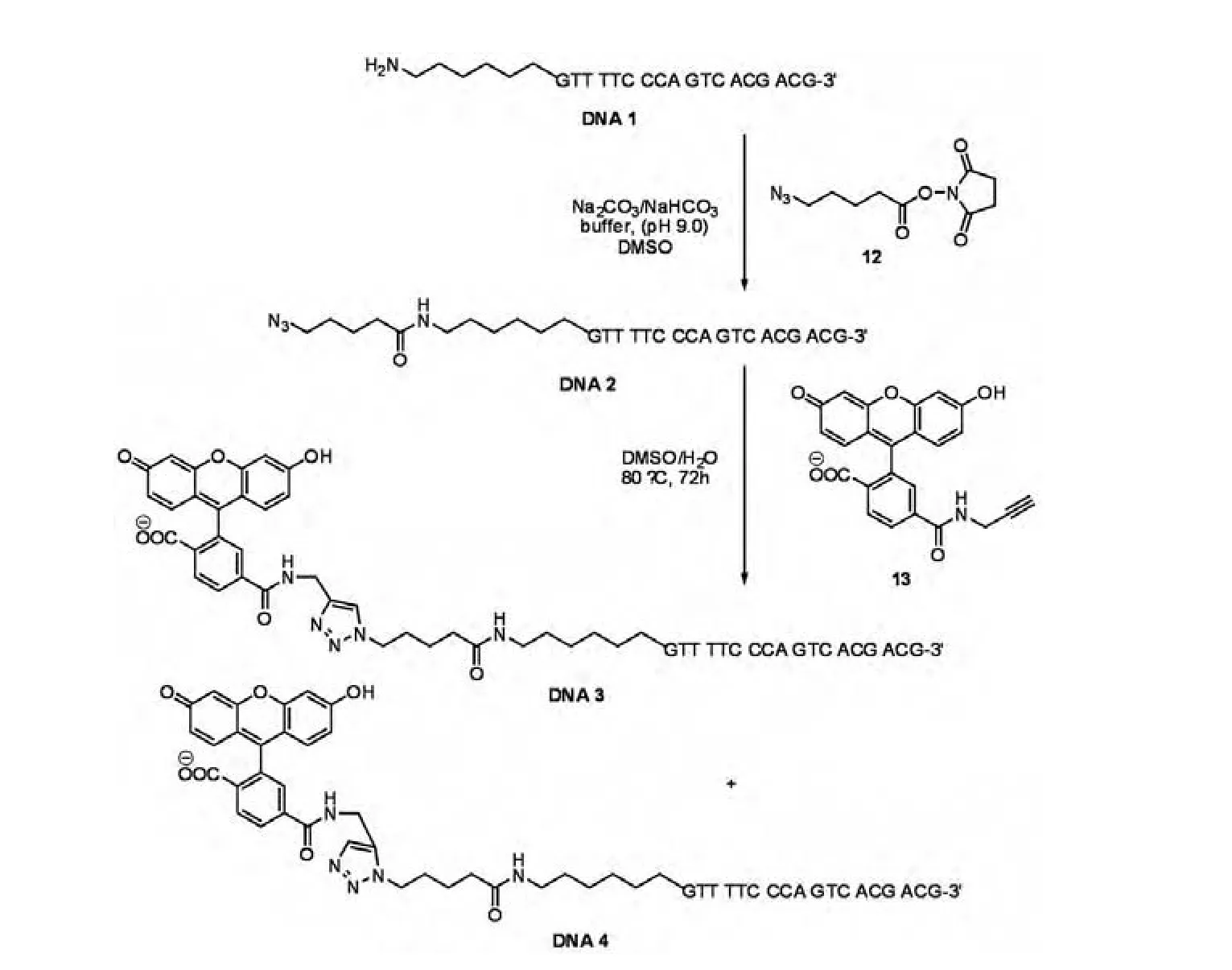
Scheme 4 Synthesis of fluorescent DNA
Salic and Jao recently reported the in vivo labeling of both DNA and RNA.In these studies,both deoxyuridine 14[20]and uridine 15[21]containing a propargyl group onto the uracil moiety were developed(Fig.2(A)).These nucleosides were effectively biosynthetically incorporated into DNA and RNA,respectively,and subsequently labeled with fluorescent tags via"click"chemistry to achieve optical detection of DNA and RNA(Fig.2(B)).Noteworthy is the fact that labeling was not only efficient in cultured cells but also in living mice.

Fig.2 In vivo labeling of DNA and RNA.(A)Alkyne-tagged deoxynucleoside(14)and nucleoside(15);(B)Selective"click"chemistry-based postlabeling of DNA containing alkynyl-nucleotide analogues
3 Labeling of Lipid in Living Cells
From membrane fusion to signal transduction lipids play important roles in a wide variety of cellular processes[22].Schultz and co-workers reported the selective labeling and optical detection of phosphatidic acid(PA)analogues bearing reactive tags(Fig.3)[23].Despite its relatively simple structure,PA is a key intermediate of phospholipid metabolism and an important signaling molecule that regulates the function of many proteins,such as Raf-1 kinase[24].In their study,PA 1,containing a cyclooctyne moiety embedded within a lipid acyl chain for reaction via copper-free"click"chemistry,was designed and synthesized.This compound also included S-acetyl-2-thioethyl(SATE)protecting groups on the headgroup that enhance membrane permeability and are enzymatically cleaved to reveal the phosphate moiety of PA 2 following cell entry.Compound PA 1 was incubated with RAW macrophages and treated with a fluorogenic coumarin-azide probe for optical visualization of the membrane in living cells.
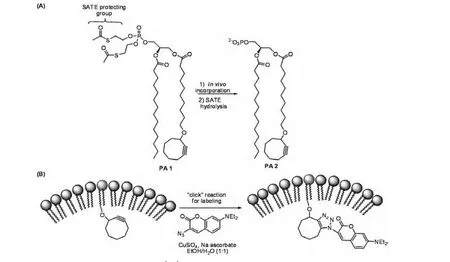
Fig.3 Strategies for in vivo imaging of lipid probes.(A)Tagged PA analogue PA 1 contains a hydrolyzable head group that assists in membrane penetration and a cyclooctyne tag for subsequent labeling;(B)Selective in vivo labeling of lipid probe PA 2 through"click"chemistry
4 Labeling of Viral Surfaces
Finn and co-workers have reported the labeling with fluorescein derivatives of the cowpea mosaic virus(CPMV),a structurally rigid assembly of 60 identical copies of a two-protein unsymmetric unit around a singlestranded RNA genome,conveniently available in gram quantities[25].In this study,60 azide or alkyne groups were introduced onto lysine residues by reaction with the sulfo-N-hydroxysuccinimide esters 16 and 17 in buffered aqueous solution(pH 7.0)(Scheme 5),followed by selective functionalization with the fluorescein derivatives 18 and 19 by"click"chemistry(Scheme 6).
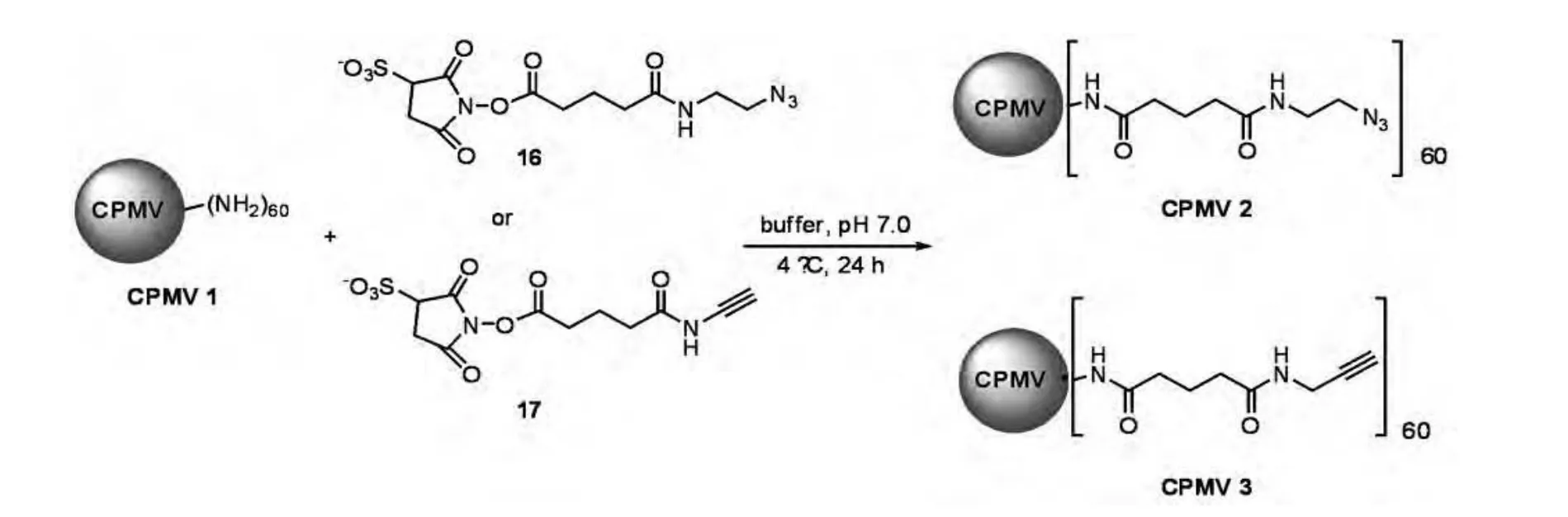
Scheme 5 Labeling virus CPMV with azide or alkyne

Scheme 6 Labeling virus CPMV with fluorescein derivatives
Since then,the derivatization of CPMV via"click"chemistry has been applied to the development of multivalent conjugates by introducing sugars,peptides,PEG,proteins[26-27],and a gadolinium-cyclen contrast agent for MRI[28].More recently,Wang and co-workers have also extended viral"click"chemistry to the tobacco mosaic virus.
5 Conclusions and Future Perspectives
As shown by this short literature overview,"click"chemistry has powerful applications in biology,especially in labeling of biomolecules,such as peptides,proteins,DNA,RNA,lipid in living cells and viral surfaces.A sufficient number and variety of examples are now in hand to inspire confidence in the ability of this reaction.Its key advantages are the synthetic accessibility,stability,and stealth of azides and alkynes as functional groups in candidate building blocks,their sluggish rates of uncatalyzed ligation,reducing the confusion and wasted effort inherent in chasing down false leads,and their potential for"locking in"useful information by an irreversible bondforming process that is easy to detect and deconvolute.With the growing popularity of azides,alkynes,and triazoles as components of functional molecules,driven by the use of the copper-catalyzed azide-alkyne ligation in many fields,we look forward to additional applications of the uncatalyzed"click"reaction for the discovery of binding agents to many types of surfaces.
[1]KOLB H C,FINN M G,SHARPLESS K B.Click chemistry:diverse chemical function from a few good reactions[J].Angew Chem Int Ed,2001,4(11):2004-2021.
[2]HOYLE C E,LOWE A B,BOWMAN C N.Thiol-click chemistry:a multifaceted toolbox for small molecule and polymer synthe⁃sis[J].Chem Soc Rev,2010,39,1355-1387.
[3]HUISGEN R.1,3-dipolar cycloadditions.past and future[J].Angew Chem Int Ed,1963,2(10):565-598.
[4]ROSTOVTSEV V V,GREEN L G,FOKIN V V,et al.A stepwise huisgen cycloaddition process:copper(I)-catalyzed regioselec⁃tive"ligation"of azides and terminal alkynes[J].Angew Chem Int Ed,2002,41(14):2596-2599.
[5]TORNOE C W,CHRISTENSEN C,MELDAL M.Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides[J].J Org Chem,2002,67(9):3057-3064.
[6]GOLAS P L,MATYJASZEWSKI K.Marrying click chemistry with polymerization:expanding the scope of polymeric materials[J].Chem Soc Rev,2010,39:1338-1354.
[7]AMBLARD F,CHO J H,SCHINAZI R F.Cu(I)-catalyzed huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleo⁃side,nucleotide,and oligonucleotide chemistry[J].Chem Rev,2009,109(9):4207-4220.
[8]MAMIDYALA S K,FINN M G.In situ click chemistry:probing the binding landscapes of biological molecules[J].Chem Soc Rev,2010,39,1252-1261.
[9]GIL M V,ARÉVALO M J,LÓPEZ Ó.Click chemistry-what's in a name?Triazole synthesis and beyond[J].Synthesis,2007,11,1589-1620.
[10]HEIN C D,LIU X M,WANG D.Click chemistry,a powerful tool for pharmaceutical sciences[J].Pharm Res,2008,25(10):2216-2230.
[11]CHANGA P V,BERTOZZI C R.Imaging beyond the proteome[J].Chem Commun,2012,48(71),8864-8879.
[12]PRESCHER J A,BERTOZZI C R.Chemistry in living systems[J].Nat Chem Biol,2005,1(1),13-21.
[13]WOLFBEIS O S.The click reaction in the luminescent probing of metal ions,and its implications on biolabeling techniques[J].Angew Chem Int Ed,2007,46,2980-2982.
[14]DONDONI A.Triazole:the keystone in glycosylated molecular architectures constructed by a click reaction[J].Chem Asian J,2007,2(6):700-708.
[15]VAN SWIETEN P F,LEEUWENBURGH M A,KESSLER B M,et al.Bioorthogonal organic chemistry in living cells:novel strategies for labeling biomolecules[J].Org Biomol Chem,2005,3(1):20-27.
[16]KAMARUDDIN M Z,UNG P,HOSSAIN M I,et al.A facile,click chemistry-based approach to assembling fluorescent chemo⁃sensors for protein tyrosine kinases[J].Bioorg Med Chem Lett,2011,21(1):329-331.
[17]KELE P,MEZÖ G,ACHATZ D,et al.Dual labeling of biomolecules by using click chemistry:a sequential approach[J].Angew Chem Int Ed,2009,48(2):344-347.
[18]WEISBROD S H,MARX A.Novel strategies for the site-specific covalent labelling of nucleic acids[J].Chem Commun,2008(44):5675-5685.
[19]SEO T S,LI Z M,RUPAREL H,et al.Click chemistry to construct fluorescent oligonucleotides for DNA sequencing[J].J Org Chem,2003,68(2):609-612.
[20]JAO C Y,SALIC A.Exploring RNA transcription and turnover in vivo by using click chemistry[J].Proc Natl Acad Sci U S A,2008,105(41):15779-15784.
[21]WOSCHOLSKI R.Phospholipid signalling:mediators in need of interdisciplinary techniques[J].Signal Transduction,2006,6(2):77-79.
[22]NEEF A B,SCHULTZ C.Selective fluorescence labeling of lipids in living cells[J].Angew Chem Int Ed,2009,48(8):1498-1500.
[23]ANDRESEN B T,RIZZO M A,SHOME K,et al.The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade[J].FEBS Lett,2002,531(1):65-68.
[24]WANG Q,CHAN T R,HILGRAF R,et al.Bioconjugation by copper(I)-catalyzed azide-alkyne[3+2]cycloaddition[J].J Am Chem Soc,2003,125(11):3192-3193.
[25]SEN GUPTA S,KUZELKA J,SINGH P,et al.Accelerated bioorthogonal conjugation:a practical method for the ligation of di⁃verse functional molecules to a polyvalent virus scaffold[J].Bioconjugate Chem,2005,16(6):1572-1579.
[26]KALTGRAD E,O'REILLY M K,LIAO L A,et al.On-virus construction of polyvalent glycan ligands for cell-surface receptors[J].J Am Chem Soc,2008,130(14):4578-4579.
[27]PRASUHN D E,YEH R M,OBENAUS A,et al.Viral MRI contrast agents:coordination of Gd by native virions and attachment of Gd complexes by azide–alkyne cycloaddition[J].Chem Commun,2007,12(1):1269-1271.
[28]BRUCKMAN M A,KAUR G,LEE L A,et al.Surface modification of tobacco mosaic virus with"click"chemistry[J].Chem Bio Chem,2008,9(4):519-523.
