Effect of Shrimp (Litopenaeus vannamei) Farming Waste on the Growth, Digestion, Ammonium-Nitrogen Excretion of Sea Cucumber (Stichopus monotuberculatus)
2015-10-13CHENYanfengLUOPengHUChaoqunandRENChunhua
CHEN Yanfeng, LUO Peng, HU Chaoqun, *, and RENChunhua
Effect of Shrimp () Farming Waste on the Growth, Digestion, Ammonium-Nitrogen Excretion of Sea Cucumber ()
CHEN Yanfeng1), 2), LUO Peng1), HU Chaoqun1), *, and RENChunhua1)
1);,,,510301,2),,528231,.
In this study, specific growth rate (SGR), ingestion rate (IR), food conversion ratio (FCR), apparent digestion ratio (ADR) and ammonium-nitrogen excretion were determined for sea cucumber () reared in plastic containers (70L; 4 containers each diet treatment)Sea cucumbers were fed with five diets containing different amounts of farming waste from shrimp () (100%, 75%, 50%, 25% and 0) and a formulated compound (20% sea mud and 80% powdered algae). Sea cucumbers grew faster when they were fed with diet D (25% shrimp waste and 75% formulated compound) than those fed with other diets. Although IR value of sea cucumber fed with diet A (shrimp waste) was higher than those fed with other diets, both the lowest SGR and the highest FCR occurred in this diet group. The highest and the lowest ADR occurred in diet E (formulated compound) and diet A group, respectively, and the same to ammonium-nitrogen excretion. The contents of crude protein, crude lipid and total organic matter (TOM) in feces decreased in comparison with corresponding diets. In the feces from different diet treatments, the contents of crude protein and TOM increased gradually as the contents of crude protein and TOM in diets increased, while crude lipid content decreased gradually as the crude lipid content in diets increased.
sea cucumber;; shrimp;; growth; digestion; ammonium-nitrogen excretion
1 Introduction
The white shrimp,, is a predominant penaeid species, which is currently cultured worldwide (Frias-Espericueta, 2001; McGraw, 2002; Saoud, 2003; Cheng, 2006). The culture ofhas been widespread in Asia (Andriantahina, 2012). Shrimp aquaculture industry has produced a large amount of farming waste. Generally speaking, farming waste from shrimp is a mixture mainly composed of effluent containing high concentrations of nitrogen and phosphorus, and sediment containing uneaten feeds, shrimp excrement and biological remains. Additionally, it was reported that the shrimp waste was helpful for the rapid growth of phytoplankton (Tookwina and Songsangjinda, 1999). Moreover, phytoplankton in shrimp ponds mostly consisted of a variety of algae and some zooplankton existing universally in shrimp aquaculture water body (Tookwina and Songsangjinda, 1999; Maristela, 2008). Thus, it seemed that shrimp waste was mainly composed of uneaten feeds, feces, various algae debris and zooplankton remains after it was dried. In recent years, farming waste from shrimp aquaculture industry has led to serious coastal ecosystem pollution (Sansanayuth, 1996; Páez-Osuna, 1998;Páez- Osuna, 2001; Burford and Williams, 2001; Anh, 2010). Therefore, dealing with shrimp farming waste is a key measure to alleviate the pollution (Chin and Ong, 1994).
As a commercially valuablespecies, sea cucumber () is distributed widely (Fan, 2012; Massin, 2002; Rowe and Gates, 2004; Byrne, 2010). At present, the research on this species is still at the starting stage. Hu(2010) studied the larval development and juvenile growth ofsp. (curry fish), and it was identified aslater (Fan, 2012). The development of sea cucumber aquaculture industry needs knowledge of dietary requirements. However, up to now, less data regarding the feeding behavior, nutrition requirements and artificial feeds of this species are available. This has hindered the development ofaquaculture industry.
It has been demonstrated that sea cucumber can ingest uneaten feeds and feces of marine animals, even their own feces (Hauksson, 1979; Tiensongrusmee and Pontjoprawiro, 1988; Goshima, 1994; Ramofafia, 1997). In previous studies, as a feasible and cost-effective method, polyculture was often used to rear both species to larger sizes and reduce the pollution from aquaculture industry due to above feeding habit of sea cucumber (Ahlgren, 1998; Kang, 2003; Zhou, 2006; Slater and Carton, 2007; Paltzat, 2008). Although the polyculture of sea cucumber with shrimp set a precedent for the reuse of shrimp waste as a food source for sea cucumber, both species in polyculture grew more slowly than those in monoculture, and the ammonium-nitrogen concentration in polyculture system was higher than that in monoculture system, which indicated that polyculture did not improve water quality (Purcell, 2006). Moreover, Bell(2007) observed that limited growth and mass mortality of sea cucumber occurred when it was reared with shrimp, exhibiting that polyculture was not practicable. In other words, the present polyculture mode makes it difficult to remove shrimp waste by sea cucumber. Thus a new method needs to be found to treat the farming waste from shrimp aquaculture and make it the food source of sea cucumber. In fact, some studies have aimed at the reuse of farming waste from marine animals in sea cucumber aquaculture (Yuan, 2006; Slater, 2009; Maxwell, 2009; Zamora and Jeffs, 2011). However, these studies basically focused on the reuse of farming waste from shellfish and fish such as abalone, scallop, mussel, oyster and salmon. So far there has been no information concerning the use of dried farming waste from shrimp as part of food of sea cucumber.
is a blue-green filamentous microalga which is regarded as human health food and animal feed due to its high contents of proteins, vitamins and unsaturated fatty acids. Previous studies indicated that dietary administration ofcould increase the phagocytic activity of fish and enhance the resistance of aquatic animals against pathogens (Duncan and Klesius, 1996; Watanuki, 2006; Lee, 2003; Rahman, 2006; Abdel-Tawwab and Ahmad, 2009). However, to our knowledge, there is no published study regarding the use offor the culture of sea cucumber. In contrast, the macroalgaand sea mud have been widely used in sea cucumber aquaculture industry (Liu, 2009; Slater, 2009; Liu, 2010; Zhang, 2011; Xia, 2012).
The goal of this study is to determine whether farming waste from shrimp aquaculture industry can be used for culture of sea cucumber so as to seek a new approach to the reuse of shrimp farming waste.
2 Materials and Methods
2.1 Experimental Diets
Shrimp () waste was collected from eighty shrimp ponds of different shrimp farms in Guangdong Province, China. No fishery drugs were used in the process of shrimp culture. Before shrimp waste was collected, waterwheel aerators were used to aerate shrimp ponds all night to concentrate waste at the outfalls of shrimp ponds spontaneously. On next day, the outfall of the shallow well beside the shrimp ponds was blocked up, and then a part of effluent containing sedimentary waste in the shrimp ponds was discharged into the shallow well. Waste slurry was collected by 0.074mm spoon net. After the seawater in waste slurry was squeezed out, waste slurry was transferred from the spoon net to a container. These shrimp wastes from different sources were dried by baking at 55℃ and pulverized into ultra-fine powder respectively, and they were mixed well to avoid the possible difference in their nutrition contents.
The initial formulated compound contained 80% powdered algae (53.33% ofand 26.67% of) and 20% sea mud (called formulated compound for short). The nutrient in sea mud (% dry weight) included 0.87%±0.01% crude protein, 0.32%±0.00% crude lipid, 92.62%±0.05% ash.(% dry weight) included 9.19%±0.04% crude protein, 0.9%±0.01% crude lipid, 39.84%±0.03% ash;(% dry weight) included 61.4%±0.03% crude protein, 2.01%±0.02% crude lipid, 5.3%±0.06% ash.
The final diets used in this experiment were diet A, shrimp waste; diet B, 75% shrimp waste and 25% formulated compound; diet C, 50% shrimp waste and 50% formulated compound; diet D, 25% shrimp waste and 75% formulated compound; and diet E, formulated compound only.
2.2 Experiment Design and Rearing Conditions
Healthy juvenilewere obtained from Zhanjiang City (Guangdong Province, China). Prior to this experiment, sea cucumber were fed with powdered algae and acclimated for 15 days.After 48h of starvation, the initial wet body weight of the sea cucumber was measured individually. After that, 120 acclimatized animals with initial wet body weight of (3.97±0.07)g were allocated in equal numbers (=6) into 20 plastic containers (70L) to form five groups and four replicates. The five groups were fed with five kinds of experimental diets once a day to excess at about 17:00. Uneaten feeds were siphoned out 24h later from containers and dried at 65℃ to constant weight in order to determine the ingestion rate. Feces were siphoned out twice a day (at about 9:00 and 16:00) and stored at −20℃ until analysis. Seawater temperature was controlled at (29±2)℃, dissolved oxygen was maintained above 5.0mgL−1and salinity ranged from 28 to 32. During the experiment, aeration was supplied continuously, and the seawater was exchanged twice a day to ensure the water quality and about one-half volume of the seawater in each container was exchanged each time. The experiment lasted for 70d. Sea cucumbers were starved for 48h at the end of the experiment, and then weighed to calculate total specific growth rate.
2.3 Chemical Analysis of Diets and Feces
Diets and feces were analyzed according to AOAC (1990). The acid-insoluble ash (AIA) content of diets and feces was determined with the method of Atkinson(1984). The total organic matter (TOM) contents of diets and feces were determined with combustion method recommended by Byers(1978) with modifications. The samples were oven dried at 60℃ for 48h, weighed and then placed in a furnace set at 500℃ for 6h to ensure complete combustion of organic matter, and then re- weighed. Percentage of TOM was calculated by sample weight lost after combustion.
2.4 Data Processing
The mortality of sea cucumber was recorded during this experiment. Specific growth rate (SGR) and ingestion rate (IR) were calculated according to the equations of Zamora and Jeffs (2012). Food conversion ratio (FCR) was calculated according to a study of Ebrahimi(2012). Apparent digestion ratio (ADR) was calculated using the following equations (Deng, 2010):
where,1and2are initial and final total wet body weight of sea cucumber in each container;is the duration of the experiment (70d);is the number of sea cucumbers in each container; andis the dry weight of food consumed.
2.5 Ammonium-Nitrogen Excretion
Ammonium-nitrogen excretion was determined for one whole day in the middle of this experiment. Different diets supplied for the sea cucumber were put into containers after exchanging the water at 16:30. The concentration of ammonium-nitrogen each container was determined at that time and 24h later. Ammonium-nitrogen was determined by using the salicylate-hypochlorite method (Bower and Holm-Hansen, 1980). One container without sea cucumber was used as control. Ammonium-nitrogen excretion was calculated using the following equations (Xia, 2012, 2013):
2.6 Variation of Nutrition Contents in Feces
According to the research of Zamora and Jeffs (2011), the variation of nutrition contents (crude protein, crude lipid and TOM) in the feces of sea cucumbers treated with different diets was compared with that in corresponding diets, and the variation regularity of nutrition content in feces among different diet treatment groups was also analyzed.
2.7 Statistical Analysis
Statistics was performed using software SPSS 11.0 for Windows with possible difference between diets and feces in the same diet treatment group for each nutrient component (crude protein, crude lipid, ash, TOM) being tested by Independent-Samples T Test. Before ANOVA comparisons, data were tested for homogeneity of variance using the Levene’s test. Inter-treatment differences of,,,, ammonium-nitrogen excretion and nutrition contents (crude protein, crude lipid, ash, TOM) in feces were tested by one-way ANOVA. Duncan’s multiple range test was used to test the difference among different diet treatment groups. Difference was considered significant when<0.05.
3 Results
3.1 Growth
There was significant difference inamong differ- ent diet treatment groups (<0.05) except for diet C and diet E treatment group (>0.05). The highest and lowestoccurred in diet D and diet A treatment group, respectively (Fig.1). During this experiment, no mortality occurred in any groups.

Fig.1 Specific growth rate (SGR) of the sea cucumber in this experiment. Different letters indicate significant difference (P<0.05), and error bars indicate the standard deviation from mean.

Fig.2Ingestion rate () of sea cucumber in this experiment. Different letters indicate significant difference (< 0.05), and error bars indicate the standard deviation from mean.
3.2 Ingestion Rate
There was significant difference inamong different diet treatment groups (<0.05) except for diet D and diet E treatment group (>0.05). The highest and lowestoccurred in diet A and diet E treatment group, respectively. The averagevalue decreased gradually as the nutrition content of diets increased (Fig.2).
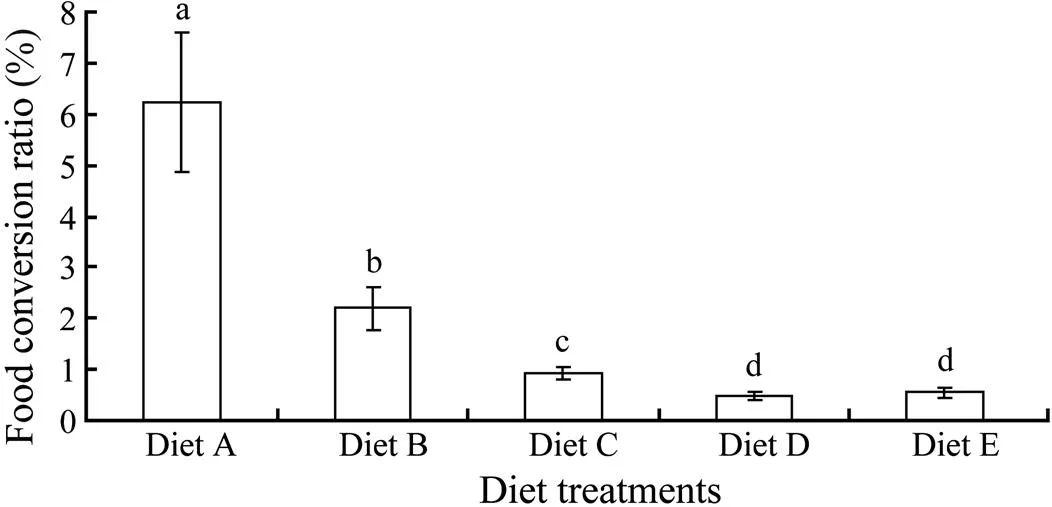
Fig.3 Food conversion ratio (FCR) of sea cucumber in this experiment. Different letters indicate significant difference (P<0.05), and error bars indicate the standard deviation from mean.
3.3 Food Conversion Ratio
The highest and lowestoccurred in diet A and diet D treatment group, respectively, and there was significant difference inamong different diet treatment groups (<0.05) except those between diet D and diet E treatment group (>0.05) (Fig.3).
3.4 Apparent Digestion Ratio
There was significant difference inamong different diet treatment groups (<0.05) except for diet C and diet D treatment group (>0.05). The highest and lowestoccurred in diet E and diet A treatment group, respectively. The averagevalue increased gradually as the content of shrimp waste in different diets decreased (Fig.4).
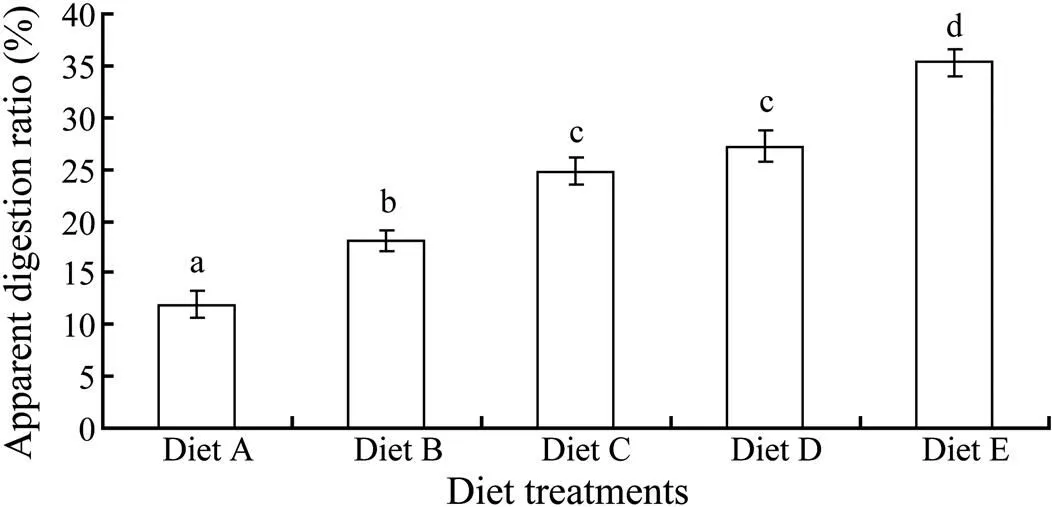
Fig.4 Apparent digestion ratio (ADR) of sea cucumber in this experiment. Different letters indicate significant difference (P<0.05), and error bars indicate the standard deviation from mean.
3.5 Ammonium-Nitrogen Excretion
There was no significant difference in ammonium-ni- trogen excretion between diet B and diet C treatment group, diet C and diet D treatment group, and diet D and diet E treatment group, respectively (>0.05). The highest and lowest ammonia-nitrogen excretion occurred in diet E and diet A treatment group, respectively. The average ammonium-nitrogen excretion value increased gradually as the crude protein content in different diets increased (Fig.5).

Fig.5Ammonium-nitrogen excretion of sea cucumber in this experiment. Different letters indicate significant difference (<0.05), and error bars indicate the standard deviation from mean.
3.6 Variation of Nutrition Contents in Feces
The average crude protein content in feces with different diet treatments increased gradually as the average crude protein content in diets increased. There was significant difference in crude protein contentamong feces with different diet treatments (<0.05). The average crude protein content in feces from different diets decreased to different extents in comparison with those in corresponding diets, and the difference of average crude protein content between feces and corresponding diets with increased gradually when the average crude protein content in diets increased.
The average crude lipid content in feces from different diets decreased gradually when the average crude lipid content in diets increased. There was significant difference in crude lipid contentamong feces from different diets (<0.05) except for those between diet A and B, diet B and C, and diet D and E. The average crude lipid content in feces from different diets decreased to different extents in comparison with those in corresponding diets. The difference of average crude lipid content between feces and corresponding diets increased gradually when the average crude lipid content in diets increased.
The average TOM content in feces from different diets increased gradually when the average TOM content in diets increased. There was significant difference in TOM contentamong feces from different diets (<0.05). The average TOM content in feces from different diets decreased in comparison with those in corresponding diets. Among diets tested, the difference of average TOM content between feces and corresponding diets was similar to each other (Table 1).

Table 1 Nutrient content in diets and feces
Notes: Data with different capital letters in the same column are significantly different between diets and feces (<0.05); Data with different small letters in the same line are significant different among the feces from different diets (<0.05).
4 Discussion
4.1 Growth Performance and Apparent Digestion Ratio
In this study, after sea cucumbers ingested pure dried shrimp waste, their total body weight increased, which was not in agreement with the result of Yuan(2006) who found that a diet of pure dried bivalve feces led to negative growth of sea cucumber ()Zhu(2005) reported that the optimum dietary protein and lipid requirements of juvenile sea cucumber were 18%–24% and 5%, respectively. Although the contents of protein and lipid in dried shrimp waste (crude protein 13.98 %, crude lipid 0.65%) used in this experiment were lower than the reported, sea cucumber could compensate for lower nutritional value by increasing the amount of food intake, resulting in weight gain (Xia, 2012).
Generally speaking, the growth of animal was affected by different diets through the interaction of,and(Yuan, 2006). In this study, thevalue of diet D was higher than that of diet E while thevalue of the former was lower than that of the latter, and theirvalues were similar to each other. Additionally, farming waste of marine animals might provide certain nutrients or digestion regulators to other animals, such as some mineral components which might be vital to the metabolism of sea cucumber (Xu, 1999).Maybe the combination of these factors induced the faster growth of sea cucumber fed diet D compared with those fed with diet E.
Different nutrition contents in diets might result in differentvalues of animals, and a negative relationship was revealed between thevalue of sea cucumber and the nutrition contents of different diets (Liu, 2009; Yuan, 2006). Thus, in this study, it was reasonable that the averagevalue with different diet treatments decreased gradually as the nutrition contents of differentdiets increased, which was in accordance with the results in previous literatures (Liu, 2010; Xia, 2012).
High growth rate was normally accompanied by low(Mercer, 1993).andin this experiment mainly reflected this negative relationship except for similarvalues in diet D and diet E treatment group.
In the present study, the average ADR value of sea cucumberdecreased gradually as the content of dried shrimp waste in differentdiets increased, indicating that dried shrimp waste was absorbed hard by sea cucumber. A similar result was also discovered when dried bivalve feces were used in culture of(Yuan,2006).
4.2 Ammonium-Nitrogen Excretion
In this study, the average ammonium-nitrogen excretion value of sea cucumber with different diet treatments increased gradually as the crude protein content in differentdiets increased. Sea cucumber could consume more protein in high-protein diets and less protein in low-protein diets (Table 1), and ammonium-nitrogen excretion could reflect the amount of ingested protein metabolism (Xia, 2012), which was a probable explanation for this result.
Maintaining an acceptable water quality is extremely important for sea cucumber. Excessive ammonium in aquaculture water body couldbe harmful to aquatic animals. In the present study, it seemed that ammonium excretion was not harmful to sea cucumber because of their high survival rate, which suggested that ammonium-ni- trogen excretion in this experiment was within a safe range.
4.3 The Variation of Nutrition Content in Feces
In this study, the differences of the nutrition contents (crude protein, crude lipid) between feces and corresponding diets with different diet treatments increased gradually as the nutrition contents (crude protein, crude lipid) in differentdiets increased, indicating that when the nutrition contents in diets fluctuated within a certain range, the nutrition contents in diets were higher, more nutrients in ingested diets were consumed by sea cucumber. However, the differences of the TOM content between feces and corresponding diets among different diet treatment groups were close to each other, indicating that sea cucumber with different diet treatments had identical ability of consuming organic matter in ingested diets.
It is well known that sea cucumber can consume organic matter from food and convert it into body tissues. During this experiment, no mortality occurred in any groups, meaning that a part of nutrient in ingested diets was used for sustaining their lives. Therefore, the nutrition contents in the excrement might be lower than those in diets, which was a probable explanation for the decrease of the contents of protein, lipid and TOM in feces in comparison with those in corresponding diets. Similar result was reported by Zhou(2006) that a small reduction of organic content in scallop lantern net waste was found when they were reared with. In other words, sea cucumber had the capability of reducing organic pollution of shrimp farming waste.
5 Conclusions
In this study, after sea cucumber ingested diets containing different quantity of dried shrimp waste, their total body weight increased without mortality. Hence, it was feasible that dried shrimp waste could be applied to sea cucumber aquaculture industry. This study showed that sea cucumber exhibited the fastest growth when they were fed with the diet containing 25% dried shrimp waste and 75% formulated compound (20% sea mud and 80% powdered algae), indicating that dried shrimp waste could become a component in the formulation of sea cucumber feeds. Moreover, this work may provide another approach to reusing the waste from shrimp aquaculture industry.
Acknowledgements
This research was supported by the Key Project of National Science & Technology Pillar Program in 12th Five-year Plan (2011BAD13B02, 2012BAD18B03), the Science & Technology Promoting Project for Oceanic & Fishery in Guangdong Province (A201100D01, A201101 D02), the Knowledge Innovation Key Project of Chinese Academy of Sciences (KZCX2-EW-Q212) and the comprehensive strategic cooperation project of Guangdong Province and Chinese Academy of Sciences (2012B09 1100269), the Cooperation Program of Guangdong Province and Chinese Academy of Sciences (2012B0911 00272), and the Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (2014KQNCX183).
Abdel-Tawwab, M., and Ahmad, M. H., 2009. Live Spirulina (Arthrospira platensis) as a growth and immunity promoter for Nile tilapia,(L.), challenged with pathogenic, 40: 1037-1046.
Ahlgren, M. O., 1998. Consumption and assimilation of salmon net pen fouling debris by the red sea cucumber: Implications for polyculture.,29: 133-139.
Andriantahina, F., Liu, X. L., Huang, H., Xiang, J. H., and Yang, C. M., 2012. Comparison of reproductive performance and offspring quality of domesticated Pacific white shrimp,,324-325: 194-200.
Anh, P. T., Kroeze, C., Bush, S. R., and Mol, A. P. J., 2010. Water pollution by intensive brackish shrimp farming in south-east Vietnam: Causes and options for control., 97: 872-882.
Association of Official Analytical Chemists (AOAC), 1990. Official methods of analysis. In:. Helrich, K., ed., 15th edition, AOAC International Publishers, Arlington, VA, 1298pp.
Atkinson, J. L., Hilton, J. W., and Slinger, S. J., 1984. Evaluation of acid-insoluble ash as an indicator of feed digestibility in rainbow trout ().,41: 1384-1386.
Bell, J. D., Agudo, N. N., Purcell, S. W., Blazer, P., Simutoga, M., Pham, D., and Patrona, L. D.,2007. Grow-out of sandfishin ponds shows that co-culture with shrimpis not viable., 273: 509-519.
Bower, C. E., and Holm-Hansen, T., 1980. A salicylate-hy- pochlorite method for determining ammonia in seawater.,37: 794-798.
Burford, M. A., and Williams, K. C., 2001. The fate of nitrogenous waste from shrimp feeding., 198: 79-93.
Byers, S. C., Mills, E. L., and Stewart, P. L., 1978. A comparison of methods of determining carbon in marine sediments, with suggestions for a standard method., 58: 43-47.
Cheng, K. M., Hu, C. Q., Liu, Y. N., Zheng, S. X., and Qi, X. J., 2006. Effects of dietary calcium, phosphorus and calcium/ phosphorus ratio on the growth and tissue mineralization ofreared in low-salinity water., 251: 472-483.
Chin, K. K.,andOng, S. L., 1994. Treatment and reuse of water for prawn cultivation., 30: 255-258.
Deng, J. M., Mai, K. S., Ai, Q. H., Zhang, W. B., Tan, B. P., Xu, W., and Liufu, Z. G., 2010. Alternative protein sources in diets for Japanese flounder(Temminck and Schlegel): II. Effects on nutrient digestibility and digestive enzyme activity., 41: 861-870.
Duncan, P., and Klesius, P.,1996. Effects of feedingon specific and non-specific immune responses of channel catfish., 8: 308-313.
Ebrahimi, G., Ouraji, H., Firouzbakhsh, F., and Makhdomi, C., 2013. Effect of dietary lipid and protein levels with different protein to energy ratios on growth performance, feed utilization and body composition of(Kamenskii, 1901) fingerlings., 9: 1447-1458.
Fan, S. G., Hu, C. Q., Zhang, L. P., Sun, H. Y., Wen, J., and Luo, P., 2012. Complete mitochondrial genome of the sea cucumbersp. and its application in the identification of this species., 43: 1306-1316.
Frias-Espericueta, M. G., Voltolina, D., and Osuna-Lopez, J. I., 2001. Acute toxicity of cadmium, mercury, and lead to whiteleg shrimp () postlarvae.,67: 580-586.
Goshima, S., Fujiyoshi, Y., Ide, N., Gamboa, R. U., and Nakao, S., 1994. Distribution of Japanese common sea cucumber,in lagoon Saroma., 42: 261-266 (in Japanese with English abstract).
Hauksson, E., 1979. Feeding biology of, a deposit-feeding holothurian., 64: 155-160.
Hu, C. Q., Xu, Y. H., Wen, J., Zhang, L. P., Fan, S. G., and Su, T., 2010. Larval development and juvenile growth of the sea cucumbersp. (Curry fish).,300: 73-79.
Kang, K. H., Kwon, J. Y., and Kim, Y. M., 2003. A beneficial coculture: Charm abaloneand sea cucumber., 216: 87-93.
Lee, Y. K., Chew, P. F., Soh, B. S., and Tham, L. Y., 2003. Enhancing phagocytic activity of hemocytes and disease resistance in the prawnby feeding., 15: 279-287.
Liu, Y., Dong, S., Tian, X., Wang, F., and Gao, Q., 2009. Effects of dietary sea mud and yellow soil on growth and energy budget of the sea cucumber(Selenka)., 286: 266-270.
Liu, Y., Dong, S. L., Tian, X. L., Wang, F., and Gao, Q. F., 2010. The effect of different macroalgae on the growth of sea cucumbers (Selenka)., 41: e881-e885.
Maristela, C., Enide, E. L., Sigrid, N. L., Eneida, E. S. A., Ralf, S., and Antônio, T. M. J., 2008. Plankton community as an indicator of water quality in tropical shrimp culture ponds., 56: 1343-1352.
Massin, C., Zulfigar, Y., Hwai, T., and Boss, S., 2002. The genus(Echinodermata: Holothuroidea) from the Johore Marine Park (Malaysia) with the description of two new species., 72: 73-99.
Maxwell, K. H., Gardner, J. P. A., and Heath, P. L., 2009. The effect of diet on the energy budget of the brown sea cucumber,(Hutton)., 40: 157-170.
McGraw, W. J., Davis, D. A., Teichert-Coddington, D., and Rouse, D. B., 2002. Acclimation ofpostlarvae to low salinity: Influence of age, salinity, endpoint and rate of salinity reduction., 33: 78-84.
Mercer, J. P., Mai, K. S., and Donlon, J., 1993. Comparative studies on the nutrition of two species of abalone,Linnaeus andIno.I. Effects of algal diet on growth and biochemical composition., 23: 75-88.
Páez-Osuna, F., Guerrero-Galván, S. R., and Ruiz-Fernández, A. C., 1998.The environmental impact of shrimp aquaculture and the coastal pollution in Mexico.,36: 65-75.
Paltzat, D. L., Pearce, C. M., Barnes, P. A., and McKinley, R. S., 2008. Growth and production of California sea cucumbers (Stimpson) co-cultured with suspended Pacific oysters (Thunberg)., 275: 124-137.
Páez-Osuna, F., 2001. The environmental impact of shrimp aquaculture: A global perspective.,112: 229-231.
Purcell, S. W., Patrois, J., and Fraisse, N., 2006. Experimental evaluation of co-culture of juvenile sea cucumbers,(Jaeger), with juvenile blue shrimp,(Stimpson)., 37: 515-522.
Ramofafia, C., Foyle, T. P., and Bell, J. D., 1997. Growth of juvenile(Holothuroidea) in captivity., 152: 119-128.
Ramofafia, C., Gervis, M., and Bell, J., 1995. Spawning and early larval rearing of Holothuria atra., 7: 2-6.
Rowe, F. W. E., and Richmond, M. D., 2004. A preliminary account of the shallow-water echinoderms of Rodrigues, Mauritius, western Indian Ocean., 38: 3273-3314.
Saoud, I. P., Davis, D. A., and Rouse, D. B., 2003. Suitability studies of inland well waters forculture.,217: 373-383.
Sansanayuth, P., Phadungchep, A., Ngammontha, S., Ngdngam, S., Sukasem, P., Hoshino, H., and Ttabucanon, M. S., 1996. Shrimp pond effluent: Pollution problems and treatment by constructed wetlands., 34: 93-98.
Slater, M. J., and Carton, A. G., 2007. Survivorship and growth of the sea cucumber(Hutton 1872) in polyculture trials with green-lipped mussel farms., 272: 389-398.
Slater, M. J., Jeff, A. G., and Carton, A. G., 2009. The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber,, 292: 219-224.
Tiensongrusmee, B., and Pontjoprawiro, S., 1988. Sea cucumber culture: Potential and prospect.. FAO, 18pp.
Tookwinas, S., and Songsangjinda, P., 1999. Water quality and phytoplankton communities in intensive shrimp culture ponds in Kung Krabaen Bay, Eastern Thailand., 30: 36-45.
Watanuki, H., Ota, H., Tassakka, ACM. A. R., Kato, T., and Sakai, M., 2006. Immunostimulant effects of dietaryon carp,., 258: 157-163.
Xia, S. D., Yang, H. S., Li, Y., Liu, S. L., Zhou, Y., and Zhang, L. L., 2012. Effects of different seaweed diets on growth, digestibility, and ammonia-nitrogen production of the sea cucumber(Selenka)., 338- 341: 304-308.
Xia, S. D., Yang, H. S., Li, Y., Liu, S. L., Zhang, L. B., Chen, K., Li, J. H., and Zou, A. G., 2013. Effects of differently processed diets on growth, immunity and water quality of the sea cucumber,(Selenka, 1867)., 19: 382-389.
Xu, Z., Bi, S., Wang, J., Wang, Z., and Wu, F., 1999. Effect of different feeds on growth and color-change of juvenile sea cucumbers., 16: 30-33 (in Chinese with English abstract).
Yuan, X. T., Yang, H. S., Zhou, Y., Mao, Y. Z., Zhang, T., and Liu, Y., 2006. The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber(Selenka) (Echinodermata: Holothuroidea)., 256: 457-467.
Zamora, L. N., and Jeffs, A. G., 2011. Feeding, selection, digestion and absorption of the organic matter from mussel waste by juveniles of the deposit-feeding sea cucumber,, 317: 223-228.
Zamora, L. N., and Jeffs, A. G., 2012. The ability of the deposit-feeding sea cucumberto use natural variation in the biodeposits beneath mussel farms, 326-329: 116-122.
Zhang, P., Dong, S. L., Wang, F., Wang, H., Gao, W., and Yan, Y., 2012. Effect of salinity on growth and energy budget of red and green colour variant sea cucumber(Selenca)., 11: 1611-1619.
Zhou, Y., Yang, H. S., Liu, S. L., Yuan, X. T., Mao, Y. Z., Liu, Y., Xu, X. L., and Zhang, F. S., 2006. Feeding and growth on bivalve biodeposits by the deposit feederSelenka (Echinodermata: Holothuroidea) co-cultured in lantern nets., 256: 510-520.
Zhu, W., Mai, K. S., Zhang, B. G., Wang, F. Z., and Xu, G. Y., 2005. Study on dietary protein and lipid requirement for sea cucumber,., 29: 54-58 (in Chinese, with English abstract).
(Edited by Qiu Yantao)
10.1007/s11802-015-2364-z
(April 8, 2013; revised May 15, 2013; accepted April 20, 2015)
. E-mail: hucq@scsio.ac.cn
ISSN 1672-5182, 2015 14 (3): 484-490
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
杂志排行
Journal of Ocean University of China的其它文章
- Research on China’s Aquaculture Efficiency Evaluation and Influencing Factors with Undesirable Outputs
- Tolerance, Oxygen Consumption and Ammonia Excretion of Ophiopholis sarsii vadicola in Different Temperatures and Salinities
- Sustainability Evaluation of Different Systems for Sea Cucumber (Apostichopus japonicus) Farming Based on Emergy Theory
- Larval and Juvenile Growth Performance of Manila Clam Hybrids of Two Full-Sib Families
- Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from Arenicola cristata
- Proline with or without Hydroxyproline Influences Collagen Concentration and Regulates Prolyl 4-Hydroxylase α (I) Gene Expression in Juvenile Turbot (Scophthalmus maximus L.)
