管状光催化反应器降解甲醛效果及其降解模型
2015-07-20刘鹏等
刘鹏等
摘要:针对建筑环境中的挥发性有机化合物甲醛,在原有管状反应器内增设带有工艺缺口的直肋片,并在密闭循环系统中对其净化效果进行分析,又利用计算流体力学(CFD)的方法得到了反应器内部的流速和光强分布.同时,基于模型计算的方法,建立了污染物循环降解模型.结果表明: 改进后的管状反应器,反应面积增加,气体停留时间延长,平衡了传质-反应能力,反应速率提高了约1倍;增设肋片后,内壁面光强有所减弱,反应器中间段光强与流速耦合较好,而两端由于气流扰动大且光强较弱,反应速率会受影响;另外,降解模型的预测值稍高于实测值,但两者变化趋势相同,该模型能较准确的预测甲醛的反应速率.
关键词:光催化氧化; 降解模型; 管状反应器; CFD模拟; 甲醛
中图分类号:O643 文献标识码:A
Abstract: A new annular photocatalytic reactor was designed for the removal of indoor formaldehyde. Three fins were added to the reactor and each fin had a triangular gap at one end, making this type reactor continuous and singlepass. The influence of fins on formaldehyde removal was examined in an airtight environmental chamber. The radiation and velocity fields of the reactors were simulated by using computational fluid dynamics (CFD) methods. A theoretical model for the degradation of formaldehyde in a recirculating system was proposed. When adding fins in the annular reactor, the reaction area and residence time were greatly increased, and the degradation rate was, therefore, obviously enhanced. The CFD simulation results showed that the radiation intensity on the internal surfaces of the exterior cylinder was nearly uniform except for the two ends and it decreased slightly for the reactor with fins. The velocity distribution was uniform in the first tube pass and became actually higher near the elbows. The UV intensity was weak while the velocity was large near the elbows, which had a negative effect on degradation efficiency there. The results obtained from the kinetic model were in agreement with experimental data. So the degradation behavior of formaldehyde could be predicted by using this kinetic model.
Key words: photocatalytic oxidation; degradation model; annular reactor; CFD simulation; formaldehyde
甲醛是室内普遍存在的挥发性有机化合物(VOCs),是造成室内空气品质下降的主要原因之一[1],会对人体健康造成危害,甚至具有致癌作用[2].光催化氧化(PCO)技术节能环保,催化活性高,降解无选择性,是去除室内VOCs的有效手段[3-5].目前,随着模型预测[6]以及计算流体力学(CFD)模拟[7]的广泛应用,它们已成为研究PCO反应的重要工具.现有报道中,PCO技术常与空调系统结合,且多采用负载网[8]或蜂窝型媒介[9]作为光催化剂载体,此结构对提高气固间的传质作用有一定效果.但实际运行的空调系统中,流速一般为2~3 m/s,上述载体不仅增大流动阻力,而且传质作用的提升也非常有限.因此,本研究设计改进了传统的管状反应器,通过增大反应面积和气体停留时间来提高其在实际空调运行条件下的净化效果.并在实验分析的基础上,建立了循环降解模型来预测反应器的降解性能,又利用CFD的方法对反应器内的流速和光强分布进行了模拟和可视化处理,以期为光催化反应器的实际应用提供帮助.
1材料与方法
1.1实验系统
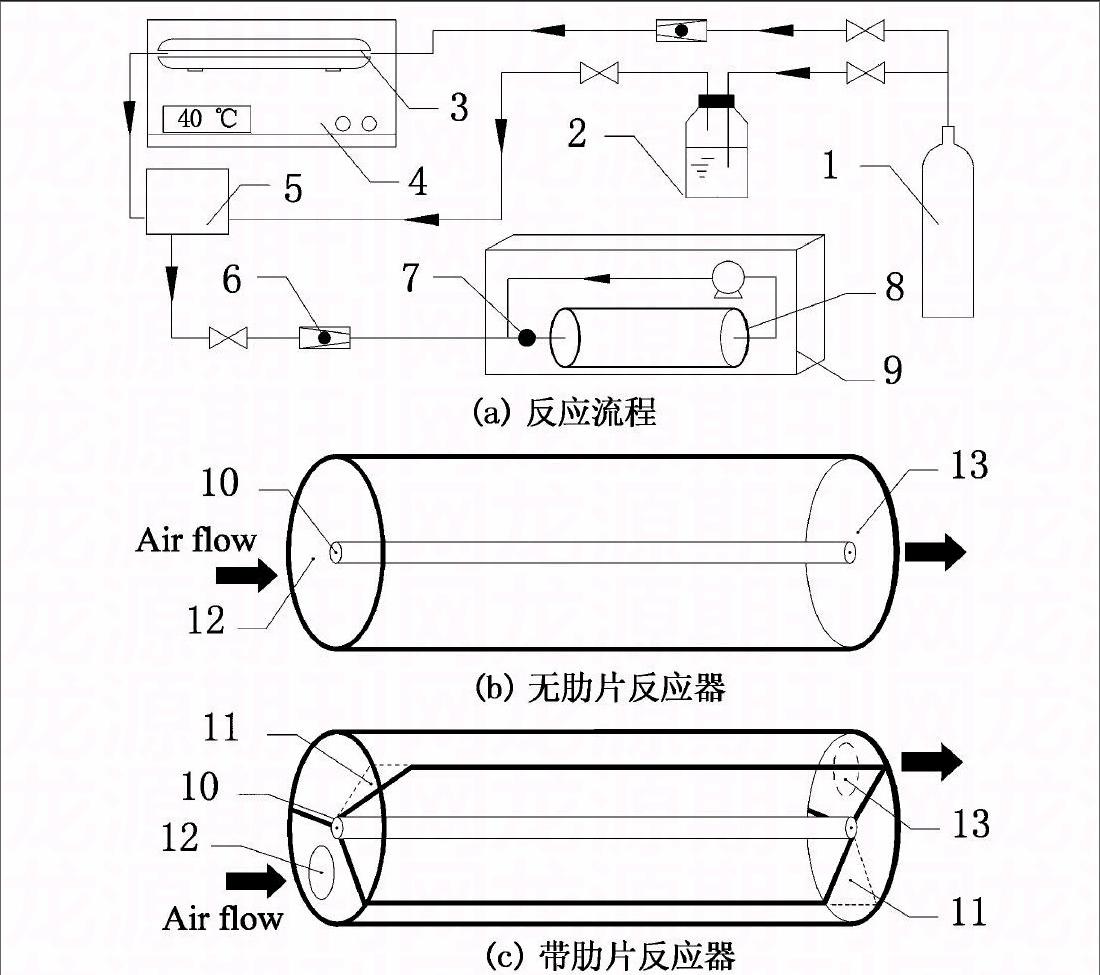
实验过程的气体流程如图1(a)所示.干洁空气(VN2/VO2×100=79∶21)分为两条气路,一条通入增湿瓶内加湿,另一条经流量计计量后流入甲醛发生器内.两条气路形成的湿空气和甲醛气体在缓冲瓶中充分混合,得到具有一定初始浓度和湿度的污染气体.该气体又在循环泵的作用下,反复流经反应器内发生光催化反应,直至降解结束.反应器的入口处设有采样口,甲醛浓度由INTERSCAN 4160甲醛分析仪测得.反应温度T和相对湿度RH由KANOMAX生产的CLIMOMASTER 6531测试仪测定, T精度±0.5 ℃, RH精度±5%.
1.2管状反应器
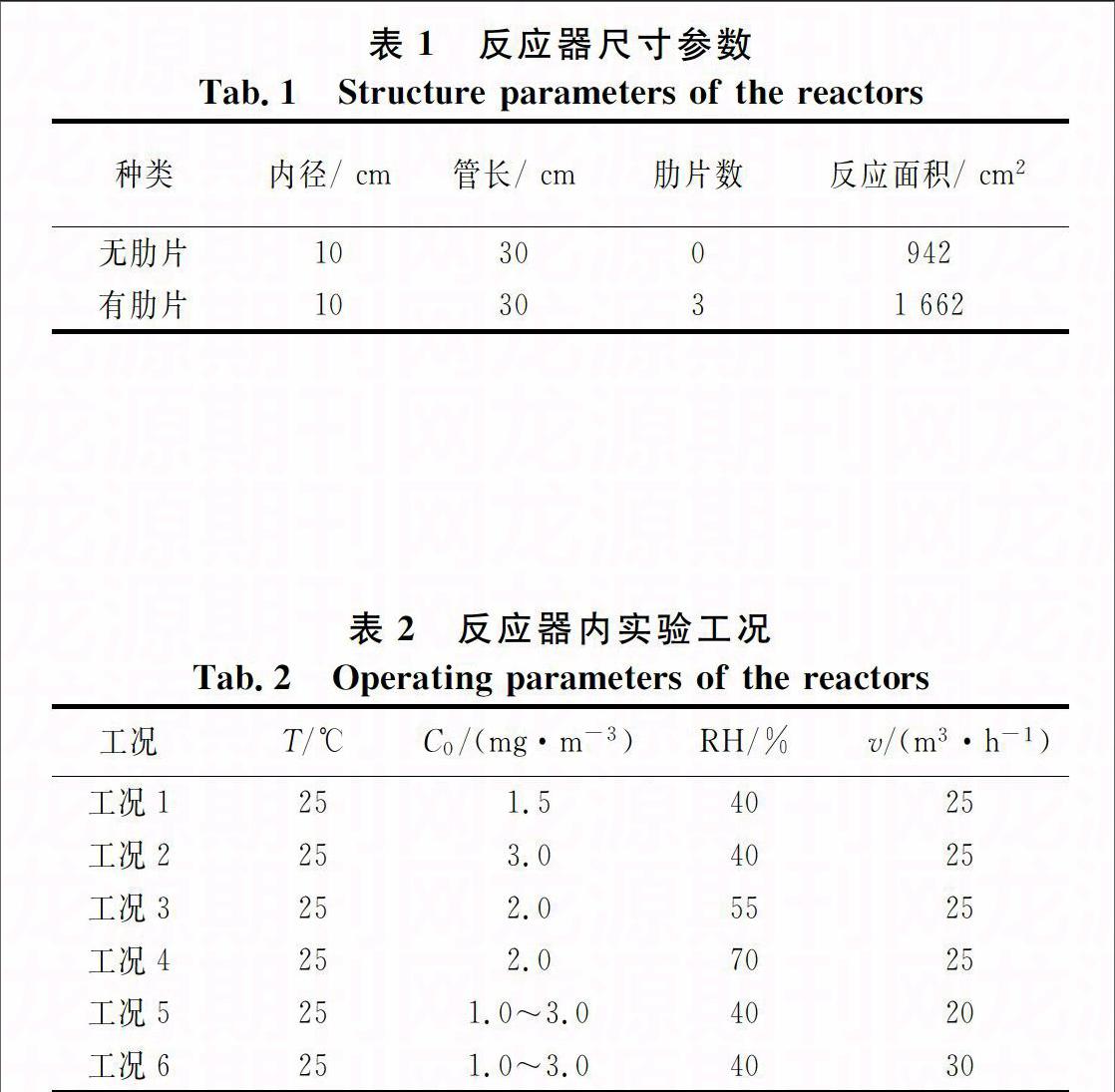
本实验设计的管状反应器共有两种,结构示意图如图1(b)和图1(c)所示,尺寸参数如表1所示.图1(b)是传统型管状反应器,在圆管内壁涂敷光催化剂,它的反应表面紫外光辐射较强且均匀,但有限的反应面积制约了其进一步发展[10].图1(c)是改进后的管状反应器,通过在传统管状反应器内壁与灯管之间添加多个直肋片得到.肋片沿管轴方向布置,且每个肋片的一端带有工艺缺口,在管内形成若干条连通的气道,使气体在进口和出口间呈多管程流动.该类型反应器的内壁面和直肋片正反表面均涂有光催化剂.所用光催化剂为Degussa P25型TiO2,负载量为1.2 mg/cm2.紫外光源选择功率20 W,波长254 nm的紫外杀菌灯.
1:干洁空气; 2:增湿瓶; 3: 甲醛发生器; 4: 恒温水浴箱;
5: 缓冲瓶; 6: 流量计; 7: 采样口; 8: 管状反应器; 9: 密闭舱;
10:紫外灯; 11:工艺缺口; 12: 进气口; 13:出气口
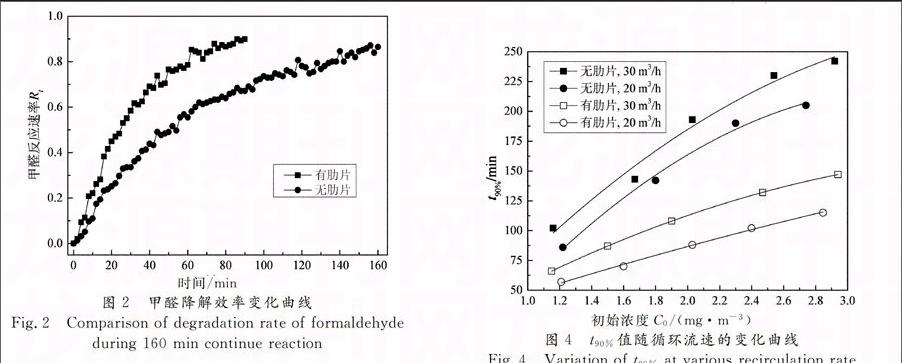
从图3中可以看出,反应器增设肋片后,各工况下t90%的值减少了约50%左右,反应速率基本提高1倍.对比工况1,工况3和工况4,工况4的相对湿度较高,t90%的值也明显大于工况1和工况3.这与Assadi [11]等研究管状反应器时的结论一致.过多的水分子会与甲醛分子在TiO2表面竞争吸附点位,且水分子会加速TiO2的电子空穴对复合,导致高相对湿度下单位时间内的降解效率降低.
2.2循环流速对反应速率的影响
为研究不同循环流速下反应速率的变化,选择工况5和工况6进行分析,结果如图4所示.其中, 20 m3/h和30 m3/h的循环流速分别对应2.1 m/s和3.2 m/s的面速度.
初始浓度C0/(mg·m-3)
从图4中可以看出,在两种反应器内,较高流速下的t90%值较大,反应速率较低.一般来说,提高流速会引起两种不同的结果:提高表面传质效果,对反应速率提升有利;减少气体停留时间,对反应速率提升不利.一般的空调系统中,面速度通常为2~3 m/s,在这种较大的流速范围内,传质作用并无明显变化[6],可以通过增加停留时间来提高气体分子与TiO2表面的接触概率,从而提高反应速率.因此,本实验中20 m3/h的循环流速对应的气体停留时间更长,反应速率更高.
为进一步分析流速对降解性能的影响,根据CFD的方法,利用Fluent 6.3软件模拟了反应器内的流速分布.图5为带肋片的管状反应器,在工况1时的径向剖面及轴向剖面速度分布云图.
从图5中可以看出, 轴向剖面上的流速分布较为均匀, 在气体通过三角形工艺缺口时出现明显扰动,此扰动可使反应气体充分混合.另外,各管程的中心区域流速相对较大,而内壁面和肋片表面附近由于阻力作用而流速较小,结合前面的分析,这种分布有利于提高反应速率.
2.3光强测定与模拟分析
表面光强在光催化反应之前测定,测试仪为UVC紫外辐照计.根据反应器内部空间的对称性,取如图6所示的单元体对反应表面光强I进行分析.图7为单元体内光强沿管轴方向的测定结果.
图8中的模拟分布与图7中实测光强基本相同.经计算,增加肋片后,内壁面光强减少了约35%,但从图3中得到的反应速率却提升了1倍左右,紫外光的利用效率明显提高.
当光强较强区域的流速较大时,该区域的传质反应更加平衡,降解效率也会较高[13].对比图5和图8中的流速与光强分布,反应器的中间段光强与流速耦合较好,降解效率将会较高,而反应器的前后两端由于气流扰动大且光强较弱,降解效率会受影响.
2.4循环降解模型
假设催化剂表面只吸附目标污染物和水,且氧化时无副产物生成,则反应物遵循单一组分的LangmuirHinshelwood (LH)降解模型:
从图9中可知,模型数据与实验结果基本相符,该降解模型基本可以反映甲醛降解的实际情况. 另外,模型计算值均处于实测浓度值的上方,这可能是因为本模型涉及的单一组分LH方程没考虑反应过程产生的副产物,导致光催化剂表面的实际与理论吸附量有所差别[16],从而产生一定误差.
3结论
1)通过增设带有工艺缺口的直肋片,使得管状反应器内的反应面积增大且气体停留时间延长,从而平衡了传质反应能力,反应速率提高了约1倍.
2)内壁面光强分布较均匀,而肋片表面分布极不均匀,且增加肋片后内壁面光强有所衰减. 反应器中间段光强与流速耦合较好,两端的气流扰动大且光强较弱,反应速率会受影响;
3)循环降解模型的预测值要稍高于实测结果,但两者变化趋势相同,该模型能较准确的预测甲醛的反应速率.
参考文献
[1]PASSALA C, ALFANO O M, BRANDI R J. A methodology for modeling photocatalytic reactors for indoor pollution control using previously estimated kinetic parameters[J]. Journal of Hazardous Materials, 2012, 211/212:357-365.
[2]BARAN T, MACYK W. Photocatalytic oxidation of volatile pollutants of air driven by visible light[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2012, 241:8-12.
[3]DERICHTER R K, MING T, CAILLOL S. Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors[J]. Renewable and Sustainable Energy Reviews, 2013, 19:82-106.
[4]WU Yiting, YU Yihui, NGUYEN V H, et al. Enhanced xylene removal by photocatalytic oxidation using fiberilluminated honeycomb reactor at ppb level[J]. Journal of Hazardous Materials, 2013, 262:717-725.
[5]FARHANIAN D, HAGHIGHAT F, LEE C S, et al. Impact of design parameters on the performance of ultraviolet photocatalytic oxidation air cleaner[J]. Building and Environment, 2013, 66: 148-157.
[6]ZHONG Lexuan, HAGHIGHAT F, BLONDEAU P, et al. Modeling and physical interpretation of photocatalytic oxidation efficiency in indoor air applications[J]. Building and Environment, 2010, 45(12): 2689-2697.
[7]余健, 王宏涛, 廖永浩,等. 气液两相在多孔介质内同向向上流动的CFD研究[J]. 湖南大学学报: 自然科学版, 2012, 39(8): 67-72.
YU Jian, WANG Hongtao, LIAO Yonghao, et al. CFD simulation of gasliquid two phase cocurrent upflow through porous media[J]. Journal of Hunan University: Natural Sciences, 2012, 39(8): 67-72.(In Chinese)
[8]DESTAILLATS H, SLEIMAN M, SULLIVAN D P, et al. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions[J]. Applied Catalysis B: Environmental, 2012, 128: 159-170.
[9]TARANTO J, FROCHOT D, PICHAT P. Photocatalytic air purification: Comparative efficacy and pressure drop of a TiO2coated thin mesh and a honeycomb monolith at high air velocities using a 0.4 m3 closeloop reactor[J]. Separation and Purification Technology, 2009, 67(2): 187-193.
[10]MO Jinhan, ZHANG Yinping, XU Qiujian, et al. Photocatalytic purification of volatile organic compounds in indoor air: A literature review [J]. Atmospheric Environment, 2009, 43(14):2229-2246.
[11]ASSADI A A, BOUZAZA A, WOLBERT D. Photocatalytic oxidation of trimethylamine and isovaleraldehyde in an annular reactor: Influence of the mass transfer and the relative humidity[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2012, 236: 61-69.
[12]MO Jinhan, ZHANG Yinping, YANG Rui, et al. Influence of fins on formaldehyde removal in annular photocatalytic reactors[J]. Building and Environment, 2008, 43(3):238-245.
[13]ZHONG Lexuan, HAGHIGHAT F, LEE C, et al. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation[J]. Journal of Hazardous Materials, 2013, 261:130-138.
[14]HOSSAIN M M, RAUPP G B, HAY S O, et al. Threedimensional developing flow model for photocatalytic monolith reactors[J]. AICHE Journal, 1999, 45(6):1309-1321.
[15]OBEE T N, BROWN R T. TiO2 photocatalysis for indoor air applications: effects of humidity and trace contaminant levels on the oxidation rates of formaldehyde, toluene, and 1,3butadiene[J]. Environ Sci Technol, 1995, 29(5): 1223-1231.
[16]MO Jinhan, ZHANG Yinping, XU Qiujian. Effect of water vapor on the byproducts and decomposition rate of ppblevel toluene by photocatalytic oxidation[J]. Applied Catalysis B: Environmental, 2013, 132/133:212-218.
