野生黄花蒿植株和土壤中的青蒿素、黄酮含量变化及其对土壤微生物的影响
2015-06-26罗世琼黄建国
李 倩,袁 玲,罗世琼,黄建国
(西南大学资源环境学院,重庆400716)
野生黄花蒿植株和土壤中的青蒿素、黄酮含量变化及其对土壤微生物的影响
李 倩,袁 玲,罗世琼,黄建国*
(西南大学资源环境学院,重庆400716)
黄花蒿在生长过程中,主要通过植株残体腐解、淋溶、根系分泌等途径向土壤中释放多种化感物质,影响邻近和后续植物的生长发育。本试验研究了野生黄花蒿植株及土壤中的青蒿素类物质和黄酮含量,以及土壤可培养微生物数量。结果表明,各生育期野生黄花蒿叶片和根区土壤中青蒿素含量的变化趋势为:现蕾期>始花期>盛花期>营养生长期;根区土壤中的去氧青蒿素平均含量最高,青蒿酸次之,青蒿素最低,三者合计516.93μg/kg干土,且3种化合物的总量根际和根表显著高于非根际。野生黄花蒿植株黄酮含量呈现出茎>叶>根系>花,根区土壤黄酮含量表现为根表>根际>非根际,且盛花期增至最大均值434.77μg/kg干土。说明根系分泌也是黄酮类化合物进入土壤的主要途径。土壤青蒿素含量与细菌和放线菌数量呈显著负相关(r=-0.508*和r=-0.478*,n=24),去氧青蒿素含量与放线菌数量呈极显著负相关(r=-0.528**,n=24)。因此,土壤中的青蒿素类物质可能抑制微生物的生长繁殖,影响土壤生物化学过程。
黄花蒿;青蒿素;黄酮;土壤微生物
黄花蒿(Artemisia annua)属菊科一年生草本植物,是提取青蒿素的唯一原料药材[1]。而黄花蒿在生长过程中,主要通过枯枝落叶、雨水淋溶和根系分泌3种途径向土壤生态系统中释放萜类、酚酸类和香豆素类等化感物质[2],严重影响土地生产力。重庆市三峡库区是我国黄花蒿的主栽区,小麦(Triticum aestivum)、油菜(Brassica campestris)和青菜(Brassica chinensis var.chinensis)等邻近和后茬作物的产量可降低20%以上[3]。
黄花蒿体内的化感物质进入土壤之后,对植物、土壤动物和水藻产生选择性毒性效应[4-9]。黄花蒿的水浸提液显著抑制小麦、燕麦(Avena sativa)、萝卜(Raphanus sativus)、油菜等多种作物种子萌发及幼苗生长[10];Maggi等[11]和Cala等[12]发现,黄花蒿叶片的醇提取物主要为青蒿素和多甲氧基黄酮类化合物,可以显著降低瓢虫(Epilachna paenulata)和南部灰翅夜蛾(Spodoptera eridania)幼虫存活率;低浓度的青蒿素对蚯蚓具有驱赶作用,高浓度则产生致死效应[13]。青蒿素抑制牛肝菌(Suillus luteus)和松乳菇(Lactarius delicious)等外生菌根真菌的生长、养分吸收及有机酸的分泌,干扰菌根的形成,进而影响森林树木生长发育和生态系统的功能[14-15]。目前,关于黄花蒿化感作用的研究多在室内开展,如用培养法检测青蒿素溶液对种子萌发和幼苗生长的影响[13,16-18],叶片法了解昆虫对黄花蒿叶片及其醇提取物的拒食性[19-21];固(液)体培养法研究青蒿素和黄花蒿提取物对土壤微生物生长繁殖的抑制作用等[22-23]。由于自然生态系统的复杂性,以上研究结果与自然生境条件下的结果必然存在显著差异。
微生物是土壤的重要成分,驱动土壤的生物化学反应,如有机质矿化,毒物降解和养分转化与供应等。大量的研究表明,化感物质可直接抑制周围和后茬植物生长发育,亦可通过干扰土壤微生物的生长和代谢活动,对植物产生直接和间接影响。因此,探讨黄花蒿对土壤微生物的影响,对于科学评估黄花蒿种植造成的生态风险,减轻其对周围和后茬作物的危害,保持土地生产力有重要意义。在重庆市三峡库区,野生黄花蒿在局部地区形成大量的优势群落而聚集生长,为研究自然状态下黄花蒿对土壤微生物的影响提供了理想的研究材料。
1 材料与方法
1.1 样地概述
本试验样地位于重庆市北碚区西南大学后山,年平均气温18.2℃,1月平均气温7.4℃,7月平均气温28.7℃,≥5℃的年均日数为356.6 d,≥10℃的年活动积温高达5979.5℃,年降水量1105.4 mm,蒸发量1181.1 mm,全年平均日照1276.7 h,无霜期335 d。1号样地海拔230.6 m,壤土(<0.01 mm土粒含量为21.95%),2号样地海拔356.1 m,沙土(<0.01 mm土粒含量为32.95%),均为多年生黄花蒿群落,簇生。土壤基本理化性状见表1。
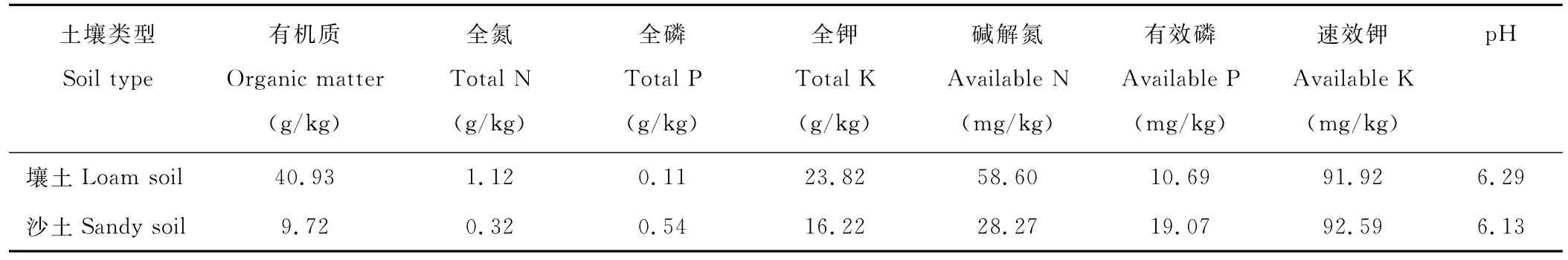
表1 土壤基本理化性状Table 1 The physical and chemical properties of soils
1.2 样品采集
2014年6-10月,分别于黄花蒿营养生长期(vegetative growth period,VGP)、现蕾期(squaring period,SP)、始花期(first flowering period,FFP)、盛花期(full-bloom period,FBP),用抖根法和洗涤法采集土壤样品[24]。在采样时,先小心抖落根系周围的土壤,收集非根际土壤;再用小毛刷将附着在根系上的根际土壤轻轻刷下,获得根际土壤。每个样地选取15个采样点,每5个混匀,得到3个样品。然后将仍粘附少量根表土壤的黄花蒿根系装入无菌塑料袋,同步采集黄花蒿植株样品,备测有关指标。
1.3 测定项目与方法
称取5.00 g新鲜的根际和非根际土,分别置于装有少量石英砂和45 m L无菌水的三角瓶中,121 r/min振荡30 min备用。将粘附根表土壤的根系剪下,称重(W1)后置于装有石英砂和45 m L无菌水的三角瓶中,振荡10 s,取出根系,121 r/min振荡30 min,得到根表土壤的菌悬液。采用稀释平板涂布法测定土壤可培养细菌(牛肉膏蛋白胨培养基)、真菌(马丁氏培养基)、放线菌(高氏一号培养基)、自生固氮菌(Ashby无氮培养基)、磷细菌(磷酸钙+植酸培养基)和钾细菌(铝土矿培养基)数目[25]。用吸水纸吸干根表水分,称重根系(W2),W1-W2即为根表土质量。烘干测定土壤含水量,用于计算微生物数量(CFU/g干土)。
40℃烘干黄花蒿根、茎、叶,分别磨碎过1 mm筛,参照Jessing等[26]的方法测定青蒿素含量。植株青蒿素的提取:精密称取250 mg植株样品粉末,置于100 m L磨口带塞三角瓶中,加入25 m L 95%乙醇,密封后,121 r/min振荡20 h,过滤获得待测液。土壤青蒿素类物质的提取:将仍粘附少量根表土壤的黄花蒿根系置于15 m L 95%乙醇溶液中轻晃5 s,提取方法同植株,抽滤浓缩至2 m L待测液,以滤纸上的土壤量为基准,计算根表土壤中青蒿素类物质含量,精确称取5.00 g新鲜根际和非根际土于25 m L离心管中,参照上述方法提取和浓缩青蒿素类物质。采用GC-MS测定植株和土壤待测液中的青蒿酸、去氧青蒿素及青蒿素含量[27]。色谱分析条件为:DB-5 MS(30 m×0.25 mm×0.25μm)色谱柱;升温程序:50~220℃(15℃/min,保持5 min),220~230℃(15℃/min,保持10 min);载气:氦气(恒流,31.4 cm/s);分流比:10∶1;检测器温度:250℃;离子源温度:250℃。
另称取10.00 g新鲜的根际和非根际土,分别置于100 m L离心管中,加入70%乙醇(土壤∶乙醇=1∶5),超声提取黄酮(80℃,3 h),抽滤浓缩至5 m L。将粘附少量根表土壤的黄花蒿根系置于70%乙醇溶液中轻晃5 s,参照根际和非根际土的方法提取和浓缩,获得根表土壤的黄酮待测液。以芦丁为标准品,硝酸铝显色后,270 nm比色测定土壤黄酮含量。参照土壤黄酮测定方法,测定黄花蒿植株黄酮含量[28-29]。
1.4 数据分析
采用Excel 2003对数据进行基本计算,SPSS 18.0进行统计分析,Origin 8.5作图,Duncan法进行差异显著性检验(P<0.05),Pearson相关分析法分析黄花蒿根区土壤青蒿素类物质、黄酮含量与可培养微生物数量之间的关系。
2 结果与分析
2.1 野生黄花蒿的生长状况
由表2可见,随生育期的推移,野生黄花蒿植株的株高和总生物量持续增加,盛花期增至最大值,与营养生长期相比,平均值分别增加了0.83倍和1.10倍。叶片生物量从营养生长期到现蕾期增至最高,然后大幅度降低,盛花期比现蕾期的平均值降低55.13%。壤土生长的黄花蒿长势优于沙土。
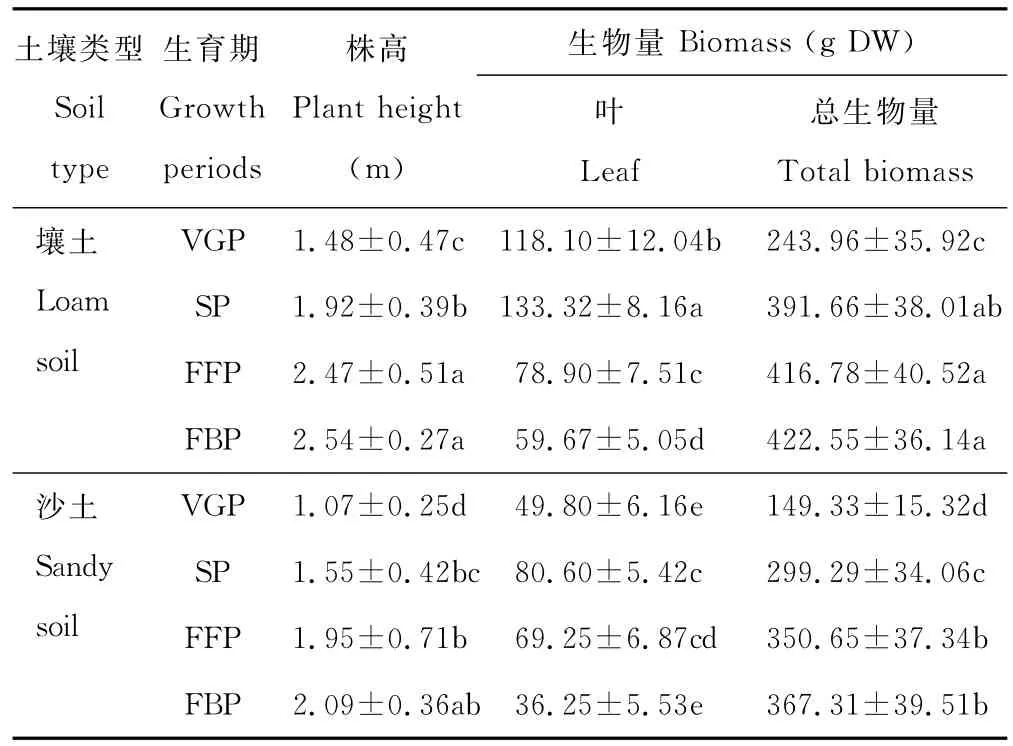
表2 不同生育期野生黄花蒿植株的株高和生物量变化Table 2 The biomass and plant height of wild A.annua in different growth periods
2.2 野生黄花蒿植株青蒿素和黄酮含量
2.2.1 叶片青蒿素含量 野生黄花蒿叶片青蒿素含量表现为现蕾期>始花期>盛花期>营养生长期,平均值高低相差1.64倍。此外,叶片青蒿素含量沙土高于壤土,平均值分别为5.35和4.61 mg/g DW(图1)。
2.2.2 植株黄酮含量 与叶片青蒿素含量的变化类似,黄花蒿植株黄酮含量呈单峰曲线变化,从营养生长期到现蕾期增至最大值,然后逐渐降低(图2)。此外,黄花蒿植株黄酮含量呈现出茎>叶>根系>花,壤土生长植株黄酮含量显著的高于沙土生长植株,平均值分别为101.64和78.43 mg/100 g DW。
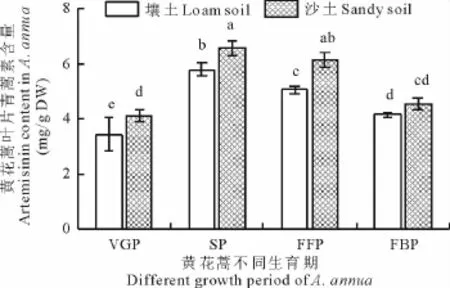
图1 黄花蒿叶片中的青蒿素含量Fig.1 Artemisinin content in the leaves of A.annua
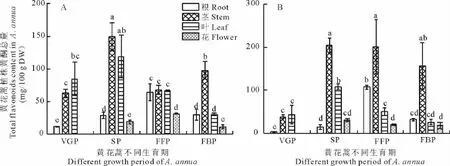
图2 野生黄花蒿植株总黄酮含量变化Fig.2 The changes of total flavonoids content in A.annua
2.3 根区土壤青蒿素类物质和黄酮含量
2.3.1 土壤青蒿素类物质 由表3可见,在黄花蒿生长的根区土壤中,去氧青蒿素的平均含量最高,青蒿酸次之,青蒿素最低,平均值依次为347.81,139.66和74.46μg/kg。在黄花蒿根表和根际土壤中,3种化合物的总量显著高于非根际。从黄花蒿生育期看,现蕾期根区土壤中这3种化合物的总量最高,盛花期降至最低。此外,在两种类型的黄花蒿根区土壤中,3种化合物的总量差异显著,沙土比壤土高了41.79%。
2.3.2 土壤黄酮 在黄花蒿根表土壤中,黄酮含量最高,根际土壤次之,非根际最低,高低相差35.1倍。此外,随黄花蒿生育进程的递进,根区土壤黄酮含量呈增加趋势,盛花期增至最大值(平均值=434.77μg/kg干土),且沙土中的黄酮平均含量显著高于壤土,前者是后者的2.33倍(图3)。
2.4 黄花蒿根区土壤可培养微生物数量
由表4可见,黄花蒿非根际土壤可培养细菌、放线菌和自生固氮菌数量最高,根际次之,根表最低,但真菌、无机磷细菌和钾细菌数量变化呈多态性。随黄花蒿生育期的推移,根区土壤中可培养细菌和放线菌数量持续增加,自生固氮菌数量先增加后减少,无机磷细菌和钾细菌数量先增加后减少再增加。
2.5 黄花蒿根区土壤青蒿素类物质、黄酮含量与可培养微生物数量的相关关系
由表5可见,在野生黄花蒿生长的土壤中,青蒿酸含量与可培养微生物数量无显著相关性;去氧青蒿素含量与放线菌数量呈极显著负相关(r=-0.528**,n=24);青蒿素含量与细菌和放线菌数量呈显著负相关(r=-0.508*和r=-0.478*,n=24);土壤黄酮含量与细菌、真菌、放线菌数量之间均无显著相关性。

表3 野生黄花蒿根区土壤青蒿素类物质含量Table 3 The changes of artemisinin compounds content of A.annua in root region soils μg/kg dry soil
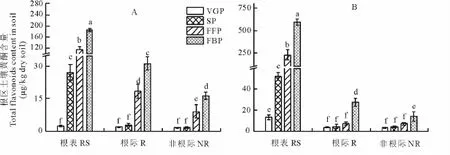
图3 野生黄花蒿根区土壤中的黄酮含量Fig.3 The changes of total flavonoids in root region soils of A.annua
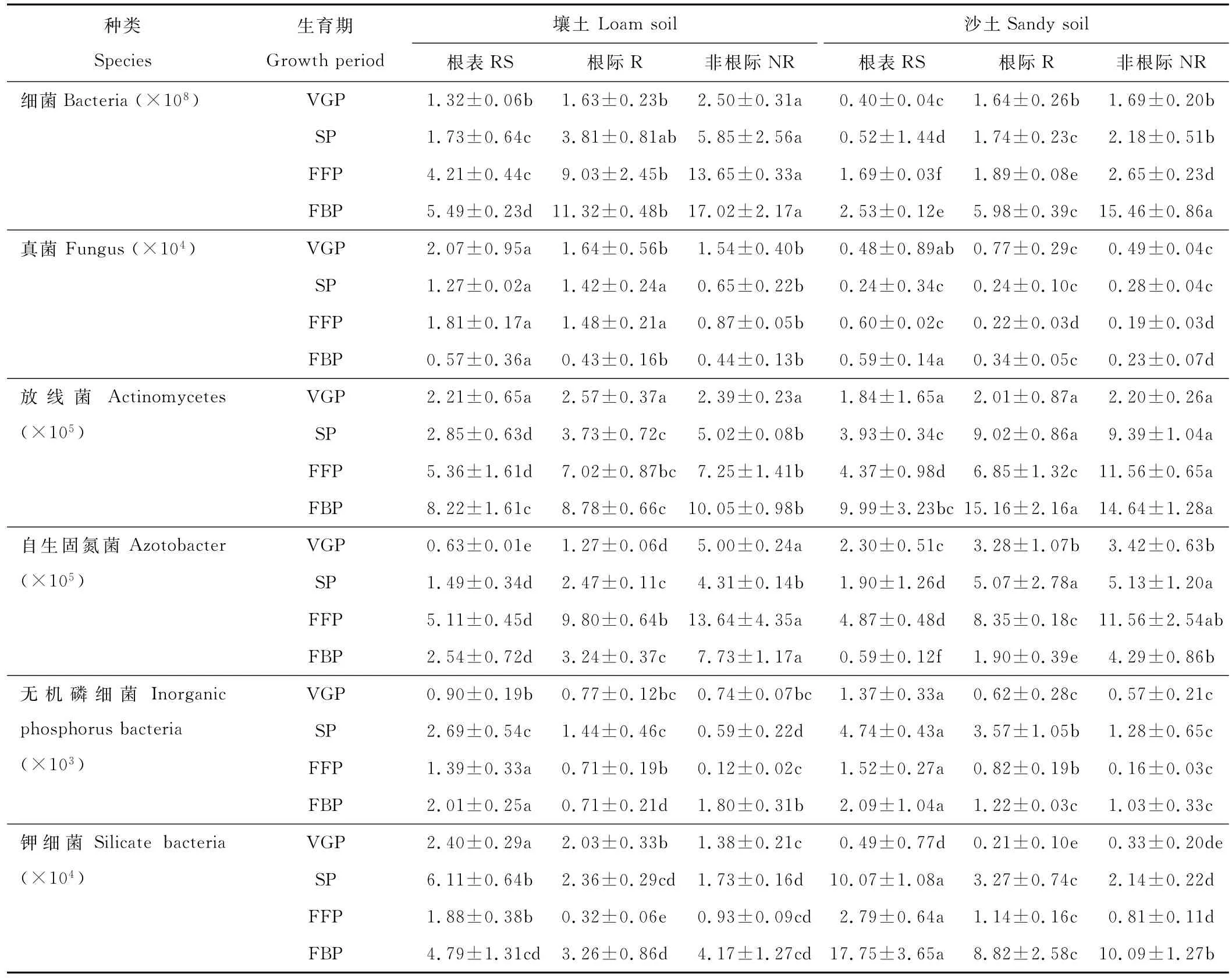
表4 黄花蒿根区土壤可培养微生物数量Table 4 The number of culturable microorganisms changes in root region soils of A.annua CFU/g dry soil
3 讨论
在黄花蒿生长过程中,叶、花、种子和果实极易脱落,向土壤释放多种化感物质,严重影响植物和土壤动物的生长代谢[30]。在本项研究中,现蕾期叶片生物量开始显著下降,降幅最高达到55.13%。在重庆市三峡库区,黄花蒿现蕾后进入当地的高温伏旱期,叶片容易凋落腐解,进入土壤的化感物质尤其是青蒿素类物质大幅增加,可能抑制黄花蒿周围及后茬作物的生长发育,造成减产。
在野生黄花蒿现蕾期,植株叶片青蒿素含量最高,根区土壤青蒿素类物质表现为去氧青蒿素>青蒿酸>青蒿素,合计高达516.93μg/kg干土。这与Jessing等[2,31]利用原位硅胶管微萃取法,证实黄花蒿根毛能分泌青蒿素的结果一致,由此也可解释青蒿素类物质含量在根表、根际>非根际土壤的现象。Duke等[16]发现,33μmol/L青蒿素即可抑制杂草生长;1μmol/L的青蒿素能干扰水葫芦(Eichhornia crassipes)的光合作用,月牙藻(Pseudokirchneriella subcapitata)和小叶浮萍(Lemna minor)的半最大效应浓度(Concentration for 50% of maximal effect,EC50)仅0.24和0.19 mg/kg[13]。因此,在野生黄花蒿群落的根际土壤中,高浓度的青蒿素类物质可能抑制其他植物生长,这对于黄花蒿形成单优种群和保持生态优势具有重要作用。此外,土壤青蒿素浓度与叶片青蒿素含量呈极显著正相关[32],在三峡库区普遍栽培的黄花蒿品种中,青蒿素含量一般为10.00~15.00 mg/g DW,是野生黄花蒿的2~3倍[33-34],由此推测,在人工栽培黄花蒿的土壤中,青蒿素类物质的含量可能更高,对周围和后续作物生长发育抑制作用更强。相关分析结果显示,土壤青蒿素含量与细菌和放线菌数量呈显著负相关(r=-0.508*和r=-0.478*,n=24),去氧青蒿素含量与放线菌数量呈极显著负相关(r=-0.528**,n=24)。培养试验也表明,在根瘤菌、无机磷细菌和外生菌根真菌等土壤微生物的培养液中,添加青蒿素后,它们的繁殖速率、固氮酶活性和溶磷能力下降,菌根真菌菌丝生物量、有机酸分泌速率和养分吸收量均显著减少[14-15,22,35]。
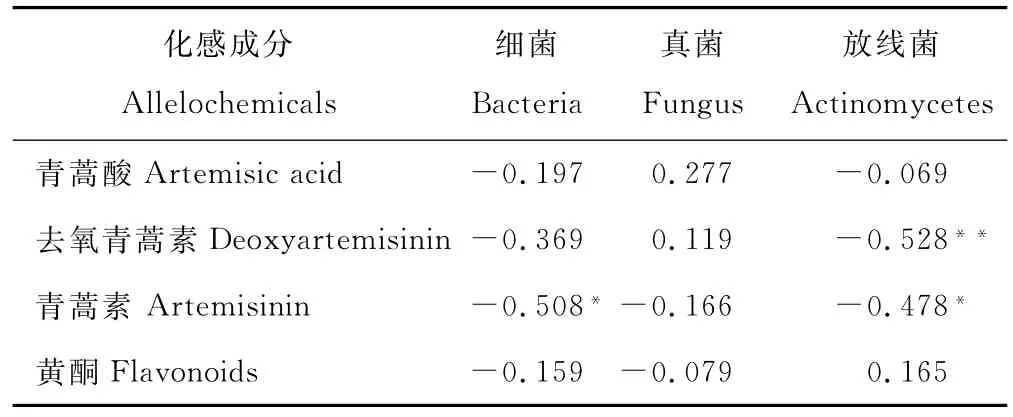
表5 黄花蒿根区土壤青蒿素类物质、黄酮含量与可培养微生物数量的相关关系Table 5 Correlation coefficients between the content of artemisinin derivatives,flavonoids and the number of soil microorganism in root region soils of A.annua
随野生黄花蒿生育期的推移,植株黄酮含量下降,根表和根际土壤的黄酮总量则大幅度增加,表明黄酮类物质也可通过植株根系分泌进入土壤。据报道,黄酮类物质对土壤微生物具有广谱杀抑作用,包括常见的细菌如枯草杆菌(Bacillus subtilis)、金黄色葡萄球菌(Staphylococcus aureus)、蜡样芽胞杆菌(Bacillus cereus)和变形杆菌(Staphylococcus epidermidis)等[36]。但是,在本试验中,土壤黄酮含量与细菌、真菌、放线菌数量之间均无显著相关性,不同于前人的研究结果[37-38]。在野外复杂的自然条件下,多种土壤因素影响土壤微生物的繁殖生长,各因素的作用大小也不一样[39],这可能是土壤黄酮与土壤微生物之间无显著相关性的原因之一。此外,青蒿素类物质能抑制原生动物和微生物繁殖生长,而甲氧基黄酮类物质与青蒿素具有协同效应,可促进青蒿素与血晶素的反应,增强青蒿素的抗疟活性,提高杀灭疟原虫的效果;在黄酮存在的条件下,青蒿素破坏细菌原生质膜的作用增强,使胞内物质大量外渗[40]。因此,土壤中的黄酮也可能与青蒿素协同,对微生物繁殖生长产生抑制作用。
综上所述,野生黄花蒿植株通过叶片凋落和根系分泌向土壤释放大量的青蒿素类和黄酮类物质,对微生物产生化感作用,选择性的干扰土壤微生物的生长繁殖,影响土壤质量和生产力。
[1] Ma J,Xiang J Q,Yang Y K,et al.Current situation of breeding and progress of systematic breeding of new varieties of Artemisia annua.Hubei Agricultural Sciences,2014,53(19):4520-4524.
[2] Jessing K K,Cedergreen N,Mayer P,et al.Loss of artemisinin produced by Artemisia annua L.to the soil environment.Industrial Crops and Products,2013,43:132-140.
[3] Zhang X B,Guo L P,Huang L Q.Climate suitable rank distribution of artemisinin content of Artemisia annua in China.Acta Pharmaceutica Sinica,2011,46(4):472-478.
[4] Zhao H M,Yang S Y,Guo H R,et al.Allelopathy of Artemisia annua on 4 receptor plants.Acta Botanica Boreali-Occidentalia Sinica,2007,27(11):2292-2297.
[5] Wu Y K,Yuan L,Huang J G,et al.Allelopathic effect of artemisinin on green algae.China Journal of Chinese Materia Medica,2013,38(9):1349-1354.
[6] Wang S,Mu X Q,Yang C,et al.Allelopathy and it’s mechanism of extract solution of Artemisia annua L.on wheat.Journal of Northwest A&F University(Natural Science Edition),2006,34(6):106-110.
[7] Yang P,Zhang S L,Dong Y.Advances in inhibitory effects of alkaloids on growth of algae.Fisheries Science,2012,31(12):754-758.
[8] Yang Z G,Zhang Y Q,Ding W,et al.Comparison of acaricidal bioactivity of natural and cultivated Artemisia annua leaves acetone extracts against Tetranychus cinnabrinus.Chinese Journal of Ecology,2011,30(7):1398-1402.
[9] Yao A Q,Liang D H.Biological activity of extract Innamomum camphora sieb and Artemisia annua L.against cabbage butterfly larva.Modern Agrochemicals,2004,3(2):28-30.
[10] Ma Y.Studies of allelopathy of Artemisia annua L[D].Shanxi:North West Agriculture and Forestry University,2007.
[11] Maggi M E,Mangeaud A,Carpinella M C,et al.Laboratory evaluation of Artemisia annua L.extract and artemisinin activity against Epilachna paenulata and Spodoptera eridania.Journal of Chemical Ecology,2005,31(7):1527-1536.
[12] Cala A C,Ferreira J F S,Chagas A C S,et al.Anthelmintic activity of Artemisia annua L.extracts in vitro and the effect of an aqueous extract and artemisinin in sheep naturally infected with gastrointestinal nematodes.Parasitology Research,2014,113(6):2345-2353.
[13] Jessing K K,Cedergreen N,Jensen J,et al.Degradation and ecotoxicity of the biomedical drug artemisinin in soil.Environmental Toxicology and Chemistry,2009,28(4):701-710.
[14] Li Q,Yuan L,Huang J G.Allelopathic effects of artemisinin on ectomycorrhizal fungal isolates in vitro.Pedobiologia,2014,57(4-6):271-276.
[15] Li Q,Yuan L,Wang M X,et al.Allelopathic effects of artemisinin on ectomycorrhizal fungi.Acta Ecologica Sinica,2013,33(6):1791-1797.
[16] Duke S O,Vaughn K C,Croom E M,et al.Artemisinin,a constituent of annual wormwood(Artemisia-Annua),is a selective phytotoxin.Weed Science,1987,35(4):499-505.
[17] Lydon J,Teasdale J R,Chen P K.Allelopathic activity of annual wormwood(Artemisia annua)and the role of artemisinin.Weed Science,1997,45(6):807-811.
[18] Bai Z,Huang Y,Huang J G.Allelopathic effects of artemisinin on seed germination and seedling growth of vegetables.Acta Ecologica Sinica,2013,33(23):7576-7582.
[19] Tripathi A K,Bhakuni R S,Upadhyay S,et al.Insect feeding deterrent and growth inhibitory activities of scopoletin isolated from Artemisia annua against Spilarctia obliqua(Lepidoptera:Noctuidae).Insect Science,2011,18(2):189-194.
[20] Wang Z J,Yang S M,Shen H,et al.Bioassay of insecticidal effect of extract from Artemisia annua on Lipaphis erysimi.Journal of West China Forestry Science,2014,43(4):136-139.
[21] Zhang Y Q,Ding W,Zhao Z M,et al.Studies on acarcidal bioactivities of the extracts from Artemisia annua L.against Tetranychus cinnabarinus bois.(Acari:Tetranychidae).Scientia Agricultura Sinica,2008,41(3):720-726.
[22] Luo S Q,Yuan L,Huang J G.In vitro effects of artemisinin on inorganic phosphate-solubilizing bacteria.African Journal of Microbiology Research,2013,7(6):525-532.
[23] Shoeb H A,Tawfik A F,Shibl A M,et al.Antimicrobial activity of artemisinin and its derivatives against anaerobicbacteria.Journal of Chemotherapy,1990,2(6):362-367.
[24] Lin X G.Principles and Methods of Soil Microbiology Research[M].Beijing:Higher Education Press,2010.
[25] Zhou D Q.Microbiology Experiment(2nd edition)[M].Beijing:Higher Education Press,2006.
[26] Jessing K K,Juhler R K,Strobel B W.Monitoring of artemisinin,dihydroartemisinin,and artemether in environmental matrices using high-performance liquid chromatography-tandem mass spectrometry(LC-MS/MS).Journal of Agricultural and Food Chemistry,2011,59(21):11735-11743.
[27] Luo S Q,Shi A D,Yuan L,et al.Changes in antimalarial compounds and antioxidation activities of Artemisia annua in response to fertilization.Acta Prataculturae Sinica,2014,23(1):339-345.
[28] Bilia A R,Melillo de Malgalhaes P,Bergonzi M C,et al.Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L.obtained from a commercial sample and a selected cultivar.Phytomedicine,2006,13(7):487-493.
[29] Li Z L,Guo K X,Zhou S B,et al.Effects of light intensity on biological characteristics,physiological indexes and flavone content of Knlimeris indica.Acta Prataculturae Sinica,2014,23(4):162-170.
[30] Jessing K K,Duke S O,Cedergreeen N.Potential ecological roles of artemisinin produced by Artemisia annua L.Journal of Chemical Ecology,2014,40(2):100-117.
[31] Jessing K K,Bowers T,Strobel B W,et al.Artemisinin determination and degradation in soil using supercritical fluid extraction and HPLC-UV.International Journal of Environmental Analytical Chemistry,2009,89(1):1-10.
[32] Herrmann S,Jessing K K,Jorgensen N O G,et al.Distribution and ecological impact of artemisinin derived from Artemisia annua L.in an agricultural ecosystem.Soil Biology&Biochemistry,2013,57:164-172.
[33] Sun N X,Li L Y,Zhong G Y,et al.Effect of different soil water treatments on physiological characteristics and yield of Artemisia annua.China Journal of Chinese Materia Medica,2009,34(4):386-389.
[34] Yang S P,Yang X,Huang J G,et al.Effects of application of N,P and K and plant density on growth of Artemisia annua and yield of artemisinin.China Journal of Chinese Materia Medica,2009,34(18):2290-2295.
[35] Li Q,Wu Y K,Huang J G.Studies on allelopathic effect of artemisinin on rhizobium.China Journal of Chinese Materia Medica,2011,36(24):3428-3433.
[36] Orhan D D,Ozcelik B,Ozgen S,et al.Antibacterial,antifungal,and antiviral activities of some flavonoids.Microbiological Research,2010,165(6):496-504.
[37] Ma T,Li G F,Liu R J,et al.Effect of arbuscular mycorrhizal fungus on the content of phenolic acids and flavonoids in con-tinous cropping watermelon roots and rhizosphere soils.North Horticulture,2014,(8):1-4.
[38] Rong L Y,Yao T,Ma W B,et al.The inoculants potential of plant growth promoting rhizobacteria strains to improve the yield and quality of Trifolium pretense cv.Minshan.Acta Prataculturae Sinica,2014,23(5):231-240.
[39] Yang X J,Wang Y S,Duan L D,et al.Changes of soil microbial biomass and enzymatic activities among restoration stages of Langshan Forest Park,Hunan Province.Acta Prataculturae Sinica,2014,23(1):142-148.
[40] Elford B C,Roberts M F,Phillipson J D,et al.Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones.Transactions of the Royal Society of Tropical Medicine and Hygiene,1987,81(3):434-436.
参考文献:
[1] 马进,向极钎,杨永康,等.黄花蒿新品种选育现状及其系统选育研究进展.湖北农业科学,2014,53(19):4520-4524.
[3] 张小波,郭兰萍,黄璐琦.我国黄花蒿中青蒿素含量的气候适宜性等级划分.药学学报,2011,46(4):472-478.
[4] 赵红梅,杨顺义,郭鸿儒,等.黄花蒿对4种受体植物的化感作用研究.西北植物学报,2007,27(11):2292-2297.
[5] 吴叶宽,袁玲,黄建国,等.青蒿素对绿藻的化感效应.中国中药杂志,2013,38(9):1349-1354.
[6] 王硕,慕小倩,杨超,等.黄花蒿浸提液对小麦幼苗的化感作用及其机理研究.西北农林科技大学学报(自然科学版),2006,34(6):106-110.
[7] 杨攀,张树林,董阳.生物碱类化合物对藻类生长抑制作用的研究进展.水产科学,2012,31(12):754-758.
[8] 杨振国,张永强,丁伟,等.栽培型和野生型黄花蒿提取物对朱砂叶螨杀螨活性比较.生态学杂志,2011,30(7):1398-1402.
[9] 姚安庆,梁德华.樟树和黄花蒿浸提物对菜粉蝶幼虫的生物活性.现代农药,2004,3(2):28-30.
[10] 马燕.黄花蒿化感作用的研究[D].陕西:西北农林科技大学,2007.
[15] 李倩,袁玲,王明霞,等.青蒿素对外生菌根真菌化感效应.生态学报,2013,33(6):1791-1797.
[18] 白祯,黄玥,黄建国.青蒿素对蔬菜种子发芽和幼苗生长的化感效应.生态学报,2013,33(23):7576-7582.
[20] 王振吉,杨申明,沈慧,等.黄花蒿提取物对菜蚜的杀虫活性研究.西部林业科学,2014,43(4):136-139.
[21] 张永强,丁伟,赵志模,等.黄花蒿提取物对朱砂叶螨生物活性的研究.中国农业科学,2008,41(3):720-726.
[24] 林先贵.土壤微生物研究原理与方法[M].北京:高等教育出版社,2010.
[25] 周德庆.微生物学实验教程(第2版)[M].北京:高等教育出版社,2006.
[27] 罗世琼,石安东,袁玲,等.黄花蒿抗疟相关成分及抗氧化活性对施肥方式的响应.草业学报,2014,23(1):339-345.
[29] 李中林,郭开秀,周守标,等.光强对马兰形态生理及黄酮类化合物含量的影响.草业学报,2014,23(4):162-170.
[33] 孙年喜,李隆云,钟国跃,等.不同生长期土壤水分处理对黄花蒿生理特性及产量的影响.中国中药杂志,2009,34(4):386-389.
[34] 杨水平,杨宪,黄建国,等.氮磷钾肥和密度对青蒿生长和青蒿素产量的影响.中国中药杂志,2009,34(18):2290-2295.
[35] 李倩,吴叶宽,黄建国.青蒿素对根瘤菌化感效应研究.中国中药杂志,2011,36(24):3428-3433.
[37] 马通,李桂舫,刘润进,等.AM真菌对连作西瓜根内和根围土壤酚酸类物质和黄酮含量的影响.北方园艺,2014,(8):1-4.
[38] 荣良燕,姚拓,马文彬,等.岷山红三叶根际优良促生菌对其宿主生长和品质的影响.草业学报,2014,23(5):231-240.
[39] 杨贤均,王业社,段林东,等.湖南崀山森林公园不同植被条件下土壤微生物量及酶活性研究.草业学报,2014,23(1):142-148.
Artemisinin and flavonoids in wild Artemisia annua and surrounding soil and the influence on soil microbes
LI Qian,YUAN Ling,LUO Shi-Qiong,HUANG Jian-Guo*
College of Resources and Environment,Southwest University,Chongqing 400716,China
Artemisia annua releases various kinds of allelochemicals into soils via rain leaching,root exudation and dead tissue decomposition during the growing season,with resulting inhibition of the growth and development of adjacent plants and succeeding crops.The present experiments were thus conducted to detect artemisinin derivatives,flavonoids and effects on soil microbes in wild A.annua and surrounding soil.The allelochemical concentrations ranked:bud break period>early flowering period>full bloom period>vegetative growth period(artemisinin in leaves and root zone soil)and stem>leaf>root>flower(flavonoids in plants).In sampled soils,the mean concentration of deoxyartemisinin was highest,followed by artemisic acid and artemisinin in soil and the sum of these artemisinin derivatives was 516.93μg/kg dry soil.Concentrations of all three compounds tested were highest in root surface soil and much higher in the root surface soil and rhizosphere soil than in non-rhizosphere soil.Soil flavonoid concentrations increased steadily during the growing season of A.annua and reached their highest levels at full-bloom stage(434.77μg/kg dry soil).Hence it is concluded that fla-vonoids are released into soils through root exudation.The numbers of bacteria and actinomycetes showed significant negative correlations with artemisinin concentration(r=-0.508*and-0.478*,n=24).There was also a negative correlation between deoxyartemsinin contents and actinomycete numbers(r=-0.528**,n=24).In summary,artemisinin and its derivatives released from A.annua appear to inhibit microbial growth and reproduction,and are therefore likely to influence biochemical reactions in soils.
Artemisia annua;artemisinin;flavonoids;soil microorganism
10.11686/cyxb2014510 http://cyxb.lzu.edu.cn
李倩,袁玲,罗世琼,黄建国.野生黄花蒿植株和土壤中的青蒿素、黄酮含量变化及其对土壤微生物的影响.草业学报,2015,24(11):29-37.
LI Qian,YUAN Ling,LUO Shi-Qiong,HUANG Jian-Guo.Artemisinin and flavonoids in wild Artemisia annua and surrounding soil and the influence on soil microbes.Acta Prataculturae Sinica,2015,24(11):29-37.
2014-12-08;改回日期:2015-02-11
国家973计划项目(2013CB127405)和国家自然科学基金(41461053)资助。
李倩(1987-),女,河南郑州人,在读博士。E-mail:qianqingzi@qq.com
*通讯作者Corresponding author.E-mail:huang99@swu.edu.cn
