Effect of heat-disturbance on microbial biomass carbon and microbial respiration in Chinese f i r(Cunninghamia lanceolata)forest soils
2015-06-09••••
••••
ORIGINAL PAPER
Effect of heat-disturbance on microbial biomass carbon and microbial respiration in Chinese f i r(Cunninghamia lanceolata)forest soils
Jianfen Guo1,2•Guangshui Chen1,2•Jinsheng Xie1,2•Zhijie Yang1,2•Yusheng Yang1,2
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Prescribed f i re has now become the usual management practice in the Chinese f i r(Cunninghamia lanceolata(Lamb.)Hook.)plantation in southern China. Heat generated during f i re may affect carbon(C)dynamics in soils.We investigated the microbial biomass C (MBC)and microbial respiration in two Chinese f i r forest soils(one is not exposed to f i re for the past 88 years,and the other is recently exposed to prescribed f i re)after soil heating(100 and 200°C)under three moisture regimes [25,50 and 75%of water holding capacity(WHC)].For both soils,signif i cant reduction in MBC with increasing heating temperature was found.Soils without exposing to fi re previously had signif i cantly greater MBC concentration than the f i re-exposed soils when heated at 100 or 200°C.Lower soil water content resulted in higher MBC concentrations in both soils.In contrast,both soils had the highest soil microbial respiration rate at 50%WHC.Soils without exposing to f i re previously had the greatest microbial respiration rates at 200°C,while the f i re-exposed soils when heated at 100°C had greatest microbial respiration rates.During 14-days post-heat incubation, soil MBC in both soils was greatest after heating at 200°C and 25%WHC.However,soil previously exposed to f i re had the lowest CO2evolution when incubated at 25% WHC.
Chinese f i r⋅Fire⋅Microbial biomass C⋅Microbial respiration
Introduction
Forest soils contain a signif i cant amount of carbon(C)on a global scale.Approximately half of Earth’s terrestrial C is in forests(1146×1015g),and about two-thirds of this amount is retained in soil pools(Dixon et al.1994).Thus change in soil C emission or sequestration under the disturbance or forest management will affect the forest C budget.Wildf i re is an important disturbance in natural forests,and prescribed f i re is potentially useful for the management of plantation forests(Johnson and Curtis 2001;Boerner et al.2009).The effect of f i re induced soil heating on C dynamics in forest mineral soils has received great attention(Hatten and Zabowski 2009;Chen and Shrestha 2012;Loehman et al.2014).It is known that f i re may impact C dynamics through changes in physical, chemical,and biological properties of the soil.These changes are related to the duration and intensity of f i re as well as to soil type and moisture,to vegetation,and to climate(Pietika¨inen and Fritze 1995;Hatten and Zabowski 2009).Temperatures higher than 50°C result in death of heat-sensitive microbes and temperatures higher than 70°C can directly affect vegetation(Neary et al.1999). Subsequently,dead plant and microbial biomass providedmore soil carbon substrate and enhanced the lability of soil organic C(SOC).Soil moisture content may become a confounding factor in assessing the impact of f i re on soil chemical and biological properties(Albini and Reinhardt 1995).For example,the lethal temperature for bacteria is 210°C in dry soil and 110°C in wet soil,whereas the lethal temperature for fungi in dry and wet soil is 155 and 100°C,respectively.The variability of soil response to f i re is one of the main factors making it diff i cult to extrapolate results from one study to another.Furthermore,the information obtained on the interaction between f i re and soil response from f i eld-based studies can be unreliable due to the diff i culty in distinguishing the real cause of the changes in the soil from several interacting effects.Thus heating soil under controlled conditions in the laboratory provides the opportunity to isolate the effects of different environmental factors(Guerrero et al.2005;Ba´rcenas-Moreno and Ba˚a˚th 2009).
Chinese f i r(Cunninghamia lanceolata(Lamb.)Hook.) is one of the most important plantation tree species in China in terms of planting area,yield and timber usage. The history of managing this species exceeds 1000 years in China(Yang 1998).As a tradition,Chinese f i r is continuously grown in pure stands at the same sites.Usually the rotation age is about 30 years.Clear-cutting mature Chinese f i r stands timber followed by prescribed burning slash has now become the usual practice in southern China.In general,this practice can impact C cycling and soil organic matter dynamics(Yang et al.2009).A better understanding of the effects of moisture,f i re intensity,and f i re history on post-f i re soil ecosystem recovery could provide a basis for improved prescriptions that better enable restoration of f i re dependent forests.
Previous research had suggested that 1–2 year postburned soil respiration was lower compared to the undisturbed Chinese f i r stand(Guo et al.2010).In addition,soil C storage in a Chinese f i r forest decreased 1 year after the prescribed f i re(Yang et al.2005).However,little is known about the short-term effect of heat-induced disturbance on soil microbial biomass C(MBC)and microbial respiration in these Chinese f i r forest soils.We conducted a laboratory experiment in which soils of different f i re history were exposed to a transient heat-disturbance.Soils were collected from areas that had been burned in a prescribed f i re six months prior to sampling and matching sites that had not been burned.The purpose of this study was to assess changes in MBC and microbial respiration as affected by soil temperature and moisture regimes to simulate the impact of low and medium intensity f i res on the mineral soil surface and compare that impact in soils of different fi re history.
Materials and methods
Site description
The study area was located at Ancaoxia Forest Farm (26°28′N,117°57′E,200 m above sea level)in Nanping, Fujian,China.It is one of the central growing areas of Chinese f i r.The region has a middle subtropical monsoonal climate,with a mean annual temperature of 19.3°C,and a mean annual precipitation of 1669 mm.The soil was classif i ed as red soil in Chinese soil classif i cation(State Soil Survey Service of China 1998),equivalent to hapludult in USDA Soil Taxonomy(Soil Survey Staff of USDA 1999).The soil was acidic and developed on deposit derived from deeply weathering products of Cretaceous Period granite and conglomerate(Guo et al.2005).The soil prof i le was well-developed and characterized by a B horizon of clay accumulation,of which was reddish or yellowish brown in color due to the accumulation of iron oxides(Zhu et al.1983).
In 2006,the 40-(mature)and 88 year-old(over-mature) Chinese f i r forests were selected.Two stands are neighbouring(<1 km apart)and have similar parent material, soil type,and topography.The 40 year-old Chinese f i r forest was located on southwestern aspect with 40°slope. Stand density averaged 1317 stems ha-1.The mean tree height and DBH(diameter at breast height)were 25.2 m and 24.3 cm respectively.Understorey vegetation was dominated byMaesa japonica,Ficus hirta,Woodwardia japonicaandDicranopteris dichotoma.The 88 year-old Chinese f i r forest was on southwestern aspect with 30° slope.Mean tree height and DBH were 32.2 m and 32.8 cm respectively.Stand density was 750 stems ha-1.Maesa japonica,Rubus buergeri,Dicranopteris dichotomaandWoodwardia japonicawere predominated in the understorey.
Soil sampling and treatment design
In December 2006,the 40 year-old Chinese f i r forest was clear-cut followed by slash burning.Three plots with each area of 20 m×20 m in size were established in this burned site and a neighboring 88 year-old forest site for soil sampling.In June 2007,six soil cores per plot were collected at the depth of 0–10 cm using an auger with inner diameter of 8 cm,and then formed into one soil sample. Following collection,a portion of bulk samples were analyzed for soil texture,total C and N.The remaining samples were passed through 2 mm sieve,air-dried,and stored for 8 months prior to use.Selected soil characteristics(0–10 cm)are provided in Table 1.
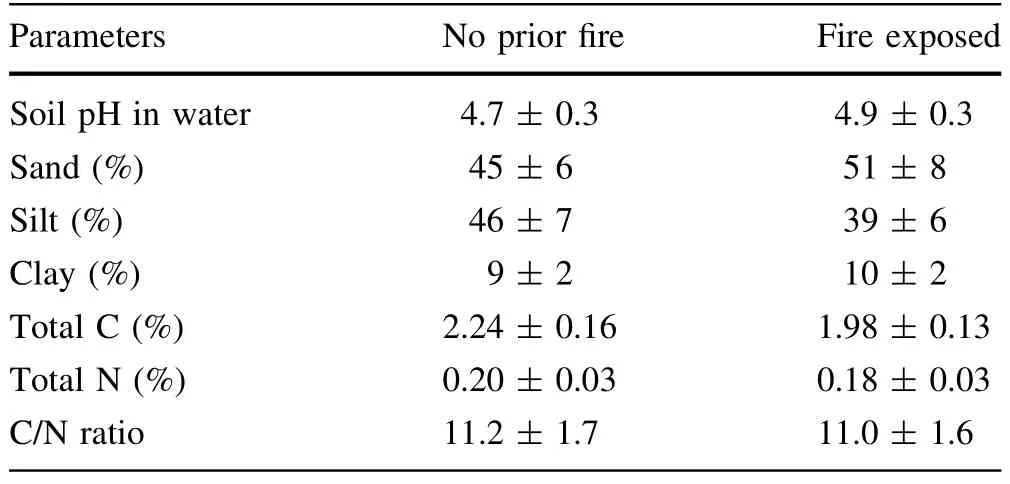
Table 1 Selected soil characteristics for two soils(0–10 cm)either not exposed to f i re for 88 years or recently exposed to prescribed f i re
In February 2008,four subsamples were drawn from the bulk soils individually,and water content at 25,50 and 75%of water holding capacity(WHC)was determined. Subsequently,soils were split into three equal parts, moistened to 25,50 and 75%WHC,and then pre-incubated for 14 days at 25°C with moisture monitored and corrected daily based on change in mass.At the end of the pre-incubation period,60 g subsamples of soil were transferred to sealed metal canisters(six replicate canisters per treatment)and placed in a preheated muff l e furnace for 30 min at 25(control),100 or 200°C.Previous measurement of soil temperature in 40 year-old Chinese f i r forest during prescribed burning(unpublished data)has showed that temperatures chosen in this study can best mimic surface temperatures associated with low and medium intensity f i res in the f i eld.Soils were allowed to cool and three replicate canisters per treatment were immediately processed and analyzed while the remaining three had their complete contents transferred to 1 L glass jars.These samples were then inoculated with 1 g of unheated soil and the moisture content returned to 25,50 and 75%WHC. The jars were then sealed and placed in a constant temperature chamber and incubated for 14 days at 25°C with moisture content checked and corrected daily based on change in mass.
Laboratory analyses
Mineral soil samples were passed through a 0.149 mm sieve,and total C and N content were determined by an elemental analyzer(Vario EL III,Elementar Analysensysteme GmbH,Hanau,Germany).The percentage of sand, silt,and clay was determined using the pipette method (Gee and Or 2002),with sand def i ned as particles>53 μm, silt between 2 and 53 μm and clay as<2 μm.
Soil samples both extracted immediately after heating (unheated control soils included)and 14-days post-heat treatment were analyzed for MBC and basal respiration. MBC was determined by the fumigation-extraction method (Vance et al.1987).MBC was calculated as the difference in extractable C before and after fumigation using a Kc factor of 0.45.CO2evolution rates of soils(25,50 and 75%WHC)incubated at 25°C were measured at the incubation time of 3,7 and 14 days using the alkali absorption method(Anderson 1982).The soil evolved CO2was collected into 20-mL traps of 0.5-M NaOH solution and titrated with 0.5-M HCl after adding 5 mL of 1.5-M BaCl2.The glass jars were regularly aerated to allow replenishment of O2.
Statistical analyses
A three-way ANOVA with post hoc Tukey’s test were used to determine signif i cant effects of prior soil f i re history,temperature,and soil moisture on MBC and microbial respiration.Statistical signif i cance was established at the 5%level,unless otherwise mentioned. Analyses were computed with the SPSS 13.0 software (SPSS Inc.2004).
Results and discussion
Immediate treatment effects
There were signif i cant immediate effects of soil f i re history,heating temperature and their interaction on microbial biomass C(MBC)across all moisture levels(P<0.01, Table 2).Soils without exposing to f i re previously had signif i cantly greater MBC concentration than the f i re-exposed soils when heated at 100 or 200°C.For both soils, signif i cant reduction in MBC with increasing heating temperature was found(Fig.1).On average,there was a 42%decrease of MBC concentration at 100°C and a 65%decrease at 200°C compared to the unheated control (Table 2;Fig.1).There were also signif i cant effects of soil water content and soil f i re history on MBC when averaged across all temperatures(P<0.01,Table 2).In contrast,the concentration of MBC was not signif i cantly inf l uenced bythe interaction between soil water content and soil f i re history(P=0.678).In both soils,the lowest MBC concentrations were at 75%WHC(Fig.2).

Table 2 Analysis of variance(P values)of the immediate effects of prior soil f i re history,soil heating temperature and soil moisture on microbial biomass C and CO2evolution

Fig.1 Soil microbial biomass C(MBC)and microbial respiration for the two Chinese f i r soils immediately after exposure to different temperatures(25,100 and 200°C)and averaged across three moisture levels(error bars indicate±SD).Signif i cant differences between temperature treatment within each soil are labeled with different lowercase letters,while differences between two soils within each temperature treatment are labeled with different capital letters (P<0.05)
Soil history,temperature and soil moisture had signif icant effects on microbial respiration rates immediately after heating(P<0.001,Table 2).Soils that have not been previously exposed to f i re had the greatest microbial respiration rates at 200°C,while the f i re-exposed soils when heated at 100°C and averaged across three moisture levels had the greatest microbial respiration rates(Fig.1).Both soils had the highest soil microbial respiration rate at 50% WHC(Fig.2).
The large decline in MBC following heating observed in this study is similar to the range of decline reported by Hernandez et al.(1997).A signif i cant decrease in soil MBC was also found after experimental f i re and it was attributed to high f i re severity(Andersson et al.2004).Fire may affect soil microorganisms directly through soil heating,or indirectly,through modif i cation of physical and chemical soil properties.Soil heating can result in a reduction of microbial biomass,depending on the temperatures reached and the soil moisture,but not all soil microorganisms will be equally susceptible(Choromanska and DeLuca 2001).Microbial mortality generally occurs between temperatures in the 50–120°C range with fungi usually more heat sensitive than bacteria(Neary et al. 1999).On the other hand,burning under wet and dry conditions can have varying effects on soil microf l ora. Consistently moist soil conditions prior to heating may increase microbial susceptibility to heat effects(Dunn et al. 1985).For example,Klopatek et al.(1990)found that 90 days following a burn,soil microbial biomass was lower when burned under wet than under dry conditions.In the present study,the highest decline in MBC was observed at the more moist condition,as a result of more effective latent heat penetration and of faster heat transmission, compared to drier soils(Campbell et al.1994).In addition, higher rates of survival in dry soils may have been,in part,a result of spore formation and microbial adaptation to stress in dry soils during the pre-treatment incubation period(Dunn et al.1985).

Fig.2 Soil microbial biomass C(MBC)and microbial respiration for the two Chinese fi r soils at 25,50 and 75%of water holding capacity (WHC)immediately after exposure to heat treatments and averaged across three temperatures(error bars indicate±SD).Signi fi cant differences between moisture levels within each soil are labeled with different lowercase letters,while differences between two soils within each moisture level are labeled with different capital letters (P<0.05)
Our observed differences in microbial respiration from the two soils perhaps resulted from the difference of C substrate initially present in the two soils(Yang 1998). Low microbial respiration rates in soils previously exposed to f i re may be a result of C substrate loss or f i re-induced microbial mortality(Choromanska and DeLuca 2002). Signif i cantly enhanced respiration rate with increasing heat temperature was found in soils without exposing to f i re previously(Fig.1).Andersson et al.(2004)and De Marco et al.(2005)also observed a short-term increase in basal soil respiration after experimental f i res and attributed to changes in soluble C.In contrast,reduced levels of MBC following heating in the same soil suggest that elevated temperatures can increase microbial activity—but not necessarily microbial biomass(Banning and Murphy 2008).Santruckova and Straskraba(1991)noted that soil respiration was independent of microbial biomass,since it remained almost constant over a range of microbial biomass contents,resulting in a low microbial biomass with a high CO2evolution.Another possibility that higher basal respiration corresponds to lower MBC is changes in the community composition,in terms of abundance of fungalv.bacterial components.Usually,the fungal:bacterial biomass ratio decreased after heating(Sakamoto and Oba 1994).The effect of the fungal:bacterial biomass ratio on the relationship between CO2evolution and the size of the soil microbial biomass had been examined by Sakamoto and Oba(1994)and found a high negative correlation between this ratio and the metabolic quotient(qCO2). Overall,the divergent responses of soil CO2f l ux and soil microbial biomass to experimental f i re in our study suggest that changes in soil respiration activity can occur without large changes in the biomass of soil microbes.This ecological response is an important consideration in linking microbial activity to carbon f l ux measurements.
Treatment effects 14-days after heating
MBC and microbial respiration during the 14-days incubation were signif i cantly inf l uenced by soil f i re history, initial heat treatment,and soil water content(P<0.01, Table 3 and Fig.3).There were also signif i cant interactions between soil f i re history and heating temperature,soil fi re history and soil moisture,and heating temperature and soil moisture for MBC.Heating to 100 or 200°C resulted in signif i cantly lower MBC concentrations in the soils without exposing to f i re previously when compared to the unheated control(Fig.3).When both soils were heated to 200°C,soil MBC was greatest at 25%WHC while the lowest MBC concentrations were observed at 75%WHC (P<0.05,Fig.3).During 14 days of incubation the observed decrease in MBC concentrations following heating in this study is supported by earlier f i ndings which showed that heating of soils causes mortality for some microorganisms(Acea et al.2003;Badı´a and Martı´2003). Some authors have related the decrease in MBC with the low re-establishment of fungi,which are the main contributors to the soil microbial biomass(70–80%;Anderson and Domsch 1975).Except for post-heat soils incubated at 25%WHC,the 200°C-exposed soils had less MBC than those exposed to 100°C(Fig.3).This was probably due to the decrease in extractable organic C at higher heating temperature(Guerrero et al.2005).In the different way, Pietika¨inen et al.(2000)found an increase of MBC after 1 month of incubation in 160°C-heated soils compared to 100°C-heated samples.
The interaction between soil f i re history,heating temperature and soil moisture played an important role in basal respiration except for the interaction between soil f i re history and soil moisture(Table 3).CO2evolution rate was generally greatest at 75%WHC in both soils with the exception of the unheated control soil without exposing to fi re previously(Fig.3).The lowest CO2evolution was observed in the f i re-exposed soils and incubated at 25% WHC.These differences in respiration likely resulted from differences in both microbial community structure and the availability of substrate(Pietika¨inen et al.2000).
Conclusion
Our study indicated that soil f i re history and soil moisture content at the time of heating were confounding factors in the interpretation of the inf l uence of heat on soil MBC and on basal respiration.The immediate effect of soil heating was a decrease in MBC.The loss of MBC at 200°C was signif i cantly higher than at 100°C.A signif i cant difference in the immediate loss of MBC between the f i re-exposed soils and those that were not previously exposed to f i re was also observed.The higher soil water content,in both soils, affected greatly MBC loss.Compared with f i re-exposed soils,the CO2evolution rate,following the heat-disturbance,was greater in soils without exposing to f i re previously.During 14 days of incubation,the lowest basal respiration and the minor MBC amount were observed in post-heat soils,incubated at 25%WHC and 75%WHC respectively.Even if the temperatures used in this experiment are lower than those commonly found in natural mineral soils,below 10 cm depth,our results clearly demonstrate the important role of soil moisture and prior fi re history on the effects of f i re on soil biochemical properties.Further investigations are needed to verify the effects over time.

Table 3 Analysis of variance (P values)of the effects of prior soil f i re history,soil heating temperature and soil moisture on microbial biomass C and CO2evolution in a 14-days post-heat treatment
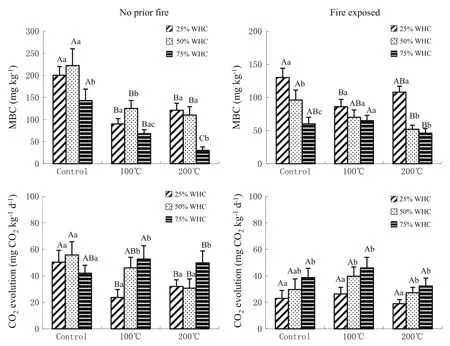
Fig.3 Soil microbial biomass C(MBC)and microbial respiration at 25,50 and 75%of water holding capacity(WHC)after the 14 days incubation of the two Chinese f i r soils exposed to different temperatures(25,100 and 200°C)(error bars indicate±SD). Signif i cant differences between moisture levels within each temperature treatment are labeled with different lowercase letters,while differences between three temperatures within each moisture level are labeled with different capital letters(P<0.05)
AcknowledgmentsThis work was f i nanced by the National Natural Science Foundation of China(No.31370615 and 31130013)and National Key Basic Research Program of China(2014CB954003). We thank Chengfang Lin and Xiaofei Liu for f i eld assistance and Weisheng Lin for lab assistance.We also thank the editor and anonymous reviewers for their helpful comments and suggestions.
Acea MJ,Prieto-Ferna´ndez A,Diz-Cid N(2003)Cyanobacterial inoculation of heated soils:effect on microorganisms of C and N cycles and on chemical composition in soil surface.Soil Biol Biochem 35:513–524
Albini FA,Reinhardt ED(1995)Modeling ignition and burning rate of large woody natural fuels.Int J Wildland Fire 5:81–91
Anderson JPE(1982)Soil respiration.In:Page AL,Miller RH,Keeny DR(eds)Methods of soil analysis,Part 2.Chemical and microbiological properties.American Society of Agronomy,Soil Science Society of America,Madison,pp 831–871
Anderson JPE,Domsch KH(1975)Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils.Can J Microbiol 21:314–322
Andersson M,Michelsen A,Jensen M,Kjøller A(2004)Tropical Savannah woodland:effects of experimental f i re on soil microorganisms and soil emissions of carbon dioxide.Soil Biol Biochem 36:849–858
Badı´a D,Martı´C(2003)Effect of simulated f i re on organic matter and selected microbiological properties of two contrasting soils. Arid Land Res Manag 17:55–69
Banning NC,Murphy DV(2008)Effect of heat-induced disturbance on microbial biomass and activity in forest soil and the relationship between disturbance effects and microbial community structure.Appl Soil Ecol 40:109–119
Ba´rcenas-Moreno G,Ba˚a˚th E(2009)Bacterial and fungal growth in soil heated at different temperatures to simulate a range of f i re intensities.Soil Biol Biochem 41:2517–2526
Boerner REJ,Huang J,Hart SC(2009)Impacts of Fire and Fire Surrogate treatments on forest soil properties:a meta-analysis approach.Ecol Appl 19:338–358
Campbell GS,Jungbauer JD Jr,Bidlake WR,Hungerford RD(1994) Predicting the effect of temperature on soil thermal conductivity. Soil Sci 158:307–313
Chen HYH,Shrestha BM(2012)Stand age,f i re and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests.Soil Biol Biochem 50:149–157
Choromanska U,DeLuca TH(2001)Prescribed f i re alters the effect of wildf i re on soil biochemical properties in a ponderosa pine forest.Soil Sci Soc Am J 65:232–238
Choromanska U,DeLuca TH(2002)Microbial activity and nitrogen mineralization in forest mineral soils following heating:evaluation of post-f i re effects.Soil Biol Biochem 34:263–271
De Marco A,Gentile AE,Arena C,Virzo de Santo AV(2005) Organic matter,nutrient content and biological activity in burned and unburned soils of a Mediterranean marquis area of southern Italy.Int J Wildland Fire 14:365–377
Dixon RK,Brown S,Houghton RA,Solomon AM,Trexler MC, Wisniewski J(1994)Carbon pools and f l ux of global forest ecosystems.Science 263:185–190
Dunn PH,Barro SC,Poth M(1985)Soil moisture effects on survival of microorganisms in heated chaparral soil.Soil Biol Biochem 17:143–148
Gee GW,Or D(2002)Particle-size analysis.In:Dane JH,Topp GC (eds)Methods of soil analysis,Part 4.Physical methods.Soil Science Society of America,Madison,pp 255–293
Guerrero C,Mataix-Solera J,Go´mez I,Garcı´a-Orenes F,Jorda´n MM (2005)Microbial recolonization and chemical changes in a soil heated at different temperatures.Int J Wildland Fire 14:385–400
Guo JF,Yang YS,Chen GS,Lin P(2005)Dissolved organic carbon and nitrogen in precipitation,throughfall and stemf l ow fromSchima superbaandCunninghamia lanceolataplantations in subtropical China.J Forest Res 16:19–22
Guo JF,Yang YS,Chen GS,Xie JS,Gao R,Qian W(2010)Effects of clear-cutting and slash burning on soil respiration in Chinese f i r and evergreen broadleaved forests in mid-subtropical China. Plant Soil 333(1–2):249–261
Hatten JA,Zabowski D(2009)Changes in soil organic matter pools and carbon mineralization as inf l uenced by f i re severity.Soil Sci Soc Am J 73:262–273
Hernandez T,Garcia C,Reinhardt I(1997)Short-term effects of wild fi re on the chemical,biochemical,and microbiological properties of Mediterranean pine forest soils.Biol Fertil Soils 25:109–116
Johnson DW,Curtis PS(2001)Effects of forest management on soil C and N storage:meta analysis.For Ecol Manag 140:227–238
Klopatek CC,DeBano LF,Klopatek JM(1990)Impact of fi re on the microbial processes in pinyon-juniper woodlands:management implications.In:Effects of fi re management of southwestern natural resources.USDA For.Serv.Gen.Tech.Rep.RM-191, pp.197–205
Loehman RA,Reinhardt E,Riley KL(2014)Wildland fi re emissions, carbon,and climate:seeing the forest and the trees—a crossscale assessment of wild fi re and carbon dynamics in fi re-prone, forested ecosystems.For Ecol Manag 317:9–19
Neary DG,Klopatek CC,DeBano LF,Ffolliott PF(1999)Fire effects on belowground sustainability:a review and synthesis.For Ecol Manag 122:51–71
Pietika¨inen J,Fritze H(1995)Clear-cutting and prescribed burning in coniferous forest:comparision of effects on soil fungal and total microbial biomass,respiration activity and nitri fi cation.Soil Biol Biochem 27:101–109
Pietika¨inen J,Hiukka R,Fritze H(2000)Does short-term heating of forest humus change its properties as a substrate for microbes? Soil Biol Biochem 32:277–288
Sakamoto K,Oba Y(1994)Effect of fungal to bacterial biomass ratio on the relationship between CO2evolution and total soil microbial biomass.Biol Fertil Soils 17:39–44
Santruckova H,Straskraba M(1991)On the relationship between speci fi c respiration activity and microbial biomass in soils.Soil Biol Biochem 23:525–532
Soil Survey Staff of USDA(1999)Soil taxonomy:a basic system of soil classi fi cation for making and interpreting soil surveys. United States Department of Agriculture(USDA),Natural Resources Conservation Service,Washington
SPSS Inc.(2004)SPSS 13.0.SPSS Inc,Chicago
State Soil Survey Service of China(1998)China soil.China Agricultural Press,Beijing(in Chinese)
Vance ED,Brookes PC,Jenkinson DS(1987)An extraction method for measuring soil microbial biomass C.Soil Biol Biochem 19:703–707
Yang YS(1998)Studies on the sustainable management of Chinese fi r plantations.China Forestry Press,Beijing,pp 24–41(in Chinese)
Yang YS,Guo JF,Chen GS,Xie JS,Gao R,Li Z,Jin Z(2005)Carbon and nitrogen pools in Chinese fi r and evergreen broadleaved forests and changes associated with felling and burning in midsubtropical China.For Ecol Manag 216:216–226
Yang YS,Guo JF,Chen GS,Yin YF,Gao R,Lin CF(2009)Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China.Plant Soil 323:153–162
Zhu HJ,Guo CD,Tan BH,Lin ZS,Chen ZG(1983)The fundamental characteristics of mountainous and hilly soils in southeast Fujian province.Acta Pedol Sin 20:225–237(in Chinese)
30 April 2014/Accepted:11 July 2014/Published online:22 July 2015
Project funding:This work was f i nanced by the National Natural Science Foundation of China(No.31370615 and 31130013)and National Key Basic Research Program of China(2014CB954003).
The online version is available at http://www.springerlink.com
Corresponding editor:Chai Ruihai.
✉Jianfen Guo jfguo@fjnu.edu.cn
1Key Laboratory for Subtropical Mountain Ecology,School of Geographical Science,Fujian Normal University, Fuzhou 350007,China
2Institute of Geography Science,Fujian Normal University, Fuzhou 350007,China
杂志排行
Journal of Forestry Research的其它文章
- Drone remote sensing for forestry research and practices
- Life cycle environmental impact assessment of biochar-based bioenergy production and utilization in Northwestern Ontario, Canada
- Growth rates of Eucalyptus and other Australian native tree species derived from seven decades of growth monitoring
- Effect of f i rst thinning and pruning on the individual growth of Pinus patula tree species
- The inf l uence of selective cutting of mixed Korean pine(Pinus koraiensis Sieb.et Zucc.)and broad-leaf forest on rare species distribution patterns and spatial correlation in Northeast China
- Modeling forest f i res in Mazandaran Province,Iran
