Non-structural carbohydrate levels of three co-occurring understory plants and their responses to forest thinning by gap creation in a dense pine plantation
2015-06-05ZheWangWeikaiBaoXiaoliYan
Zhe Wang•Weikai Bao•Xiaoli Yan
Non-structural carbohydrate levels of three co-occurring understory plants and their responses to forest thinning by gap creation in a dense pine plantation
Zhe Wang1,2,3•Weikai Bao1•Xiaoli Yan1
We investigated non-structural carbohydrates (NSC)levels and components(starch,glucose,fructose and sucrose)in the leaves of three typical co-occurring forestfloor plants,moss Eurhynchium savatieri(ES),fern Parathelypteris nipponica(PN)and forb Aruncus sylvester (AS)in a 30-year-old Chinese pine(Pinus tabulaeformis) plantation forest on the eastern Tibetan Plateau.We also explored their responses to three gap creation treatments (controland two gap creations of 80 and 110 m2)based on NSC levels.PN had the highest leaf NSC levelof the three plants,with AS second and ES lowest.Starch was the predominant component of NSC and the contents of glucose were higher than those of fructose or sucrose for all three species.The NSC level of ES in intermediate gaps was significantly higher than at control sites.PN also hadhigher NSC levels in both small and intermediate gaps than in control sites.But the differences between treatments were not obvious for AS.Our results suggest that ES and PN benefit from gap formation while the two species have different NSC response sensitivities to gap size,butthe leaf NSC level of AS is less sensitive to the disturbance.
Forest-floor plant·Gap thinning·Light radiation·Moss·Non-structural carbohydrates
Introduction
Non-structural carbohydrate(NSC)level is an important indicator to elaborate the carbon source and sink capacities of plants,and to a certain extent,can reflect their ecophysiological and growth status(Ko¨rner 2003;Wurth et al. 2005).Many researchers focused on trees and shrubs in forest in recent decades(Ko¨rner 2003;Wurth et al.2005; Palacio et al.2007;Li et al.2008;Palacio et al.2008). Nevertheless,we know little about the NSC levels of forest-floor herbaceous plants.Most previous studies only concerned species from single groups and comparative studies of plants with different growth forms have been few.Therefore,the first objective of the current research was to quantify and compare the NSC levels of understory plants in different growth form groups.
Forest thinning can improve environmental conditions of habitats such as the diurnal patterns,air temperature, vapor pressure deficit,and illumination,and can have great impacts on understory plants(Rambo and North 2009). However,most previous studies only focused on climatic fluctuation and vegetation renewalin the gap sites(Kariuki et al.2006;Milakovsky et al.2011).Thus we have limitedinsightinto the effectof gap formation on the physiological status of forest-floor herbaceous plants such as mosses, ferns and forbs.Therefore,our second objective was to study the responses to gap thinning of forest-floor herbaceous plants according to their NSC levels.
We addressed the following two questions:Whatare the differences in NSC levels and NSC components[Soluble sugars(SS)and starch]between different growth forms (moss,fern and forb);and what are the different responses of these plants to forestthinning according to their levels of NSC and its components.We hypothesized that in comparison to ferns and forbs,the NSC level of the shadetolerant mosses would be lower because its photosynthetic capacity and NSC accumulation ability are relatively weak (Glime 2007;Waite and Sack 2010).Furthermore,since light is most likely to be the limiting factor in dense plantations,we presumed that NSC levels of these plants should be promoted by slightthinning because the resulting higher light intensity and longer irradiation duration could increase the NSC concentration in plant leaves(Montgomery and Chazdon 2002;Kuptz etal.2010;Zhang et al. 2013).We also hypothesized that NSC levels of mosses would be lower in intermediate-sized gaps compared than in small gaps because the poikilohydric mosses might be more sensitive to the increased evaporation rate and light intensity caused by larger gap size(Marschall and Proctor 2004;Glime 2007).
Materials and methods
Focal species
We planned to select at least 12 understory plants(including mosses,ferns,forbs and sedges)growing in the experimentalforest.However,due to the dense tree canopy cover,only a few understory species grew.Finally only three co-occurring forest-floor plants,Eurhynchium savatieri(ES),Parathelypteris nipponica(PN)and Aruncus sylvester(AS)were chosen.ES is a slender moss forming loose or dense mats on the soil,tree bases and rocks in moist habitats.PN is a fern with long creeping rhizomes, 40–60 cm in height and with leaves about 30-40 cm in length and 7–10 cm in width.This plant mainly grows under mature forest in hilly regions at elevations between 400 and 2500 m.And AS is a perennial forb,about 50–70 cm in height with leaves 5–13 cm in length and 2–8 cm in width.AS usually lives in open habitats under mixed forests on montane slopes and in valleys.Our nomenclature follows Flore of China and Flora Bryophytorum Sinicorum.Specimens were deposited in the herbarium at the Chengdu Institute of Biology,CAS.
Experimental layout and sample collection
We undertook field work at the thinning experimental sites of a 30-years-old Chinese pine(Pinus tabulaeformis) plantation forest near the Maoxian Mountain Ecosystem Research Station of Chinese Academy of Sciences at Fengyi Township of Maoxian County,Sichuan,China (103°54′E,31°42′N,1826 m a.s.l.).The region has a typical temperate climate with annual sunshine time of 1373.8 h,annual mean temperature of 9.3°C,annual precipitation of 825.2 mm and annual evaporation of 968.7 mm.The thinning experiment had three treatments (control,small gap and intermediate gap with gap areas of 0,80 and 110 m2,respectively)and three replicates(totally six gaps and three controls)on similar blocks(Jiang et al. 2011).
We measured fundamental environmental parameters at the sites before sample collection.Photosynthetically active radiation(PAR)was measured by Skye Instruments type SKR1800 dual channel radiometers(Skye Instruments,Llandrindod,Powys,UK)at several spots in the center of each site.Temperature and moisture at soil surface were also measured using a temperature/humidity data logger(DS1923 iButton,Maxim Integrated Products).All parameters were synchronously obtained.The data indicated that PAR,temperature and moisture at soil surface were significantly higher in intermediate and small gaps than in the controls(Table 1).All monitored parameters were similar at the two gap treatments.
We collected samples from three sampling points in the central zone of each site(about 2-g fresh weight for each sample)on 21 September 2010,a sunny day.We collected only mature leaves at the top layer of PN and AS,and ES tissues without cover from other plants or litter.The samples were stored in a refrigerator(about 4°C)and brought to the station laboratory within 3 h.Only green tissues of ES,the 10–20th pinna from the leaf apexes of PN and the firstpair of leaves behind the spiry leaf of AS were selected as the final samples.All samples were swiftly washed with deionized water and put into a pre-heated oven at 115°C for 10 min to stop physiological activities. Samples were then oven-dried at 65°C for 48 h.Dried samples were ground and stored at-4°C.
Chemical analysis
The method of Baninasab and Rahemi(2006)was adopted for sample pre-treatment.HPLC with evaporative lightscattering detector was used to measure the concentrations of sugars:glucose,fructose and sucrose(Sun et al.2004). Starch content was determined using an anthrone colorimetric protocol following Li et al.(2008).All results were recorded on a dry weight basis(mg g-1).

Table 1 Photosynthetically active radiation(PAR),soil temperature and moisture at soil surface in three thinning treatments of Chinese pine plantation forest at Maoxian County,southwestern China
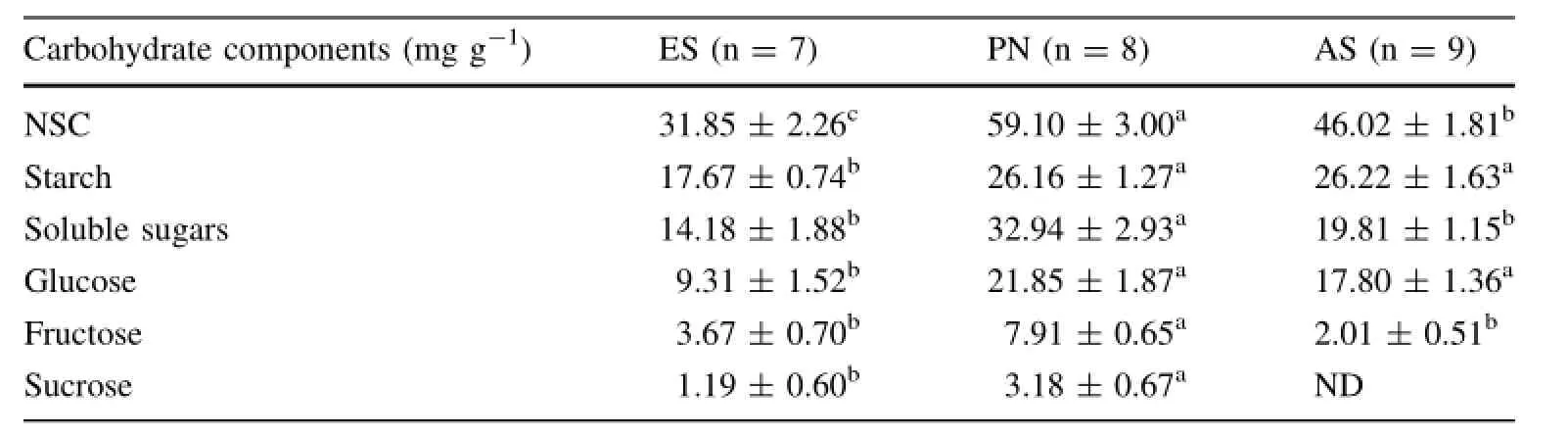
Table 2 Non-structural carbohydrate(NSC) concentrations for three understory plant species from Chinese pine plantation forestat Maoxian County,southwestern China
Statistical analysis
The SS contentof each sample was calculated as the sum of glucose,fructose and sucrose.And NSCs were counted by summing values for starch and SS.Data for allcomponents (NSC,starch,SS,glucose,fructose and sucrose)were checked for normality and homoscedasticity prior to statistical analyses.Sucrose concentration values were transformed by ln(y+1)to meet the requirement of normal distribution.Data were analyzed by two-way ANOVA with species and thinning treatment as the main factors.LSD multiple comparison tests were used to compare differences between means and the Games-Howell post hoc test was used when the data did notmeetthe ANOVA assumption of homogeneity of variance.All statistical analyses were performed using SPSS(version 16.0)and Origin(version 8.6).Allvalues were considered significantwhen p≤0.05.
Results
NSC level and proportion

Fig.1 Non-structural carbohydrate(NSC)proportions of three understory species in Chinese pine plantation forests at Maoxian County,southwestern China.ES:Eurhynchium savatieri;PN: Parathelypteris nipponica;AS:Aruncus Sylvester

Fig.2 The ef fec ts of ga p c rea ti on on con cen tr ati ons of no n-str uct ura l car boh ydr ate(NSC)and it s c omp on ent s(m g g-1)fo r t hre e u nde rsto ry pla nt spe ci es und er a p in e p lan tat ion a t Maox ian Co un ty, Sou thw est ern Ch in a.Eurh ync hiu m sav ati eri—■,Pa rat hel ypt eri s nip pon ica—▲a nd Ar unc us syl ve ste r—○.Th e l ow erc ase le tte rs ind ica te dif fer en ces am ong th e thr ee tre atm en ts for ea ch sp eci es (p≤0.0 5)
Levels of NSC and its components varied significantly among the three understory plants(Table 2).PN had the highest NSC level,with AS second and ES lowest.PN and AS had similar starch levels and both had significantly higher starch concentrations than ES.ES and AS had similar SS levels,both significantly lower than that of PN (Table 2).Three species also had different levels of glucose and fructose,ranking as PN≅AS>ES and PN>AS≅ES,respectively.PN had higher sucrose levels than ES and sucrose concentrations in the leaves of AS were too low to be detected(Table 2).For all of these understory species,starch was the predominant component of NSC and the level of glucose was higher than the other two sugars(Fig.1).Starch contents of ES and AS were higher than SS while the pattern was reversed for PN.
Response of NSC to thinning
The NSC level of ES was higher at intermediate gaps than at control sites but there were no significant differences between the NSC levels at small gaps and at the other two treatments.PN had higher NSC level at the thinning gaps than at the control sites.NSC levels for AS were similar at alltreatments(Fig.2a).There were no differences in starch levels among treatments for all three plants(Fig.2b; Table 3).Much higher contents of glucose,fructose,sucrose and thus SS were recorded at the two gap treatments than at the control sites for PN but not for the other two species(Fig.2c–e).The glucose level of AS at intermediate gaps was lower than at control sites but the difference was not significant(p=0.053).For all three species,levels of NSC and its components were similar for two gap treatments(Fig.2).

Table 3 Statisticalresults of two-way ANOVA analysis on effects of species and thinning treatments for the levels of non-structural carbohydrates(NSC)and its components
Discussion
Levels of NSC and its components among species
The varied NSC levels of the co-occurring understory plants reflect their different carbon supply status(Wurth et al.2005).Here we firstly focus on moss and compare it with other herbaceous species under the weak light environment.Our results supported the initial hypothesis that the moss ES had a lower NSC level than fern or forb (Table 2).The leaf NSC concentration of a plantis closely associated with its inherent photosynthetic trait,source/ sink capacity and the environmental light condition (Ko¨rner 2003;Wurth etal.2005;Palacio etal.2007,2008). We deduce thatthe low NSC concentration of the moss can be attributed to three reasons.The first is that the light saturation points and net photosynthetic rates of most mosses are relatively low(Gabriel and Bates 2003;Waite and Sack 2010).Second,given a lack of nutritive tissue and a typically slow growth rate,the carbon sink demand of moss is lower and easier fulfilled than that of vascular plants(Glime 2007).Third,weak light radiation may also account for the low NSC level of ES because this moss lives on the soil surface while the leaves of PN and AS were about 50 cm above the ground surface at our study sites.Moreover,PN had higher NSC levels in leaves than did AS under the dense pine forest.
Responses of NSC to thinning
Forest thinning by gap creation can significantly increase light intensity,prolong photoperiod,extent the diurnal range of temperature and vapor pressure deficit(Rambo and North 2009)thus greatly improving the survival and growth of understory plants(Milakovsky et al.2011).Our results clearly revealed that different forest-floor plants (moss,fern and forb)showed distinct responses to the thinning treatments according to their levels of NSC and its components.For ES,although there were no significant differences in starch,glucose,fructose,sucrose and SS among treatments,the NSC level at intermediate gaps was significantly higher than at control sites(Fig.2).This suggests that the shade under the dense pine plantation is not the optimal environment for ES and it can adapt to better lighted habitats.The similarity of NSC levels of ES at small gaps and at control sites indicates that creation of smallgaps was insufficientto increase the NSC levelin ES. In contrast,PN had a significantly higher NSC level at small gaps than at control sites suggest that it was more sensitive than ES to gap thinning.Furthermore,except for starch,the levels of glucose,fructose,sucrose and thus SS of PN were all increased with increasing gap sizes(Fig.2 b–f).These results demonstrate that the metabolic activity of PN is stronger in gaps than in understory because the increase of NSC levels is due to SS,not starch(Palacio et al.2007).AS did not differ in any of the NSC components among the three treatments,indicating that this species was less sensitive to the impact of moderate thinning. Moreover,for the three species,the similar NSC levels between the intermediate gaps and the small gaps(Fig.2a) could be attributed to light saturation due to increasing gap size(Zhu et al.2010)or perhaps due to the sink regulation of the photosynthetic process(Paul and Foyer 2001;Kasai 2008).Taken together,our results only support part of the initial prediction that leaf NSC levels of ES and PN would be promoted by slight thinning.However,there was no indication that the intermediate gap restricted the accumulation of NSC for the moss.Conversely,the gap creation at intermediate size may be a better scheme for ES and PN than creation of smaller gaps.
In conclusion,the moss ES had the lowestlevels of NSC and its components while the fern PN had highest levels among the three species under the pine plantation forest (Table 2).Furthermore,the NSC levels of ES in intermediate gaps and PN at both gap sites were significantly higher than at control sites.There were no significant differences among treatments for the forb AS.Our results suggest that ES and PN can benefit from the lighter and warmer habitats created by gap formation in the understory of the pine plantation.In contrast,AS proved less sensitive to gap formation.
AcknowledgmentsWe greatly appreciate Mr.Yufei Lifor assisting HPLC analysis,Dr.Xueyong Pang and Qihua He for providing the background information.
Baninasab B,Rahemi M(2006)Possible role of non-structural carbohydrates in alternate bearing of pistachio.Eur J Hortic Sci 71:277–282
Gabriel R,Bates JW(2003)Responses ofphotosynthesis to irradiance in bryophytes of the Azores laurel forest.J Bryol25:101–105
Glime JM.2007.Vol.1.Physiological Ecology.Chapter 6 Limiting factors and limits of tolerance.Bryophyte Ecology.Ebook sponsored by Michigan Technological University and the International Association of Bryologists.Accessed on 16 August 2010 at http://www.bryoecol.mtu.edu/
Jiang Y,Pang X,Bao W(2011)Soil microbial biomass and the influencing factors under Pinus tabulaeformis and Picea asperata plantations in the upper Minjiang River.Acta Ecol Sin 31:801–811(In Chinese)
Kariuki M,Kooyman RM,Smith RGB,Wardell-Johnson G,Vanclay JK(2006)Regeneration changes in tree species abundance, diversity and structure in logged and unlogged subtropical rainforestover a 36-Year period.For Ecol Manag 236:162–176
Kasai M(2008)Regulation of leaf photosynthetic rate correlating with leaf carbohydrate status and activation state of rubisco under a variety of photosynthetic source/sink balances.Physiol Plant 134:216–226
Ko¨rner C(2003)Carbon limitation in trees.J Ecol 91:4–17
Kuptz D,Grams TEE,Gunter S(2010)Light acclimation of four native tree species in felling gaps within a tropical mountain rainforest.Trees 24:117–127
Li MH,Xiao WF,Shi PL,Wang SG,Zhong YD,Liu XL,Wang XD, Cai XH,Shi ZM(2008)Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations.Plant,Cell Environ 31:1377–1387
Marschall M,Proctor MCF(2004)Are bryophytes shade plants? Photosynthetic lightresponses and proportions of chlorophyll a, chlorophyll b and total carotenoids.Ann Bot 94:593–603
Milakovsky B,Frey BR,Ashton MS,Larson BC,Schmitz OJ(2011) Influences of gap position,vegetation management and herbivore control on survival and growth of white spruce(Picea glauca(Moench)Voss)Seedlings.Forest Ecol Manag 261:440–446
Montgomery R,Chazdon R(2002)Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps.Oecologia 131:165–174
Palacio S,Maestro M,Montserratmarti G(2007)Seasonal dynamics of non-structural carbohydrates in two species of mediterranean sub-shrubs with different leaf phenology.Environ Exp Bot 59:34–42
Palacio S,Milla R,Albuixech J,Pe´rez-Rontome´C,Camarero JJ, Maestro M,Montserrat-Martı´G(2008)Seasonal variability of dry matter content and its relationship with shoot growth and nonstructuralcarbohydrates.New Phytol 180:133–142
Paul MJ,Foyer CH(2001)Sink regulation of photosynthesis.J Exp Bot 52:1383–1400
Rambo TR,North MP(2009)Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest.For Ecol Manag 257:435–442
Sun YA,Wang GQ,Zhang YJ,Yan KY(2004)Determination of the water-soluble carbohydrates in tobacco by Hplc-Elsd.Fenxi Kexue Xuebao 20:531–533(in Chinese)
Waite M,Sack L(2010)How does moss photosynthesis relate to leaf and canopy structure?Trait relationships for 10 Hawaiian species of contrasting light habitats.New Phytol 185:156–172
Wurth MK,Pelaez-Riedl S,Wright SJ,Korner C(2005)Nonstructural carbohydrate pools in a tropical forest.Oecologia 143:11–24
Zhang M,Zhu JJ,Li MC,Zhang GQ,Yan QL(2013)Different light acclimation strategies oftwo coexisting tree species seedlings in a temperate secondary forest along five natural light levels.For Ecol Manag 305:234–242
Zhu XG,Long SP,Ort DR(2010)Improving photosynthetic efficiency for greater yield.Annu Rev Plant Biol 61:235–261
21 February 2014/Accepted:21 March 2014/Published online:30 April 2015
ⒸNortheast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Project funding:This work was financially supported by the Strategic Priority Research Program of the CAS(No.XDA05070306)and the National Science&Technology Pillar Program in 12th 5-year Plan of China(No.2011BAC09B0402).
The online version is available at http://www.springerlink.com
Corresponding editor:Yu Lei
✉Weikai Bao baowk@cib.ac.cn
1Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization,Chengdu Institute of Biology, Chinese Academy of Sciences,Chengdu 610041,China
2Key Laboratory for Bio-Resource and Eco-environment of Ministry of Education,College of Life Science,Sichuan University,Chengdu 610064,China
3University of Chinese Academy of Sciences,Beijing 100049, China
杂志排行
Journal of Forestry Research的其它文章
- Production of mahogany sawdust reinforced LDPE wood-plastic composites using statistical response surface methodology
- Self-thinning lines and allometric relation in Chinese fir (Cunninghamia lanceolata)stands
- Estimation of above-ground biomass and carbon stock of an invasive woody shrub in the subtropical deciduous forests of Doon Valley,western Himalaya,India
- Carbon stock and rate of carbon sequestration in Dipterocarpus forests of Manipur,Northeast India
- Cadmium and lead effects on chlorophyll fluorescence, chlorophyll pigments and proline of Robinia pseudoacacia
- Variability in cone,seed and seedling characteristics of Pinus kesiya Royle ex.Gordon
