Inhalation of nanoparticle-based drug for lung cancer treatment:Advantages and challenges
2015-05-16WingHinLeeChingYeeLooDanielaTrainiPaulYoung
Wing-Hin Lee*,Ching-Yee Loo,Daniela Traini,Paul M.Young
Respiratory Technology,Woolcock Institute of Medical Research and Discipline of Pharmacology,Sydney Medical School,The University of Sydney,NSW 2037,Australia
Inhalation of nanoparticle-based drug for lung cancer treatment:Advantages and challenges
Wing-Hin Lee*,Ching-Yee Loo,Daniela Traini,Paul M.Young
Respiratory Technology,Woolcock Institute of Medical Research and Discipline of Pharmacology,Sydney Medical School,The University of Sydney,NSW 2037,Australia
ARTICLE INFO
Article history:
Received 24 June 2015
Received in revised form 9 August 2015
Accepted 16 August 2015
Available online 9 September 2015
Inhalation
Ever since the success of developing inhalable insulin,drug delivery via pulmonary administration has become an attractive route to treat chronic diseases.Pulmonary delivery system for nanotechnology is a relatively new concept especially when applicable to lung cancer therapy.Nano-based systems such as liposome,polymeric nanoparticles or micelles are strategically designed to enhance the therapeutic index of anti-cancer drugs through improvement of their bioavailability,stability and residency at targeted lung regions.Along with these bene fi ts,nano-based systems also provide additional diagnostic advantages during lung cancer treatment,including imaging,screening and drug tracking.Nevertheless,delivery of nanobased drugs via pulmonary administration for lung cancer therapy is still in its infancy and numerous challenges are expected.Pharmacology,immunology,toxicology and largescale manufacturing(stability and activity of drugs)are some aspects in nanotechnology that should be taken into consideration for the development of inhalable nano-based chemotherapeutic drugs.This review will focus on the current inhalable nano-based drugs for lung cancer treatment.
©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Cancer is a leading cause of disease worldwide.Lung,female breast,colorectalandstomachcancersaccountedformorethan 40%ofcancercasesdiagnosedworldwide;withtheWorldHealth Organization reporting an estimated 14.1 million new cancer cases worldwide in 2012[1,2].Among them,lung cancer is one of the most common,with 16.7%of all new cases diagnosed in men[1].In Australia alone,lung cancer has accounted for over 11,000 new cancer cases in 2012.Additionally,lung cancer is the most common cause of cancer-related death for men and women and the fi nancial burden to the healthcare system is estimated at>100 million dollars annually in Australia[2]. Importantly,lung cancer has the highest mortality rate of all common cancers and a miserable dismal rate of less than 5 years[2].Out of the 8.2 million deaths caused by cancer in 2011 globally,mortality from lung cancers contributed the highest,with 1.3 million deaths alone[2].Historically lung cancer has been linked to smoking and consequently classi fi ed as a social disease with a stigma attached[3].Contrary to popular belief, lung cancer not only affects smokers but also non-smokers. For example,in women,only 65%of cancer deaths can be attributed to smoking,withlungcancerkillingmorewomenthan breast,uterine,and ovarian cancers combined in women.Irrespective of cause,mortality from lung cancer is high;with only15%oflungcancerpatientssurvivingformorethan5years after diagnosis[4].Clearly there is a lack of diagnostics and effective treatment regimes.
Surgery,chemotherapy and radiation are standard treatment options for lung cancer depending on the stage of malignancy,resectability and overall performance[5].Chemotherapy is a fi rst-line treatment for advanced stage of lung cancer in which chemotherapeutic drugs are usually administered intravenously for systemic circulation[6,7].The use of chemotherapeutic drug is based on the principle of toxic compounds to inhibit the proliferation of cells growing at an abnormal rate.Combination of gemcitabine(FDA approved chemotherapeutic agent)with cisplatin has been widely used for fi rst or second line treatments of patients with advanced or metastatic lung cancer[8].In addition,common chemotherapeutic drugs such as paclitaxel,docetaxel and gemcitabine,and vinorelbine are widely used in combination with platinumbased drugs to(i.e.cisplatin)improve therapeutic index[8-10]. However,it should be noted that majority of chemotherapy drugs is associated with side effects such as pain,nerve damage and skin allergic reactions.Therefore,minimizing the side effects of chemotherapy drugs remains a challenge in the fi eld of cancer chemotherapy.
2. Challenges and advantages involved in the delivery of inhalation therapeutic drugs
Lung offers numerous advantages as a delivery route for noninvasive drugs especially for localized therapy,i.e.lung cancer and treatment of airway diseases such as asthma,cystic fibrosis and chronic obstructive pulmonary disease(COPD)[11]. Compared to other delivery methods such as oral or intravenous injection,it is envisaged that the bioavailability of drugs in lung could be enhanced using pulmonary delivery since lung possesses limited intracellular and extracellular drugmetabolizing enzyme activities unlike gastrointestinal tract and liver[12].On top of that,this option also reduces non-reversible tissue damage caused by drugs’cytotoxicity[12].In addition, higher absorption rate,reduced drug doses and rapid onset of action are among the advantages of pulmonary administration[11,12].The bio-barriers existing in the respiratory airway systems such as mucus,ciliated cells and resident macrophages are effective to limit the localization,penetration and adsorption of drugs in the lung[13].The clearance mechanisms of inhaled drugs are activated depending on the location of deposited drug.Drug localized at the upper airways are removed by ciliated cells in the epithelia region while those in lower airways were protected by resident alveolar macrophages[14,15].Resident alveolar macrophages detect the presence of foreign particles,followed by engulfment via phagocytosis and fi nally digestion in lysosomal of macrophages.The bioavailability of anti-cancer drugs to cancer cells provides an indirect re fl ection of success rate of therapy.To achieve this, we should determine the key factors that affect the bioavailability of drug in lungs such as aqueous solubility,dissolution rate,ef fl ux of drugs and drug clearance by alveolar macrophages.Table 1 outlines the factors involved in determining the bioavailability of drugs into tumor cells.
For an effective drug targeting involving lung,various parameters matter which include:(i)deposition and localization of drugs onto targeted area or cells,(ii)penetrability of drugs through airway mucus,(iii)ability to escape from mucociliary clearance,(iv)transportable across epithelial cells into blood stream for systemic exposure,(v)low entrapment and inactivation of drugs by bacterial bio fi lms in cases of infections, (vi)tunable phagocytic activity by alveolar macrophages and (vii)minimal host protein-drug molecule interactions[13,23]. In physiological conditions,mucus,a viscoelastic gel,is secreted to protect cellular surfaces and maintain water balance. These mucus layers act as fi lter to remove pathogens while allowing gas and nutrient exchange within the underlying epithelial cells(extensively reviewed in[24]).Therefore,mucus production by epithelial cells is a physical barrier and ratelimiting step for drug targeting[25].For instance in CF patients, the over-production of highly viscous mucus effectively limits thebioavailabilityofdrugmolecules.Additionallyinhaleddrugs may be trapped within the mucus and subsequently removed with multiple clearance mechanism.Adhesion interactions usually occur between mucus and drug particles via electrostatic,hydrophobicandhydrogenbonding[26].Itisdocumented that mucus in CF patients is negatively charged and displayed highbindingaf fi nitiestowardpositivelychargedaminoglycosides (tobramycin)[27].More than 10-fold increases in mucus production were also observed in chronic bronchitis patients compared to healthy subjects.It is therefore useful to manipulatethephysicochemicalpropertiesofdrugssuchassurface charges or hydrophobicity to avoid the entrapment of drugs in the mucus and increase the penetration of drugs across the mucus layers.Following the entrapment of deposited drug particles or foreign particles in the mucus is the elimination viamucociliary clearance(extensively reviewed in[24]).The major roles of mucociliary agents are(i)to act as mechanical fi lter to entrap particles in the surface liquid onto the airway epithelium and clear by ciliary action.Most insoluble particles,with aerodynamic diameter larger than 6 μm,are eliminated by mucociliaryclearance.Meanwhile,nano-sizedparticlesareable to travel faster to reach bronchial epithelial region and escape the action,(ii)to provide antioxidant activities with its surface liquid,and(iii)toprovideasurfacebiologicalinteractionbetween microorganisms with luminal in fl ammation cells in order to prevent bacterial migration to airway epithelial cells.The clearance mechanistic is facilitated by hair-shaped structure like cilia,which are present on the topside of epithelial cells.Once foreign particles are trapped in mucus,cilia beat in a coordinated direction(pharynx)to remove the freight either by coughing or swallowing(extensively reviewed in[28]and[29]). Besides mucociliary clearance,phagocytosis is another major mechanism that is involved in the clearance of foreign particles in the lungs[30].
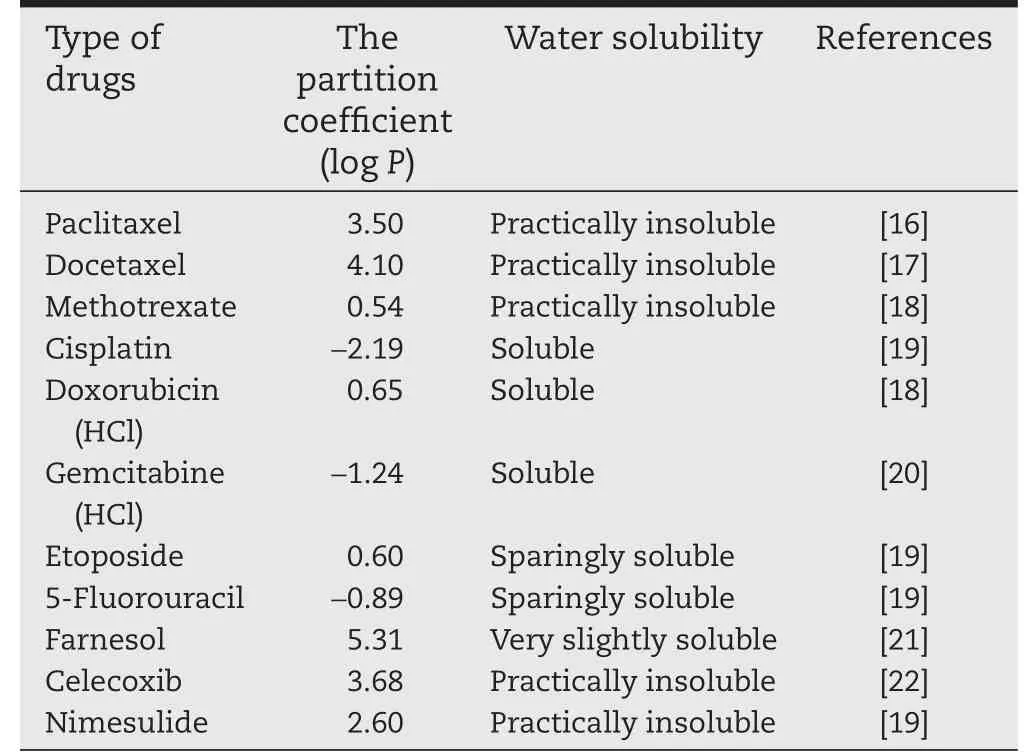
Table 1-Water solubility and partition coef fi cient of anti-cancer drugs for lung cancer treatment.
2.1. Formulation aspects:physicochemical properties of anti-cancer drugs
The physiochemical properties of drugs play an important role in therapy index.For that,most anti-cancer drugs are poorly soluble in aqueous physiological condition at pH 7.4.These include taxane-based drugs(paclitaxel and docetaxel)and camptothecin derivatives(9-nitrocamptothecin).The fundamental properties of anticancer drug such as log P and pKa values are important for designing the delivery methods as well as clearance from the lungs.According to Lipinski’s rule,the solubility of anti-cancer drug would affect their permeability and potency of the cancer treatment[31,32].Table 2 provides the water solubility and log P values of the anti-cancer drugs that are used in lung cancer treatment.
2.2. Host related response:ef fl ux proteins
There are fi ve major modalities of anticancer drug resistance:decreased drug in fl ux,increased drug ef fl ux,activation of DNA repair,detoxi fi cation and inactivation of apoptosis pathway.ATP-binding cassette(ABC)transporters represent a large family of trans-membrane proteins,which is an ATP-dependent ef fl ux system expelling anticancer drugs from cytoplasm out from the cells.This is a normal defense mechanism from cancer cells to maintain their survival rate as ef fl ux proteins act to pump out or decrease the intracellular concentration of anti-cancer drugs in cancer cell.However,due to their defense mechanism,severe multidrug resistance issue has occurred for cancer treatment.This means that the ef fl ux of the anti-cancer drugs is regulated with transmembrane ef fl ux proteins including P-glycoprotein(P-gp),multidrug resistant associated proteins(MRPs)and breast cancer resistant protein (BRCP)in cancer cells.P-gp is encoded by multidrug resistance-1 (MRP-1)gene and is one of the most studied ef fl ux transporters for drug delivery into lung.It is present on the apical membrane of the bronchial and bronchiolar epithelium,in the endothelial cells of the bronchial capillaries and alveolar macrophages.It is accepted that interaction of anti-cancer drug with drug-binding domains of the transporter resulted in ATP hydrolysis at nucleotide-binding domains of the protein.Such hydrolysis reduced the af fi nity of drug and stimulated drug expelling from the cancer cells.In addition,treatment of other lung disease such as cystic fi brosis contributed to the upregulation of P-gp level but this situation was not present for patients with COPD.This information is relatively important for lung cancer treatments,as most of the lung cancer patients are associated with COPD.
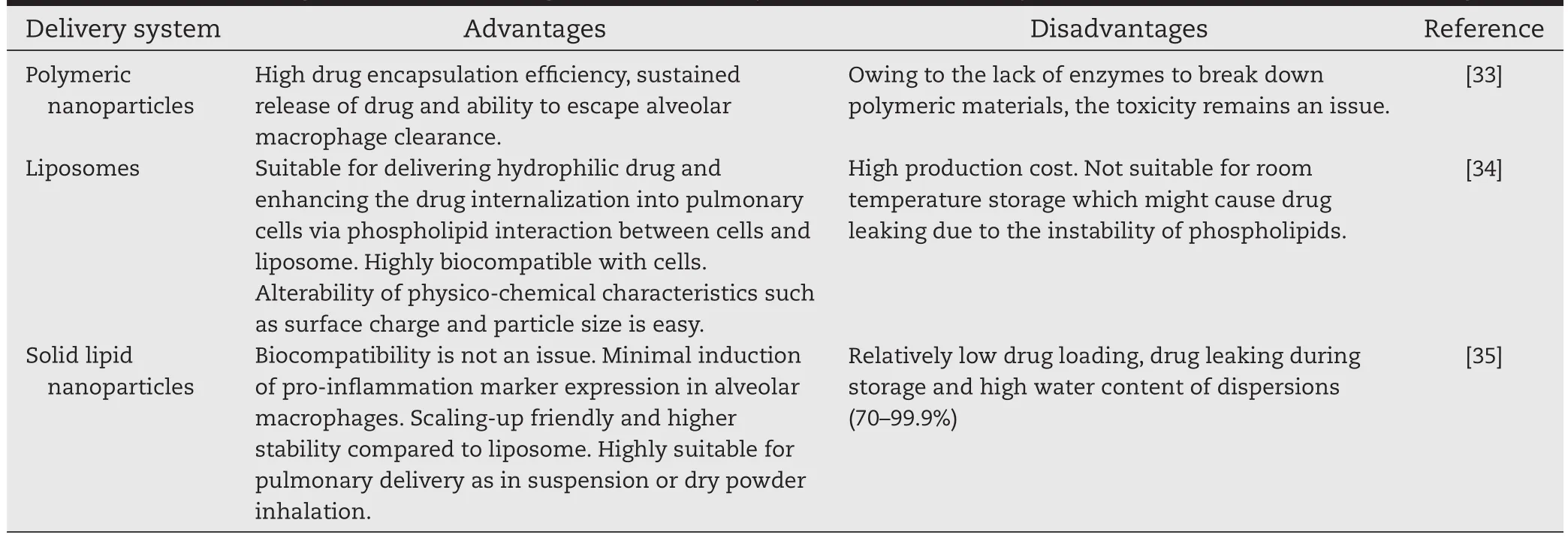
Table 2-The advantages and disadvantages of few nanosystems for pulmonary administration of anti-cancer drugs.
2.3. Aerosol drug delivery approaches
Systemic administration of anti-cancer drugs is a commonly used route to treat lung cancer even though this method often results in sub-optimal therapeutic concentration of drugs at tumor regions and damages to healthy cells/organs.Therefore local delivery via inhalation is a suitable alternative to deliver higher local drug concentration to the speci fi c target side.Another advantage of pulmonary delivery is that it enables the delivery of low doses of inhaled drugs to lungs for localized effect while signi fi cantly reduces the toxicity of drugs toward organs or healthy cells.To date,four clinically successful aerosol pulmonary delivery systems based on devices includedry powder inhaler(DPI),nebulizer,pressurized metered dose inhaler(pMDI)and soft-mist inhalers,and have been described in detail elsewhere[36].pMDI and nebulizers are liquidbased aerosol systems whereby only the former requires the use of a propellant such as hydro fl uoroalkanes(HFAs)and released through an ori fi ce as spray upon actuation at a high velocity of 0.30 msec.However,it is reported that 10-20%of emitted aerosol could reach the lung parenchyma probably owing to lack of hand-mouth coordination and inspiratory fl ow rate.Meanwhile nebulized drug formulation often exists as solution or suspension without propellant and then atomized into droplets.
Nebulizers have been used many years in hospital setting to treat respiratory diseases and are feasible for elderly as well as young children(under 2 years of age).This technique is based on the principle of generating aerosol droplets from suspension or solution drugs and does not require specialized inhaled coordination.In contrast to pMDI and DPI,nebulizers can be used in cases where patients are unable to control their breathing or while receiving mechanical ventilation.In the case for treating lung cancer,nebulizers present stronger attractive possibilities for the delivery of chemotherapeutic drugs,especially for drug formulations developed as nano-sized particles in suspensions.Nebulizers are preferred as they could deliver larger amount of aerosolized drug in small droplets continuously over extended time periods.In theory nebulizers should generate high fi ne particle fraction(FPF)for satisfactory lung deposition,thus ensuring low doses required.In addition an ideal nebulizer should maintain drug stability and does not provoke any changes in formulation during nebulization process[37]. Several parameters have been identi fi ed to affect the ef ficiency of nebulized solution such as solution pH,viscosity, surface tension,drug concentration,and osmolarity.Other factors include the design of the device itself such as emptying(nebulization)rate and aerosol fl ow rate.Soft-mist inhalers are novel inhalers and are poised to overcome limitations of pMDI,DPI and nebulizers.Soft-mist inhalers are propellantfree,spacer-free and utilize the mechanical energy from the spring to actuate metered drug solution.It has been reported that the optimal aerosol generation by soft-mist inhaler is inspiratory fl ow rate independent while simultaneously could generate higher FPF compared to pMDI,DPI or nebulizers.In addition,the aerosol generated travelled much slower and had longer duration than aerosol from a pMDI[38].
The development of DPI has originally meant for overcoming poor actuation-inhalation coordination to treat lung diseases such as asthma and COPD.The advantages of DPI include high stability,non-invasive,sustained release pro fi le to achieve modifi ed pharmacokinetics,rapid onset of action,and without the use of propellant.In addition,for delivery of hydrophobic drugs with low tolerance to shear,formulations in the form of dried powder seem to be ideal.Compared to jet nebulizers,DPI is more convenient to use and does not require storage in cold conditions or reconstitution into solution prior to nebulization.A DPI formulation contains either respirable active drug or drugs blended with non-respirable excipients/carrier such as lactose.To date the only FDA-approved non-respirable carrier in the US is lactose.Meanwhile,several non-respirable carriers have been used(outside US)including mannitol,glucose, xylitol,sorbitol,raf fi nose,sucrose and maltitol.It is expected that excipient used must possess close resemblance to endogenous substances in lung to evade premature clearance.
3. Rationale of development of inhalable nanoparticle-based drug for lung cancer
3.1. Size of nanoparticles
Although nano-sized particle is believed to be effective for cancer treatment,speci fi c range of particles size is crucial to determine the effectiveness of the treatment,as some of them might be eliminated by organ or engulfed by alveolar macrophage.For example,nanoparticles with the size lower than 10 nm are likely to be eliminated via glomerular capillary by kidney,liver,spleen and lymph nodes,which is also known as reticuloendothelial system(RES).In the lung,alveolar macrophages clear the nanoparticles in the range of more than 100 nm[39].
3.2. Enhanced permeability and retention(EPR)
The EPR effect has an important role to retain drug in tumor tissues using tumor lymphatic drainage.As demonstrated in other published fi ndings,there are some differences in terms of vascular cut-off pore size between tumors and healthy cells. Depending on the type and stage of cancer,the vasculature size of tumors ranged from 10 nm to 1000 nm.Meanwhile normal vascular cells are only permeable to particles less than 2 nm[40].It has been proven that EPR in fl uenced the accumulation of anti-cancer drug in tumors via passive tumor targeting.A particle size of 10-100 nm was found to be effectively targeted tumor based on animal model[39].
3.3. Surface properties
Surface properties of nanoparticles are known to in fl uence the bioavailability of anti-cancer drug in tumor cells.The coating of hydrophilic polymer layers such as poly(ethylene oxide),polyethylene glycol and poloxamine could prevent phagocytosis ingestion by macrophages.Nanoparticles with positive charges could penetrate easily into tumor cells as higher binding activity between tumor cells and particles was noted.On top of that,the stability of nanoparticles in suspension is greatly improved,when the zeta potential values are above±30 mV,as the aggregations of particle are minimized[39].
3.4. Challenges for nanoparticle-based drug delivery in lung cancer therapy
The past few decades have witnessed the expedite development of nanoparticle-based medicine such as nanoparticle, liposomes,micelles in applications covering cancer diagnosis,imaging,detection and treatment.The ability to design nanoparticles as personalized medicine is seductive and ideal for lung cancer therapies.Combinational approaches with intricate balance between targeting moieties and anti-cancer agents have been widely documented in recent years.In a nutshell,multicomponent nanoparticle systems are usuallydesigned to encapsulate and stabilize poorly soluble anticancer agents while simultaneously anchored with speci fi c targeting moiety on the surface to impart selective targeting at desired sites.However,the translation of nanoparticlebased drug delivery in lung cancer therapy speci fi cally as inhalation medication to clinic is extremely challenging.Firstly, synthesizing nanoparticles designed with speci fi c size distribution and ability to evade clearance as well as residing for suf fi cient time at targeted site has always been tricky and complicated.Therefore the identi fi cation of appropriate physicochemical characteristics(size,surface chemistry and shape)of nanoparticles is imperative to determine the biological behavior of particles in physiological environments.As such,crucial information such as particle-particle interactions,aggregation behavior,adsorption of proteins on nanoparticles and tendency to elicit immunological response in cells would be bene fi cial to design nanoparticulate systems to achieve the highest drug delivery ef fi ciency.In addition,currently there is lack of established rules and regulations for the testing of nanoparticle-based medicine which include manufacturing,functional testing and safety evaluation.In theory a nanoparticle-based medicine should be evaluated in the same manner of any newly discovered drug that includes optimal design,reproducible manufacturing process,clear analytical methods for characterization,pharmacology,pharmacokinetic and toxicology pro fi les,and fi nally ef fi cacy in clinical trial. However it should be noted that unlike conventional anticancer drugs which exist as single component,nanoparticles are complex and contain multicomponent.Therefore such variations should warrant the modi fi cation of standard testing of nanoparticles.
4. Application of nanotechnology for delivery of inhaled chemotherapeutic drugs
4.1. Polymeric nanoparticles
Poly(lactic-co-glycolic acid)(PLGA),a biocompatible and noncytotoxic polymer,is the most commonly used either as carrier or excipient in drug delivery to achieve sustained release of drug.In a study by Tomoda and co-workers,nanoparticles-inmicroparticles dry powder was prepared to ensure effective drug deposition into deep lung.For this PLGA nanoparticles loaded with anti-cancer drug(TAS-103)with average size diameter of approximately 200 nm were fi rst synthesized as primary particle and subsequently spray-dried in the presence of trehalose as excipient[41].Higher aerosol performance coupled with sustained release pro fi le was achieved for PLGA nanoparticles loaded with 5%of TAS-103.In comparison,the FPF value for spray-dried formulation was 14.35%while only 0.79%was found for primary TAS-103 loaded PLGA nanoparticles.As expected the powdered formulation was more potent against A549 cells compared to free drug probably owing to enhanced internalization of drug nanoparticles via endocytosis or passive diffusion.It was hypothesized that the dry powder was completely decomposed into nanoparticle dispersion in the presence of cell medium[41].The in vivo administration of spray-dried TAS-103 loaded PLGA nanoparticles via inhalation to rats demonstrated higher retention of drugs in the lung(approximately 13-times higher)compared to those delivered via intravenous injection[41].A similar approach was employed to prepare a DPI formulation through incorporation of doxorubicinloaded nanoparticle into inhalable carrier[42].Doxorubicin was incorporated into poly(butylcyanoacrylate)nanoparticles using emulsion techniques and coated with both polysorbate 80 and dextran.These puri fi ed doxorubicin-loaded nanoparticles with mean particle size of 173 nm were then co-spray freezedried with lactose at low temperature to avoid decomposition and loss of drug activity.The mass median aerodynamic diameter(MMAD)of the DPI formulation measured using Andersen Cascade Impactor(ACI)at fl ow rate of 60 L/min was 3.41±0.22 μm.Similarly,this DPI formulation containing doxorubicin was more cytotoxic against A549 and H460 lung cancer cells compared to free drug[42].
A highly porous PLGA microparticle loaded with doxorubicin was recently synthesized in double w/o/w emulsi fi cation method in the presence of ammonium bicarbonate[43].These particles showed desirable aerosol characteristic with MMAD of 3.6±0.4 μm and were retained in the lungs of C57BL/6 mice up to 2 weeks of administration using a dry powder insuf fl ator.Remarkably,these porous microparticles signi fi cantly reduced the masses of B16F10 bearing metastatic lung tumors while exerting negligible toxicity to healthy cells[43].To increase the selective targeting of cancer cells,the same group attempted to functionalize the surface of porous doxorubicinloaded PLGA microparticles with Apo2L/TRAIL(tumor necrosis factor(TNF)-related apoptosis-inducing ligand).Apo2L/TRAIL is reported to speci fi cally bind to death receptors such as DR4/ TRAIL-R1,which are commonly overexpressed in cancer cells but not healthy cells[44].The pulmonary administration of these particles resulted in the deposition in mouse lungs in which they remained in situ for a week.Furthermore,the reduction in the volume of BALB/c nu/nu mice bearing H226 metastatic lung cells was more pronounced,thus suggesting a synergistic apoptotic relationship between doxorubicin and Apo2L/TRAIL[44].Recently the synergistic effect of doxorubicin and paclitaxel incorporated into porous PLGA microparticles was evaluated for treatment of metastatic lung[45].Combination of doxorubicin and paclitaxel in solubilized form with ratio of 5 to 1 achieved the optimal synergistic therapeutic effect against B16F10 cells in vitro.The in vivo co-delivery of doxorubicin and paclitaxel by PLGA particles using insuf fl ation was far superior in terms of reducing the lesion and volume of lung tumor compared to single therapy.For instance,the average lung weight of melanoma bearing mice treated with both drugs was 284.0±19.49 mg while both mice treated with either doxorubicin orpaclitaxelalonewere360±33.17 mgand 420±35.36 mg,respectively[45].
4.2. Liposomes
Liposome is one the most successful nano-based drug delivery systems to date with several FDA-approved liposomal formulations in the market[46].Liposomes are self-assembled carrier with the presence of an outer hydrophobic lipid layer and a hydrophilic core.Liposome can be prepared in different sizes ranging from 50 nm to more than 1000 nm,depending on the composition of phospholipid and cholesterol molecules used.The presence of phospholipid layers makesliposome an ideal carrier to encapsulate water insoluble drugs, especially most chemotherapeutic drugs such as paclitaxel and doxorubicin[39,47,48].
A multi-component liposomal formulation containing two cytotoxic agents(etoposide and docetaxel)and p53 was recently developed directed toward enhanced effects onA549 and H-1299 cell lines[49].The enhanced synergistic activity was anticipated owing to increased apoptosis and necrosis following the restoration of p53 apoptotic function,thus leading to sensitization of lung cells toward cytotoxic agents.The liposomal formulation showed an average size of 200-350 nm and released behavior up to 24 h.Using ACI,excellent aerodynamic properties were observed for the liposomal formulation with a FPF and MMAD of 33-37%and 2-4 μm[49].
Paclitaxel liposome consisting of dilauroylphosphatidylcholine(DLPC)at drug-to-lipid ratio of 1:10(w/w)was prepared in butanol and lyophilized to prepare a DPI formulation [47].The lyophilized liposomal formulation was reconstituted in sterile water and vortexed to obtain homogenous suspension with particle sizes of 2.0-25.3 mm.Using an ACI at fl ow rate of 10 L/min and Aeromist jet nebulizer,the generated aerosol demonstrated an MMAD and a GSD of 2.2 μm and 1.9,respectively.The pharmacokinetic studies showed that the AUC in liposomal formulation delivered via nebulization was at least 20-fold higher than that of intravenous administration.Similarly,signi fi cant reduction in tumor number and increased survival time was recorded over duration of 2 weeks in aerosolized paclitaxel liposome[47].The effect of varying drug to lipid ratio(1:7.5 w/w)during drug encapsulation process did not result in appreciable changes of aerosol performance whereby both the MMAD and GSD were 1.6 μm and 2.2,respectively[50].It was however estimated that chamber aerosol exposure could yield deposition of 6.1 mg/kg paclitaxel liposomes to the lungs.Similar to previous study,the liposomal formulation containing paclitaxel demonstrated signi fi cantly lower lung mass and tumor surface areas compared to untreated mice[50].
The presence of cyclosporin A improved the anti-cancer effect of aerosolized paclitaxel liposome in mice.Using a Renca lung metastatic mouse model,BALB/c mice were injected with 100,000 Renca cells for 24 h and were subsequently treated with aerosolized paclitaxel liposome or co-cyclosporin A paclitaxel liposome.The aerosol generated using Aero-Mist jet nebulizer exhibited MMAD of approximately 2 μm.Weight loss was noted in mice with aerosolized co-cyclosporin A paclitaxel liposome by day 22 to 22 and no toxicity following treatment was observed in histopathological examination[50].
The pulmonary administrations of liposomal formulation of 9-nitrocamptothecin using nebulizers have been studied extensively both in vivo and in vitro in regard of the feasibility and therapeutic impact of aerosolized formulations against metastatic lung cancers[51].Meanwhile,in vivo studies in which rats were exposed to aerosolized 9-nitrocamptothecin liposome or empty liposome showed negligible side effects,thus demonstrating its safety and tolerability.In a study by Knight et al.,a liposomal 9-nitrocamptothecin formulation containing dilauroylphosphatidylcholine(DLPC)was administered at doses of less than 200 L g/kg daily,5 days per week to rat to evaluate the therapeutic ef fi cacy of drugs against various primary cancer xenografts in nude mice[52].Using Aerotech II nebulizer at fl ow rate of 10 L/min,the generated aerosol showed a MMAD of 1.6 μm[52].The liposomal formulation showed higher ef fi cacy compared to free drug in which tumor growth was greatly suppressed after weeks of treatment[52]. In two follow-up studies,it was found that liposomal 9-nitrocamptothecin formulations could serve as preventive treatment and effective against established lung metastasis [51].In the fi rst study,liposomal 9-nitrocamptothecin was delivered to C57BL/6 mice immediately after injection of melanoma cells and the treatment was continued for 1 h,5 days a week for 3 weeks.Fewer lung metastases in treated mice were observed compared to untreated mice,thus showing a preventive effect of drug.In a second model,the aerosol was only delivered after nine weeks of injection of osteosarcoma cells in nude mice[51].Based on the promising in vivo results,Phase I clinical trial was performed on twenty fi ve patients with either primary or metastatic lung cancer[53]. Aerosols were generated using a jet nebulizer in a HEPA-fi ltered airborne scavenging tent.This study was performed to evaluate the tolerability and safety of pulmonary administration of liposomal 9-nitrocamptothecin and to determine the recommended Phase II dosages based on an 8-week treatment regime.In the fi rst part,patients received aerosolized liposomal 9-nitrocamptothecin for 5 consecutive days/week for 1,2,4,or 6 weeks followed by 2 weeks of rest to determine feasibility.Compared to side effects which occurred using oral route,it was interesting that hematological toxicity was not noted for pulmonary administration.The dose limiting toxicity of aerosolized liposomal 9-nitrocamptothecin was grade 3 chemical pharyngitis.Other side effects include nausea,vomiting,cough,wheezing,chest congestion and sore throat.In addition,lower aerosolized dose was required to achieve similar 9-nitrocamptothecin plasma levels to those seen in oral route. Some patients involved in the trial responded partially to the treatment establishing the therapeutic potential of pulmonary administration of 9-nitrocamptothecin formulated in liposome[53].
Several preclinical studies have nudged that liposomal entrapment of cisplatin did not alter the cytotoxic properties of the drug[54].The in vitro cytotoxicity and in vivo pharmacokinetic pro fi les of aerosolized cisplatin loaded into lipid vesicles (Sustained Release Lipid Inhalation Targeting,SLIT)were previously investigated using human tumor cell line(NCI-H460) and Sprague-Dawley rats,respectively[54].The formulation of cisplatin into lipid-based vesicles showed comparable cytotoxicity activity to free cisplatin.The IC50 values of SLIT cisplatin and free cisplatin were 0.55 and 0.49 μg/mL,respectively.Meanwhile,intratracheal instillation of SLIT cisplatin in rats exhibited higher accumulation in lungs and reduced exposure to kidney, thus minimizing the risk of nephrotoxicity.In addition,no histopathological changes were observed in lungs,kidney or bone marrow following 14 days of inhaled administration of SLIT cisplatin.In terms of anti-cancer potential of SLIT cisplatin, inhaled administration of the formulation dramatically reduced the burden of tumor in metastatic model.A phase I clinical study was set up to investigate the safety pro fi le,maximum tolerated dose and pharmacokinetics of aerosolized SLIT cisplatin in seventeen patients with lung carcinoma[55].Doseescalating regime of SLIT cisplatin was aerosolized to patients during 1-4 consecutive days in a 21-day treatment cycle.Theaerosols generated using PARI LC Star nebulizer had a MMAD of 3.7 μm and GSD of 1.9.No dose limiting toxicity was observed at the maximum delivered dose.The common systemic toxicity related to cisplatin such as hematologic toxicity,nephrotoxicity,ototoxicity and neurotoxicity was not observed.The most common side effects were nausea and vomiting as well as a grade 1-2 decrease in FEV1[55].
In another study,a phase I study was designed to evaluate the toxicity and suitability of inhaling interleukin(IL)-2 liposomes to patients with pulmonary metastases[56].The main objective of the study was to determine the maximum doses tolerated which would be biologically active while not exerting toxic effects in an outpatient setting.The liposome containing IL-2 was nebulized using a Puritan twin jet nebulizer for approximately 20 min,3 times a day.All patients completed at least 8 days of treatment and could perform normal activities during treatment,thus con fi rming that signi fi cant toxicity was not present.Based on the data,the optimal dose and timing of IL-2 liposome via inhalation are 3-6×106IU and 3 times a day[56].
4.3. Gelatin based nanoparticles(GNP)
Gelatin is a denatured protein obtained either through acid or alkaline hydrolysis of animal collagen.The interesting feature of gelatin is that this polypeptide contains cationic,anionic and hydrophobic amino acid groups with ratio of 1:1:1.As such, gelatin polypeptide is~13%positively charged,~12%negatively charged and~11%hydrophobic in nature[57,58].Gelatin based nanoparticles(GNP)have been studied in detail for the delivery of hydrophilic and hydrophobic anti-cancer drugs such as paclitaxel[59],cisplatin[60],curcumin[61],resveratrol[62] and methotrexate[63].Similar to other nanotechnology approaches,GNP demonstrated enhanced anti-cancer activity, sustained drug release and exerted very low toxicity to cells [58].From the economic point of view,the cost of gelatin is low and the good reproducibility of gelatin makes the prospect of future upscaling promising[58].The superior ef fi cacy of cisplatin-loaded biotinylated-EGF-modi fi ed-GNP compared to free cisplatin solution was manifested against A549 lung adenocarcinoma cells in vitro.The IC50was as low as 1.2 μg/mL for cisplatin-loaded GNP compared to free cisplatin(2.54 μg/mL) [60,64].In addition,the accumulation of cisplatin in lung cancer following inhalation in mouse was much higher for the nanoformulation compared to free cisplatin solution[60].Analysis of aerosol droplet size following nebulization using DUST monitor revealed that 99%of the particles had MMAD(0.5-5 μm) within suitable range for airway deposition[65].
4.4. Others
An interesting concept involving the use of effervescent molecules as excipient during formulations of doxorubicin nanoparticles was published recently[66].Effervescent technology is based on the concept of employing carbonated or citric-based molecules which form gas bubbles when in contact with aqueous solutions,thus increasing the phase transition from gas to liquid and hereby achieving a more rapid action. Roa and co-workers evaluated the effectiveness of using effervescent components in their formulations containing inhalable doxorubicin nanoparticles.Tumor bearing mice treated with effervescent nanoparticles survived longer compared to those administered with non-effervescent nanoparticles[66]. Meanwhile,Al-Hallak and co-workers demonstrated that effervescent nanoparticles containing doxorubicin achieved deep lung deposition,were distributed primarily in the lung and negligible accumulation in other tissues or organs[67].
Pulmonary instillation of free doxorubicin had been shown to be completely absorbed within minutes.To prolong and liberate the exposure of lung cancers to chemotherapeutic drugs, Kaminskas and co-workers recently explored the possibility of using PEGlated polylysine dendrimer in conjugation with doxorubicin[68].After intratracheal instillation to rats,an average of 60%dendrimers was cleared from the lungs within 24 h followed by a slower clearance phase.The bioavailability of the dendrimer-conjugated doxorubicin was in the range of 10-13%and approximately 15%of drugs remained in the lungs after a week of administration.A comparison between the effi cacy of administered route demonstrated that dendrimerconjugated doxorubicin delivered via pulmonary led to a>95% reduction in lung tumor burden after 2 weeks.Meanwhile,only 30-50%reduction was observed in rats receiving drugs via intravenous administration[68].
As mentioned,apoptotic TRAIL protein exhibits high affi nities toward over-expressed death receptors in abnormal cells. In a recent study,hydrophobic self-assembled nanoparticles consisting of doxorubicin,octyl aldehyde and human serum albumin(HSA)were functionalized with TRAIL protein to increase synergistic apoptotic effect[69].Using an aerosolizer, TRAIL-doxorubicin-HSA conjugates were well deposited in mouse lung and demonstrated gradual release of drug over 3 days[69].Recently,Taratula and co-workers explored the feasibility of mesoporous silica nanoparticles(MSN)as carrier for inhalation therapy with speci fi c targeting to lung cancer[70]. The formulation contained fi ve main components:MSN,chemotherapeutic drugs(cisplatin and doxorubicin),suppressors of pump(MRP1)and non-pump(BCL2)drug resistance and targeting ligand(LHRH peptide)speci fi c for lung cancer.The simultaneous delivery of chemotherapeutic drugs and suppressor proteins via inhalation to A549-bearing NCR nude mice led to higher accumulation of nanoparticles in the lung.In addition,the delivery route preserved the activities of both chemotherapeutic drugs and siRNA,which in turn resulted in enhanced apoptotic actions in lung cells[70,71].
In another study,the encapsulation of COX-2 inhibitor, celecoxib into nanostructured lipid carriers using triglycerides such as compritol and miglyol,was reported via high pressure homogenization method[72].The suspension of celecoxib-loaded nanoparticles showed an average diameter of 217±20 nm and demonstrated controlled release kinetics in vitro for extended period of time.The aerosol performance of the formulation was determined using ACI with Pari LC Star jet nebulizer.The nebulized droplets presented an FPF,MMAD and GSD of 75.6%,1.6 μm and 1.2,respectively[72].In addition,nebulization ofcelecoxib loaded nanoparticles demonstrated 4-fold higher area-under-curve(AUC)in lung tissues,and thus improving its bioavailability in vivo[72].
The in vivo pharmacokinetics of 5- fl uorouracil lipid coated nanoparticles(LNP)in hamster following inhalation was investigated to determine its feasibility for lung cancer therapy[73].Approximately 30 mg LNP/kg body weight(1.5 mg/kg 5- fl uorouracil)was delivered using atomizer at 1.7 MHz ultrasonic drive.It was found that the nanoparticles were mainly deposited in the respiratory tracts,speci fi cally in the lung, trachea and larynx.The nanoparticles were cleared away from respiratory tract within 24 h[73].
Acknowledgements
W.H.Lee is the recipient of Early Career Fellowships from Cancer Institute New South Wales(CINSW 14/ECF/1-12).
③数据同步:配置了数据库服务器和应用服务器,实现车载数据与省指挥中心数据同步,提供装备大型应用系统的软硬件环境,极大地提高了应急指挥所指挥决策能力。
REFERENCES
[1]Cancer Research UK crukorg/cancerstats 2013.
[2]Jemal A,Bray F,Center MM,et al.Global cancer statistics, vol.61.CA:A Cancer Journal for Clinicians;2011.p.69-90.
[3]Centers for Disease Control and Prevention(US).The Health Consequences of Smoking:A Report of the Surgeon General. Of fi ce of the Surgeon General(US);Of fi ce on Smoking and Health(US).Atlanta,GA;2004.
[4]Rodriguez E,Lilenbaum R.Small cell lung cancer:past, present,and future.Curr Oncol Rep 2010;12:327-334.
[5]Chang A.Chemotherapy,chemoresistance and the changing treatment landscape for NSCLC.Lung Cancer 2011;71:3-10.
[6]Paumier A,Le Péchoux C.Radiotherapy in small-cell lung cancer:where should it go?Lung Cancer 2010;69:133-140.
[7]Arriagada R,Bergman B,Dunant A,et al.Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer.NEJM 2004;350:351-360.
[8]Sandler AB,Nemunaitis J,Denham C,et al.Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer.J Clin Oncol 2000;18:122.
[9]Schiller JH,Harrington D,Belani CP,et al.Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer.NEJM 2002;346:92-98.
[10]Wozniak AJ,Crowley JJ,Balcerzak SP,et al.Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer:a Southwest Oncology Group study.J Clin Oncol 1998;16:2459-2465.
[11]Karathanasis E,Ayyagari AL,Bhavane R,et al.Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery.J Control Release 2005;103:159-175.
[12]Loira-Pastoriza C,Todoroff J,Vanbever R.Delivery strategies for sustained drug release in the lungs.Adv Drug Deliv Rev 2014;75:81-91.
[13]Ruge CA,Kirch J,Lehr CM.Pulmonary drug delivery:from generating aerosols to overcoming biological barrierstherapeutic possibilities and technological challenges. Lancet Respir Med 2013;1:402-413.
[14]Sung JC,Pulliam BL,Edwards DA.Nanoparticles for drug delivery to the lungs.Trends Biotechnol 2007;25:563-570.
[15]Fels AOS,Cohn ZA.The alveolar macrophage.J Appl Physiol 1986;60:353-369.
[16]Dhanikula AB,Panchagnula R.Localized paclitaxel delivery. Int J Pharm 1999;183:85-100.
[17]Carstens MG,de Jong PHJLF,van Nostrum CF,et al.The effect of core composition in biodegradable oligomeric micelles as taxane formulations.Eur J Pharm Biopharm 2008;68:596-606.
[18]Solubility AA.Absorption and drug development.John Wiley &Sons,Inc.;2003.p.91-115.
[19]Hansch C,Leo A,Hoekman D.Exploring QSAR:hydrophobic, electronic,and steric constants American Chemical Society; 1995.
[20]Stella B,Arpicco S,Rocco F,et al.Encapsulation of gemcitabine lipophilic derivatives into polycyanoacrylate nanospheres and nanocapsules.Int J Pharmaceut 2007;344:71-77.
[21]Cantrell CL,Franzblau SG,Fischer NH.Antimycobacterial plant terpenoids.Planta Med 2001;67:685-694.
[22]Seedher N,Bhatia S.Solubility enhancement of cox-2 inhibitors using various solvent systems.Aaps Pharmscitech 2003;4:36-44.
[23]Hamman JH,Enslin GM,Kotze AF.Oral delivery of peptide drugs:barriers and developments.Biodrugs 2005; 19:165-177.
[24]Todoroff J,Vanbever R.Fate of nanomedicines in the lungs. Curr Opin Colloid Interface Sci 2011;16:246-254.
[25]Rubin BK.Mucus,phlegm,and sputum in cystic fi brosis. Respir Care 2009;54:726-732.
[26]Ensign LM,Schneider C,Suk JS,et al.Mucus penetrating nanoparticles:biophysical tool and method of drug and gene delivery.Adv Mater 2012;24:3887-3894.
[27]Hunt BE,Weber A,Berger A,et al.Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother 1995;39:34-39.
[28]Cone RA.Barrier properties of mucus.Adv Drug Deliv Rev 2009;61:75-85.
[29]Sigurdsson HH,Kirch J,Lehr C-M.Mucus as a barrier to lipophilic drugs.Int J Pharm 2013;453:56-64.
[30]d’Angelo I,Conte C,La Rotonda MI,et al.Improving the ef fi cacy of inhaled drugs in cystic fi brosis:challenges and emerging drug delivery strategies.Adv Drug Deliv Rev 2014;75:92-111.
[31]Patton JS,Fishburn CS,Weers JG.The lungs as a portal of entry for systemic drug delivery.Proc Am Thorac Soc 2004;1:338-344.
[32]Schanker LS,Less MJ.Lung pH and pulmonary absorption of nonvolatile drugs in the rat.Drug Metabol Dispos 1977;5:174-178.
[33]Lü J-M,Wang X,Marin-Muller C,et al.Current advances in research and clinical applications of PLGA-based nanotechnology.Expert Rev Mol Diagn 2009;9:325-341.
[34]Gabizon AA,Shmeeda H,Zalipsky S.Pros and cons of the liposome platform in cancer drug targeting.J Liposome Res 2006;16:175-183.
[35]Mukherjee S,Ray S,Thakur RS.Solid lipid nanoparticles:a modern formulation approach in drug delivery system. Indian J Pharm Sci 2009;71:349-358.
[36]Dolovich M.B.,Dhand R.Aerosol drug delivery: developments in device design and clinical use.Lancet 2011;377:1032-1045.
[37]Respaud R,Vecellio L,Diot P,et al.Nebulization as a delivery method for mAbs in respiratory diseases.Expert Opin Drug Deliv 2015;12:1027-1039.
[38]Anderson P.Use of respimat(®)soft Mist™inhaler in COPD patients.Int J Chronic Obstruct Pulmon Dis 2006;1:251-259.
[39]Lee W-H,Loo C-Y,Young PM,et al.Recent advances in curcumin nanoformulation for cancer therapy.Expert Opin Drug Deliv 2014;11:1183-1201.
[40]Davis ME,Chen Z,Shin DM.Nanoparticle therapeutics:an emerging treatment modality for cancer.Nat Rev Drug Discov 2008;7:771-782.
[41]Tomoda K,Ohkoshi T,Hirota K,et al.Preparation and properties of inhalable nanocomposite particles for treatment of lung cancer.Colloids Surf B Biointerfaces 2009;71:177-182.
[42]Azarmi S,Tao X,Chen H,et al.Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles.Int J Pharm 2006;319:155-161.
[43]Kim I,Byeon HJ,Kim TH,et al.Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer.Biomaterials 2012;33:5574-5583.
[44]Kim I,Byeon HJ,Kim TH,et al.Doxorubicin-loaded porous PLGA microparticles with surface attached TRAIL for the inhalation treatment of metastatic lung cancer.Biomaterials 2013;34:6444-6453.
[45]Feng T,Tian H,Xu C,et al.Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment.Eur J Pharm Biopharm 2014;88:1086-1093.
[46]Lee W-H,Loo C-Y,Traini D,et al.Nano-and micro-based inhaled drug delivery systems for targeting alveolar macrophages.Expert Opin Drug Deliv 2015;12: 1009-1026.
[47]Koshkina NV,Waldrep JC,Roberts LE,et al.Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model.Clin Cancer Res 2001;7:3258-3262.
[48]Song H.,Zhang J.,Liu X.,et al.Development of a bone targeted thermosensitive liposomal doxorubicin formulation based on a bisphosphonate modi fi ed non-ionic surfactant. Pharm Dev Technol 2015;15:1-8.
[49]Jinturkar KA,Anish C,Kumar MK,et al.Liposomal formulations of Etoposide and Docetaxel for p53 mediated enhanced cytotoxicity in lung cancer cell lines.Biomaterials 2012;33:2492-2507.
[50]Koshkina NV,Golunski E,Roberts LE,et al.Cyclosporin a aerosol improves the anticancer effect of paclitaxel aerosol in mice.J Aerosol Med 2004;17:7-14.
[51]Koshkina NV,Kleinerman ES,Waldrep C,et al. 9-nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice.Clin Cancer Res 2000;6:2876-2880.
[52]Knight V,Kleinerman ES,Waldrep JC,et al. 9-nitrocamptothecin liposome aerosol treatment of human cancer subcutaneous xenografts and pulmonary cancer metastases in mice.Ann N Y Acad Sci 2000;922:151-163.
[53]Verschraegen CF,Gilbert BE,Loyer E,et al.Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies.Clin Cancer Res 2004;10:2319-2326.
[54]Perkins W,Weers J,Meers P,et al.An inhalation formulation of liposomal cisplatin(SLIT2 cisplatin)for treatment of lung cancer.Vancouver:Lipids,Liposomes&Biomembranes; 2005.
[55]Wittgen BPH,Kunst PWA,van der Born K,et al.Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung.Clin Cancer Res 2007;13:2414-2421.
[56]Skubitz KM,Anderson PM.Inhalational interleukin-2 liposomes for pulmonary metastases:a phase I clinical trial. Anticancer Drugs 2000;11:555-563.
[57]Kommareddy S,Shenoy DB,Amiji MM.Gelatin nanoparticles and their biofunctionalization.In:Kumar CSSR,editor. Nanotechnologies for the life sciences.Weinheim:WILEYVCH Verla GmbH&Co.KGaA;2005.p.330-352.
[58]Elzoghby AO.Gelatin-based nanoparticles as drug and gene delivery systems:reviewing three decades of research. J Control Release 2013;172:1075-1091.
[59]Lu Z,Yeh T-K,Wang J,et al.Paclitaxel gelatin nanoparticles for intravesical bladder cancer therapy.J Urol 2011;185:1478-1483.
[60]Tseng C-L,Su W-Y,Yen K-C,et al.The use of biotinylated-EGF-modi fi ed gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009;30:3476-3485.
[61]Cao F,Ding B,Sun M,et al.Lung-targeted delivery system of curcumin loaded gelatin microspheres.Drug Deliv 2011;18:545-554.
[62]Karthikeyan S,Rajendra Prasad N,Ganamani A,et al. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed Prev Nutr 2013;3:64-73.
[63]Cascone M,Lazzeri L,Carmignani C,et al.Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate.J Mater Sci Mater Med 2002;13:523-526.
[64]Tseng C-L,Wang T-W,Dong G-C,et al.Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting.Biomaterials 2007;28:3996-4005.
[65]Tseng C-L,Wu SY-H,Wang W-H,et al.Targeting ef fi ciency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer.Biomaterials 2008;29:3014-3022.
[66]Roa WH,Azarmi S,Al-Hallak MHDK,et al.Inhalable nanoparticles,a non-invasive approach to treat lung cancer in a mouse model.J Control Release 2011;150:49-55.
[67]Al-Hallak MHDK,Sarfraz MK,Azarmi S,et al.Distribution of effervescent inhalable nanoparticles after pulmonary delivery:an in vivo study.Ther Deliv 2012;3:725-734.
[68]Kaminskas LM,McLeod VM,Ryan GM,et al.Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy.J Control Release 2014;183:18-26.
[69]Choi SH,Byeon HJ,Choi JS,et al.Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer.J Control Release 2015;197:199-207.
[70]Taratula O,Garbuzenko OB,Chen AM,et al.Innovative strategy for treatment of lung cancer:targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA.J Drug Target 2011;19:900-914.
[71]Taratula O,Kuzmov A,Shah M,et al.Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release 2013;171:349-357.
[72]Patlolla RR,Chougule M,Patel AR,et al.Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release 2010;144:233-241.
[73]Hitzman CJ,Wattenberg LW,Wiedmann TS. Pharmacokinetics of 5- fl uorouracil in the hamster following inhalation delivery of lipid-coated nanoparticles.J Pharm Sci 2006;95:1196-1211.
*Corresponding author.Respiratory Technology,Woolcock Institute of Medical Research and Discipline of Pharmacology,Sydney Medical School,The University of Sydney,NSW 2037,Australia.Tel.:+61 2 9114 0365;fax:+61 2 9351 4391.
E-mail address:w.lee@sydney.edu.au(W.-H.Lee).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.009
1818-0876/©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Lung cancer Liposome
Nanoparticle
猜你喜欢
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Rethinking bioequivalence and equivalence requirements of orally inhaled drug products
- Inhaled nicotine replacement therapy
- Practical,regulatory and clinical considerations for development of inhalation drug products
- Mathematical approach for understandingdeagglomeration behaviour of drug powder in formulations with coarse carrier
- The effects of surface morphology on the aerosol performance of spray-dried particles within HFA 134a based metered dose formulations
- Delivery of theophylline as dry powder for inhalation
