Sustained release donepezil loaded PLGA microspheres for injection:Preparation,in vitro and in vivo study
2015-05-16WenjiGuoPengQunLingFngDongmeiCunMingshiYng
Wenji Guo,Peng Qun,Ling Fng,Dongmei Cun,*, Mingshi Yng,b
aDepartment of Pharmaceutics,Shenyang Pharmaceutical University,110016,ChinabDepartment of Pharmacy,Faculty of Health and Medical Sciences,University of Copenhagen, Universitetsparken 2,2100 Copenhagen Ø,Denmark
Sustained release donepezil loaded PLGA microspheres for injection:Preparation,in vitro and in vivo study
Wenjia Guoa,Peng Quana,Liang Fanga,Dongmei Cuna,*, Mingshi Yanga,b
aDepartment of Pharmaceutics,Shenyang Pharmaceutical University,110016,ChinabDepartment of Pharmacy,Faculty of Health and Medical Sciences,University of Copenhagen, Universitetsparken 2,2100 Copenhagen Ø,Denmark
ARTICLEINFO
Article history:
Received 5 May 2015
Received in revised form 15 June 2015
Accepted 18 June 2015
Available online 9 July 2015
Donepezil
PLGA
Sustained release
Microspheres
In vitro and in vivo correlation
The purpose of this study was to develop a PLGA microspheres-based donepezil(DP)formulation which was expected to sustain release of DP for one week with high encapsulation efficiency(EE).DP derived from donepezil hydrochloride was encapsulated in PLGA microspheres by the O/W emulsion-solvent evaporation method.The optimized formulation which avoided the crushing of microspheres during the preparation process was characterized in terms of particle size,morphology,drug loading and EE,physical state of DP in the matrix and in vitro and in vivo release behavior.DP microspheres were prepared successfully with average diameter of 30µm,drug loading of 15.92±0.31%and EE up to 78.79±2.56%.Scanning electron microscope image showed it has integrated spherical shape with no drug crystal and porous on its surface.Differential scanning calorimetry and X-ray diffraction results suggested DP was in amorphous state or molecularly dispersed in microspheres.The Tg of PLGA was increased with the addition of DP.The release profile in vitro was characterized with slow but continuous release that lasted for about one week and fitted well with first-order model,which suggested the diffusion governing release mechanism.After single-dose administration of DP microspheres via subcutaneous injection in rats,the plasma concentration of DP reached peak concentration at 0.50 d,and then declined gradually,but was still detectable at 15 d.A good correlation between in vitro and in vivo data was obtained.The results suggest the potential use of DP microspheres for treatment of Alzheimer’s disease over long periods.
©2015 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Alzheimer’s disease(AD)is a progressive neurodegenerative disease characterized clinically by cognitive decline and memory loss.In 2013,Alzheimer’s Disease International(ADI)reported the worldwide prevalence of dementia to be more than 44 million[1-3].With the progress of AD,AD patients may start to suffer from memory loss and thinking disorders from the mild stage of AD.As a consequence,they need to get some help with their routine daily activities and health care to avoid overdosing on medicine or missing doses due to memory loss.Even worse,some psychological problems may occur to some patients with severe AD and they might refuse to take medicine. Furthermore,since the primary patient population of AD is the elderly,most of them are likely to have several other diseases apart from AD,and they may need to take various medicines,which make their medication regimen more complicated,seriously compromising their compliance.
Donepezil(DP),the second generation of cholinesterase inhibitors,approved by the FDA in 1996,was thought to be able to improve cognitive function in patients with AD,and showed less hepatotoxicity and better tolerance compared to its predecessor(tacrine),and it associated with a smaller risk of clinical trial dropout rate than rivastigmine and galantamine[4].Currently,DP is only commercially available in the oral administration dosage form of tablets and capsules.However, the difficulty in swallowing that elderly people usually have and the gastrointestinal side effects of DP may result in the patients’poor compliance and even the interruption or failure of treatment.Therefore,a long-term sustained release dosage form administrated via non-oral route is highly desirable for AD patients,with the purpose of improving patient compliance.
As an FDA-approved polymer,poly(d,l-lactic-co-glycolic acid) (PLGA),has been widely used in controlled drug delivery systems (DDSs)due to its biodegradability and biocompatibility.PLGA-based DDSs such as microspheres,nanoparticles and implants could provide long-term sustained release of payload from several days to months to meet different clinical demands.Currently,there are already several PLGA-based products commercially available for the sustained release of encapsulated small molecule drug,proteins or peptides[5].
In a previous study of Zhang et al.[6],DP was encapsulated into the matrix of PLGA microspheres and the release of DP lasted at least one month.The results clearly showed that PLGA microspheres are a promising carrier of DP for longterm sustained release.However,the physicochemical properties of these microspheres have not been studied intensively,and the formulation has not been optimized to achieve high enough drug loading and encapsulation efficiency(EE),which are critical to improve the delivery efficiency.The lack of in vitro-in vivo correlation(IVIVC)in the previous study made it impossible to predict the in vivo performance of formulations by looking at their in vitro release data and further optimize the formulation rationally.In addition,in order to fulfill various clinical demands,it is necessary to design other DP products which can achieve sustained release over a shorter period than one month.The main advantage of having another DP-loaded PLGA microspheres showing shorter release period is the better flexibility of dose adjustment.With the short release formulation, the dose could be easily regulated according to the pharmacodynamics response of the patient,which is especially important at the beginning of treatment.
2. Materials and methods
2.1. Materials and animals
PLGA 7505(lactide/glycolide 75/25,Mw 5000 Da,acid-terminated) was purchased fromWako Pure Chemical Industries,Ltd(Osaka, Japan).For the preparation of microspheres,polyvinyl alcohol (PVA)POVAL®-217SB(88%hydrolyzed,KURARAY Co.,Ltd.Osaka, Japan)were used.Methanol,acetonitrile and tetrahydrofuran (THF)were of high-performance liquid chromatography(HPLC) grade and obtained from theYuwang Pharmaceutical Co.,Ltd. (Shandong,China).Donepezil hydrochloride(DP·HCl)was purchased from Jinan Dexinjia Biological Products Co.,Ltd. (Shandong,China).All other chemicals or solvent were of analytical grade or purer and purchased from commercial suppliers.
Wistar rats(male,220-260 g)were provided by Shenyang Pharmaceutical University Experimental Animal Center.The study was approved by the Education and Research Committee and the Ethics Committee of Shenyang Pharmaceutical University(approval SYPU-IACUC-C2015-0428-001).Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, Shenyang Pharmaceutical University,and the 1996 Guide for the Care and Use of Laboratory Animals(Institute of Laboratory Animal Resources on Life Sciences,National Research Council,National Academy of Sciences,Washington DC).All efforts were made to minimize animal suffering and to limit the number of animals used.
2.2. Preparation and identification of donepezil free base
DP·HCl 1 g was dissolved completely in double distilled water (about 20 ml),in which 4%NaOH was dropwise added under agitation.After the pH of solution reached about 11,the synthetic was extracted with 20 ml ethyl acetate twice,and then sufficient amount of anhydrous sodium sulfate was added into the separated organic phase and placed for 24 h to remove residual water.With the evaporation of ethyl acetate at 40°C water bath,the product was collected.
Differential scanning calorimetry(DSC)was used to study the thermodynamic properties of the obtained product.Samples were placed in aluminium pans and heated at a scanning rate of 10°C/min from 25°C to 250°C under a nitrogen atmosphere.
2.3. Preparation of donepezil loaded PLGA microspheres
DP-loaded PLGA microspheres were prepared by a conventional O/W emulsion-solvent evaporation method[7].Briefly, 5 mg DP and 20 mg of PLGA were dissolved in 200µl of dichloromethane(DCM),then injected into 4 ml aqueous phase containing certain concentration of PVA as emulsifier and homogenized with a high speed dispersion homogenizer(FJ200-S,Shanghai specimen model Factory,China)under 1500 rpm for 4 min to form an O/W emulsion.The emulsion wassubsequently transferred to a larger volume of distilled water or PVA solution to dilute the emulsion and avoid the aggregation of particles.After the emulsion was stirred for 4 h at room temperature to evaporate the solvent,the microspheres were collected by centrifugation under 252 g(1500 rpm)for 3 min(800-centrifuge,Jintan Ronghua instrument manufacture Co.,Ltd.China),washed three times with redistilled water and then lyophilized(DRC-1000REC Freeze Dryer,Tokyo Rikakikai Co.,Ltd.Japan),stored at-20°C.The blank microspheres were prepared by following the same procedure except for the adding of DP.
2.4. Particle size and morphology
During solvent evaporation procedure optical microscope(XSP-2CA,Shanghai CSOIF Co.,Ltd.China)was used for particle observation,and photos were taken by digital camera.The size distribution was determined with Laser Particle Size Analyzer(Coulter LS-230,Beckman Coulter Co.,Ltd.USA).The volume weighted mean diameter(vol-wt mean)of microspheres is reported as particle size and the span value(SP)was calculated with the following formula:SP=(d90-d10)/d50,where d90is the particle diameter at 90%cumulative size,d10is the particle diameter at 10%cumulative size and d50is the particle diameter at 50%cumulative size.The size distribution is considered as narrow for span values<0.45.
The surface morphology of the microspheres was observed by optical microscope or scanning electron microscopy (SEM)(S-3400,Hitachi,Ltd.,Japan).With SEM,freeze-dried microspheres mounted on aluminum stubs with doublesided tape and coated with a thin layer of gold.The coated specimen was then scanned and photographed under the microscope at an acceleration voltage of 5.0 kV.
2.5. Drug loading and encapsulation efficiency determination
A drug extraction method was developed for drug loading and EE determination.About 2 mg microspheres were dissolved in 200µl acetonitrile and extracted with 4 ml of 0.012 M HCl by vortex for 4 min).The supernatant was collected by centrifugation for DP content determination.The recovery rate of the extraction procedure was 97.55±1.02%(n=6).
The DP loading and EE were calculated using the following Eqs.(1)and(2):
2.6. HPLC-UV analysis
Quantification of DP in various assay samples was carried out using an HPLC system consisting of a Hitachi L2130 HPLC pump (flow 1 ml/min),a Hitachi L-2420UV detector(UV-detection 271 nm),a Hitachi L-2200 autosampler and an ODS-C18column (Dikma,4.6 mm×200 mm,5µm).
For the analysis of in vitro assay samples,the mobile phase was 65%methanol and 35%water containing 0.5%glacial acetic acid and 1%triethylamine.Integration of peak areas was utilized for quantification of sample concentrations and calculations were performed on the basis of standard curves. The standard curve was A=35385C-5.7560(R2=0.9999,the concentration range was 0.08-20.00µg/ml).A represents the peak area and C represents the concentration of DP.The lower limit of quantification was 40 ng/ml and the lower limit of detection was 20 ng/ml.
For in vivo biologic samples,the mobile phase consisted of 60%methanol and 40%glacial acetic acid solution(0.5%)adjusted to pH 6.5 with triethylamine.The standard curve was Ai/As=0.8159C+0.0180(R2=1.0000,and the concentration range was 0.05-2.00µg/ml).Ai and As represent the peak area of internal standard and DP,respectively,and C represents the concentration of DP.The lower limit of quantification was 50 ng/ ml and the lower limit of detection was 25 ng/ml.
2.7. Differential scanning calorimetry
The thermal analysis of pure DP,PLGA,blank microspheres, physical mixture of DP and PLGA and DP microspheres was performed to determine the physical state of the drug incorporated inside the microspheres and to investigate the effect of drug on PLGA glass transition temperature(Tg).DSC was performed using METTLER TOLEDO DSC 1(METTLER TOLEDO, Switzerland).5 mg samples were placed in an aluminum pan and heated at a constant rate of 10°C/min,using dry atmosphere of nitrogen as carrier gas,in a temperature range of-40 to 150°C.The instrument temperature and energy scales were calibrated using purified indium(99.9%)as the standard reference material.
2.8. X-ray powder diffraction
DP,pure PLGA,physical mixture of DP and PLGA and microsphere products were studied by X-ray powder diffraction(XRD)technique using D/MAX-2500PC(RIGAKU,Japan).θ-2θ powder diffract meters set for an angle range of 3-50°(2θ).The step size was 0.01°(2θ)and count times were of 1 s per step.
2.9. In vitro release study
In vitro release studies were conducted with the sample and separate techniques[8].Approximately 2 mg of DP microspheres were suspended in 6.0 ml of phosphate buffer saline(PBS, pH=7.4)(sink condition can be maintained)in 10 ml capped glass centrifuge tube with round bottom and incubated at 37±0.5°C under 60 rpm horizontal shaking(WHY-2 thermostatic horizontal oscillator,Dadi auto-instrument,Ltd.Jintan, China)for 10 d.At each predetermined sampling point,the suspension was centrifuged at 2120 g(3500 rpm)for 5 min(Anke TDL-40B,Shanghai,China)and the supernatant was separated for assay by HPLC method.Fresh medium of the equal volume was added in the meantime,re-suspended and incubated again.All assays were always performed in triplicate.
2.10. In vitro degradation study
The degradation of PLGA was characterized by molecular weight change.In detail,3 mg of microspheres were incubated in 1 mlrelease media containing 0.02%NaN3at 37±0.5°C,under 60 rpm horizontal shaking with the WHY-2 thermostatic horizontal oscillator and three samples were prepared for each time point.At different time points(1,3,5,10 d),the three tubes were taken out and lyophilized,then dissolved with THF and subjected to gel permeation chromatography(GPC)analysis.
GPC analysis was carried out using a Waters liquid chromatograph equipped with pump(Waters 1515 isocratic HPLC pump)and column oven in combination with a differential refraction detector(Waters 2414 refractive index detector).A series of Chromatographic column were applied:Styragel HT3 Column, 10µm,7.8×300 mm(THF),Styragel HT4 Column,10µm, 7.8×300 mm(THF),Styragel HT5 Column,10µm,7.8×300 mm (THF)(Waters,USA).THF was used as the mobile phase with the flow of 1 ml/min,and the injection volume of assay sample was 20µl.The column temperature was set at 30°C.A universal calibration method(third-order polynomial fit, R2=0.99996)was applied to determine the molecular weight of PLGA,which was obtained from polystyrene standards ranged from 1200 to 125,000 Da in their molecular weight.Data acquisition was performed using Breeze version 3.30 SPA software (Waters).
2.11. In vivo pharmacokinetic study
In pharmacokinetic study,the rats were randomly divided into two groups.One group(n=4)received DP·HCl aqueous solution administered intravenously(30 mg/kg)while the other group(n=4)was given DP microspheres(corresponding to 280 mg DP/kg)by subcutaneous injection at the back of rats after reconstitution with a viscous aqueous vehicle(0.5%carboxymethylcellulose,w/v).At predetermined interval,blood samples(0.3 ml)were withdrawn via the orbital vein with heparinized tubes.The sampling time point for DP·HCl aqueous solution group was 5,10,15,30,60,90,120,180,240,300,360, 420,480,600,and 720 min after i.v.and 0.2,0.5,1,1.5,2,2.5,3, 3.5,4,4.5,5 d and then once a day for DP microspheres group. And then plasma was separated immediately by centrifugation and stored at-80°C until assay.
Plasma samples were processed by following the procedure below:100µl of plasma sample was mixed with 10µl of NaOH solution(0.5 M).Then 10µl of Ethylparaben(internal standard)methanol-water(60:40,v/v)solution(5µg/ml)was added to the mixture and vortexed for 1 min.The mixture was extracted with 1 ml of ethyl acetate by vortex for 5 min.After centrifugation for 5 min under the force of 17,788 g(16,000 rpm), the organic layer was taken and dried with nitrogen.The residuals after the removal of solvent were then reconstituted with 100µl of mobile phase and the content of DP in it was determined by using the established HPLC.The recovery rate of DP extraction from plasma sample was 80.61±2.27%, 85.98±4.42%,87.55±1.89%(mean±SD,n=3)under three concentration levels(0.10µg/ml,0.20µg/ml,1.00µg/ml),respectively.
2.12. In vitro/in vivo correlation
A two-stage modeling process was applied to evaluate the IVIVC for DP loaded microspheres according to a former literature [9].First,in vivo absorption data of DP microspheres in rats were obtained by the deconvolution of the plasma concentration data, in which the in vivo results of DP solution after injection administrated to rats were used as weight function.Then the in vivo absorption profile obtained is connected to the time course of the in vitro release profile.
The correlation analysis was performed with the help of the IVIVC Toolkit of WinNonlin 5.2.1(Pharsight),and correlation coefficients were examined for significance(P<0.05)using Student’s t-test.
2.13. Statistical analysis
Data were presented as mean±standard deviation.Significant differences were determined using analysis of variance (ANOVA).Results were considered significant if P<0.05.Calculations were performed using SPSS 16.0 software.
1375 胰腺癌石蜡包埋组织中微 RNA 原位杂交检测技术的优化 倪晨明,倪灿荣,金 钢,焦莉娟,李连峰,郑建明
3. Results and discussion
3.1. Preparation and identification of donepezil free base
In the market,DP is usually available in the form of DP·HCl, which is highly soluble in water.However,as far as the encapsulation efficiency(EE)of PLGA microspheres is concerned, the high water solubility of the drug is unfavorable because the drug will tend to leak into the water phase.Therefore,DP free base instead of DP·HCl was used in the current study with the purpose of achieving high EE.
DP free base was prepared simply by alkalization of DP·HCl with NaOH and then extracted with ethyl acetate.With the evaporation of solvent,some white crystal product was obtained,and the yield was over 90%.The purity of DP was determined by HPLC peak area normalization method and higher than 99%.The DSC curve of synthesized product showed a characteristic sharp endothermic peak at 87.26°C which was consistent with its melting point reported in literature[10], which clearly means the successful preparation of DP free base (Fig.1).

Table 1-Formulations and morphology of DP loaded microspheres.
3.2. Optimizing the formulation of DP microspheres
The classic O/W emulsion solvent evaporation method was employed to produce the DP loaded PLGA microspheres since DP was readily co-dissolved in DCM with PLGA and high encapsulation efficiency could be expected.However, the phenomenon of severe fragmentation of microspheres was observed in our preliminary study,and it was found that the concentration of PVA in outer water phase was the determinate factor influencing the integrity of microspheres. In order to obtain smooth and spherical-shaped microspheres, the formulation was optimized by varying the concentration of PVA.The detail of the formulations is shown in Table 1.As shown in Fig.2a,when 1%PVA was applied(formulation A), obvious fragmentation of microspheres occurred during DCM evaporation process,which might be mainly caused by the poor plasticity of the low molecular weight PLGA we used. When the primary emulsion was diluted with 0.5%PVA instead of distilled water as we did with formulation B,there were more spherical particles appearing in the field of view under optical microscopy(Fig.2b),which indicated that increasing the concentration of PVA has the potential of improving the morphology of microspheres.Subsequently, 3%PVA was chosen as the stabilizer to form the emulsion and then the emulsion was further diluted with 2%PVA as shown in formulation C.As expected,spherical and integrated microspheres were successfully prepared finally(Fig.2c) Therefore,formulation C was considered to be the optimal formulation and in the following experiment,all the microspheres were prepared with this formulation unless otherwise stated.
Irregular particles were obtained in other research when the content of PVA was low[11].It has been demonstrated that PVA bound on the microparticle surface in an irreversible manner [12].The low concentration of PVA in water phase was not enough for forming integrated coating on the surface of emulsion droplets and therefore had weaker stable effect.
3.3. Characterization of DP microspheres
The optimized PLGA microspheres were characterized in terms of particle size,size distribution,morphology,drug loading and EE.As shown in Fig.2d,the particle size distribution of prepared PLGA microspheres was narrow and monodispersed,with the mean diameter of 29.4±17.1µm,d10of 8.95µm,d50of 27.64µm,d90of 51.28µm and span value of 0.85.There were no particles larger than 100µm.It has been well known that particle size and its distribution will significantly affect the in vitro release in microspheres system.For hydrophobic drugs, the removal of drug out from boundary layer was crucial during drug release.High ratio of surface area and volume such as a decrease in the particle size always lead to a higher release. The particle size obtained was considered to be beneficial for faster drug release rate.
In order to measure the loading and EE of DP in microspheres accurately,a new method for the extraction of DP from PLGA microspheres was developed.In this method,certain amount of microspheres was dissolved by acetonitrile prior to the addition of hydrochloric acid solution.During the process of extraction by vortex,huge acetonitrile-water interface was formed,and on the interface an acid-base reaction between acid and DP happened.The reaction converted the insoluble DP into its soluble counterpart DP·HCl,which could be easily partitioned into water phase.HPLC system can be used during the determination of DP loading based on this drug extraction method.
The determined drug loading was 15.92±0.31%and the average EE was 78.79±2.56%for three batches of DP loaded microspheres,which are significantly higher than that of the previous report mentioned[6].Actually,this result was not out of expectation at all since the DP free base we used in our study has much lower solubility than DP·HCl that has been used in previous study.The solubility of encapsulated drug is one of the critical factors determining the EE when emulsion solvent evaporation method was applied.Drugs with high water solubility tend to leak into outer water phase during preparation process,which usually result in low encapsulation efficiency. The strategies such as increasing the viscosity of polymer solution by increasing the polymer concentration or molecular weight are commonly used to increase EE[13].Compared to the same previous study[6],although the molecular weight of the PLGA we used was lower,the higher concentration of PLGA resulted in much more viscous oil phase,which also could contribute to the improved EE.In addition,it was reported that the ionic interaction between basic amino residue of the drug and uncapped carboxylic acid end-group of the polymers plays an important role in the encapsulation of drugs[14-17].This is the reason why we selected the carboxyl terminal PLGA. However the information about the end group of the PLGA they used was missed,therefore it is impossible to discuss its effects on EE.
DSC is a useful technique to investigate the change in the physical state of drug in microspheres during preparation process.DSC thermograms of pure DP,blank microspheres,DP loaded microspheres and physical mixture of DP and PLGA were shown in Fig.3.It was found that both pure DP and physical mixture of DP and PLGA presented a sharp endothermic peak at the position corresponding to the melting point of DP (87.26°C),but the peak disappeared in the curve of DP loaded microspheres which indicated that DP was amorphous or molecularly dispersed in microspheres.
Thermograms obtained from the first heating cycle provided information on the actual physical and morphological changes within the particle matrix[18].So we recorded the glass transition temperatures(Tg)of PLGA from first cycle,and the value was 38.5°C for bulk PLGA,34.8°C for blank microspheres and 43.7°C for DP loaded microspheres.We can find that the Tg of PLGA was decreased about 3.7°C after blank microspheres were prepared,which could be attributed to the plasticizing effect of residual water or DCM[5].It has been reported that PVA plays an antiplasticizing effect on PLGA due to its hydroxyl groups which has the potential to interact with PLGA. It’s obvious that the antiplasticizing effect of PVA was much weaker compared with the plasticizing effect induced by residual water or DCM[18].On the other hand,it was interesting to find that the incorporation of DP within PLGA increased the Tg of PLGA from 34.8°C to 43.7°C.This is not common since in most cases,drug presented in microspheres act as a plasticizer and would reduceTg of polymer due to the interactions between drug and polymer.The same phenomenon has been observed in Gentamycin/poly(dl-lactic acid)blends in which both gentamycin base and gentamycin sulfate increasedTg of polymer[19].This strong anti-plasticizing effect of DP on PLGA must be due to the reduced mobility of PLGA chain with the involvement of DP.It was assumed that DP could act as filler to occupy the vacancy of PLGA chain,which may decrease the free volume of PLGA.However,we believed this effect was limited,and there must be other more strong effect existing in light of the great increase ofTg(about 8.9°C).We found that there are four H bond acceptors in DP molecular which may interact with carboxyl terminal of PLGA chain ends and form stable clusters and thus increaseTg.The baseline drift was observed after the glass transition peak which was attributed to the structural relaxation of PLGA[18].
Further investigation of the crystallization properties of DP in the drug-loaded microspheres was performed using XRD technique.Fig.4 shows the XRD patterns of DP,blank microspheres,DP loaded PLGA microspheres and physical mixture of DP and PLGA.The analysis by XRD of the DP powder demonstrated the presence of many diffraction bands which were characteristic of a crystalline material.The absence of characteristic peaks in XRD pattern of DP loaded microspheres confirmed the amorphous state of DP in the matrix of PLGA.
3.4.DP-loaded microspheres providing long-term sustained release both in-vitro and in-vivo

Table 2-Results of DP release profile fitting for different mathematical models.
As we mentioned above,the purpose of the current study was to develop a PLGA microspheres-based formulation for DP injection which was expected to provide sustained release for about one week.Therefore,the PLGA with the glycolic acid to lactic acid ratio of 75:25 and low molecular weight about 5000 Da was adopted to encapsulate the DP.The release behavior of prepared DP-loaded PLGA microspheres was investigated both in vitro and in vivo.From the obtained in-vitro release profile(Fig.5), it can be seen that under in-vitro conditions,DP was released slowly and continuously with no obvious initial burst release, and about 88.44%of total DP was cumulatively released over 10 d.Obviously,this release pattern was different from the typical triphasic release profile which presents an initial burst release phase followed by the lag phase and the third continuous release phase.Here,the phase of initial burst release and lag phase were missed.The in-vitro release data were fitted to various mathematical models including the first-order and Higuchi equation in an attempt to figure out the mechanism that governed the release kinetics of DP-loaded PLGA microspheres.According to the data of mathematical modeling listed in Table 2,it can be seen that the regression(R)was more than 0.99 for the models except for Higuchi equation.It seemed that all these three models(First-order,Korsmeyer-Peppas andWeibull model)were appropriate to model the drug release behavior.In the Korsmeyer-Peppas model,k is a system specifics constant and n is the diffusion exponent[20]that corresponds to the transport mechanism.In this case,n was smaller than 0.43(n=0.27),it can be suggested that the diffusion controlled drug release[21].The fitting for the Firstorder model with first-order release constant of 0.573 which also suggested the diffusion of DP driven by the concentration gradient was the main release mechanism.In the Weibull model,a link between the values of b and the diffusional mechanisms of the release have been found[22],when the value of b was 0.619,which was lower than the 0.75 release that follows Fickian diffusion.The conclusion of diffusion controlled release was also supported by the data about the change of molecular weight of PLGA during the in vitro release process (shown in Table 3).It was evident that the molecular weight of PLGA was almost constant over the 10 d of release;therefore the release was polymer degradation independent.
Drug release from microspheres was fast,and this was contributed to both aspects from PLGA and drug.First,DP dispersed in this PLGA microspheres system as an amorphous or molecular from.In both cases,the situation was that the free DP molecular provides enough diving force for diffusion.On the other hand,it has always been known that for diffusioncontrolled release the dissolved drug needs to pass through the PLGA matrix or water-filled pores[23].It has been observed that there were no significant pores both on the surface and interior of microspheres system.PLGA acts as a diffusion barrier,and its properties will significantly affect drug release.Since the Mw of PLGA we selected was small,the overall mobility of their chains was high and therefore allows DP to pass through the polymer matrix faster[24].Furthermore,carboxyl end-group further increases the hydrophilicity of PLGA and thus water can penetrate more quickly and hydrate the polymer.
The mean plasma concentration-time profiles of DP in rats after i.v.administration of pure DP·HCl solution and i.m.administration of DP loaded PLGA microspheres are shown in Fig.6 and Fig.7.The main calculated pharmacokinetic parameters are listed in Table 4.
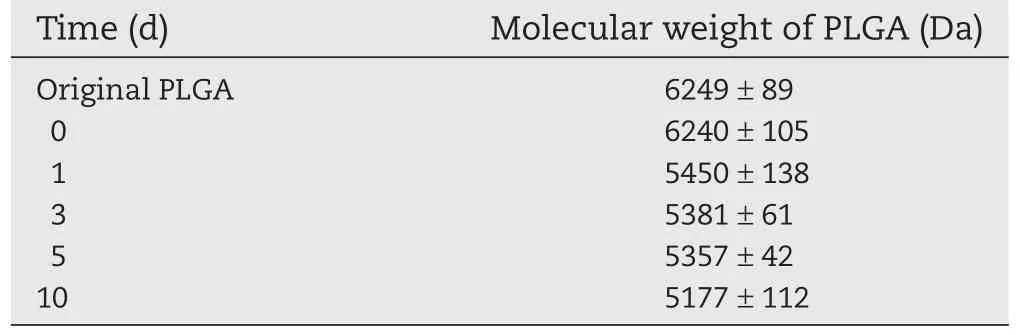
Table 3-Mw change of PLGA in DP microspheres after preparation and incubation in release media.

Table 4-Pharmacokinetic parameters of DP after intravenous injection of DP 3 mg/kg(dissolved in saline(1 mg/ml)via the marginal tail vein and subcutaneous administration(280 mg/kg)(n=4,mean±SD).
After single-dose administration of DP loaded microspheres via subcutaneous injection route,the plasma concentration of DP reached peak concentration(Cmax)563.45±84.50 ng/ml at the time point of 0.50 d,and then the concentration declined gradually,but was still was detectable at 15 d after administration.Compared to DP·HCl solution administered via i.v. injection,the MRT value of DP-MS was prolonged to 8.72±1.07 d,which was 80-fold longer than that of pure DP·HCl solution.These results further confirmed the obvious sustainable release properties of the microspheres.
IVIVC is a predictive model which can be used to describe the relationship between in vitro behavior of a dosage form and relevant in vivo response.As presented in Fig.8,the mean deconvoluted absorption profile in vivo of different rats was in agreement with the observed release profile in vitro,and the correlation coefficient was 0.9768.
A level A correlation which was the most informative and recommended by the FDA was obtained in this study.Based on the good in vivo/in vitro relationship,the in vitro drug release testing can be used to predict the in vivo performance of the corresponding formulation,which means that we could use the in vitro drug release testing to optimize the formulation at the stage of formulation development,and to control the quality of the product during the produce process.Furthermore,on the basis of the level A correlation between in vitro release and in vivo profiles,the in vitro release test cannot only serve as a tool to assure batch-to-batch uniformity for a product but also the evaluation of bioequivalence.
4. Conclusion
DP microspheres with integrated sphere morphology were prepared successfully with 3%PVA as emulsifier.Higher encapsulation efficiency of 78.79±2.56%was obtained.Selected PLGA can control DP release that last for one week in vitro.The release data fit well with first order,Korsmeyer-Peppas and Weibull model,suggesting diffusion-controlled release of this DP microspheres system.Sustained and steady plasma DP concentration was obtained after subcutaneous injection into rats,indicating the slow absorption of DP in vivo. And good in vitro-in vivo correlation was obtained.These results suggested the potential use of DP loaded PLGA microspheres for treatment of AD over long periods.
REFERENCES
[1]Cummings JL.Alzheimer’s disease.NEJM 2004;351(1): 56-67.
[2]Alzheimer’s Association.Alzheimer’s disease facts and figures.Alzheimers Dement 2012;8(2):131-168.
[3]Ballard C,Gauthier S,Corbett A,et al.Alzheimer’s disease. Lancet 2011;377(9770):1019-1031.
[4]Ritchie CW,Ames D,Clayton T,et al.Meta analysis of randomized trials of the efficacy and safety of donepezil, galantamine,and rivastigmine for the treatment of Alzheimer’s disease.Am J Geriatr Psychiatry 2004;12(4):358-369.
[5]Varde NK,Pack DW.Microshpheres for controlled release drug delivery.Expert Opin Biol Ther 2004;4(1):35-51.
[6]Zhang P,Chen L,Gu W,et al.In vitro and in vivo evaluation of donepezil-sustained release microparticles for the treatment of Alzheimer’s disease.Biomaterials 2007;28(10):1882-1888.
[7]Xie M,Zhou L,Hu T,et al.Intratumoral delivery of paclitaxel-loaded poly(lactic-co-glycolic acid)microspheres for Hep-2 laryngeal squamous cell carcinoma xenografts. Anticancer Drugs 2007;18(4):459-466.
[8]Hickey T,Kreutzer D,Burgess DJ,et al.Dexamethasone/ PLGA microspheres for continuous delivery of an antiinflammatory drug for implantable medical devices. Biomaterials 2002;23(7):1649-1656.
[9]Sun L,Cun DM,Yuan B,et al.Formulation and in vitro/in vivo correlation of a drug-in-adhesive transdermal patch containing azasetron.J Pharm Sci 2012;101(12):4540-4548.
[10]Liu N,Zhang Y,Cun D,et al.Effect of backing films on the transdermal delivery of donepezil from patches.AAPS PharmSciTech 2014;15(6):1569-1573.
[11]Jeong YI,Song JG,Kang SS,et al.Preparation of poly(DL-lactide-co-glycolide)microspheres encapsulating all-trans retinoic acid.Int J Pharm 2003;259(1-2):79-91.
[12]Lee SC,Oh JT,Jang MH,et al.Quantitative analysis of polyvinyl alcohol on the surface of poly(D,L-lactide-coglycolide)microparticles prepared by solvent evaporation method:effect of particle size and PVA concentration. J Control Release 1999;59(2):123-132.
[13]Ogawa Y,Yakamoto M,Okada H,et al.A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic acid or Copoly(lactic/glycolic)acid.Chem Pharm Bull(Tokyo)1988;36(3):1095-1103.
[14]Ogawa Y,Yamamoto M,Takada S,et al.Controlled release of leuprolide acetate from polylactic acid or copoly(lactic/ glycolic)acid microcapsules:influence of molecular weight and copolymer ratio of polymer.Chem Pharm Bull(Tokyo) 1988;36(4):1502-1507.
[15]Heya T,Okada H,Tanigawara Y,et al.Effects of counteranion of TRH and loading amount on control of TRH release from copoly(dl-lactic/glycolic acid)microspheres prepared by an in-water drying method.Int J Pharm 1991;69(1):69-75.
[16]Okada H,Yamamoto M,Heya T,et al.Drug delivery using biodegradable microspheres.J Control Release 1994;28(1-3):121-129.
[17]Okada H.One-and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv Drug Deliv Rev 1997;28(1):43-70.
[18]Rouse JJ,Mohamed F,van der Walle CF.Physical ageing and thermal analysis of PLGA microspheres encapsulating protein or DNA.Int J Pharm 2007;339(1-2):112-120.
[19]Mauduit J,Bukhb N,Vert M.Gentamycin/poly(lactic acid) blends aimed at sustained release local antibiotic therapy administered per-operatively.I.The case of gentamycin base and gentamycin sulfate in poly(DL-lactic acid)oligomers. J Control Release 1993;23(3):209-220.
[20]Ritger PL,Peppas NA.A simple equation for description of solute release I.Fickian and non-Fickian release from nonswellable devices in the form of slabs,spheres,cylinders or discs.J Control Release 1987;5(1):23-36.
[21]Jójárt I,Kása P Jr,Kelemen A,et al.Study of the compressibility of chewing gum and its applicability as an oral drug delivery system.Pharm Dev Technol 2015;12:1-7.
[22]Papadopoulou V,Kosmidis K,Vlachou M,et al.On the use of the Weibull function for the discernment of drug release mechanisms.Int J Pharm 2006;309(1-2):44-50.
[23]Wischke C,Schwendeman SP.Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles.Int J Pharm 2008;364(2):298-327.
[24]Liggins RT,Burt HM.Paclitaxel loaded poly(l-lactic acid) microspheres:properties of microspheres made with low molecular weight polymers.Int J Pharm 2001;222(1):19-33.
*Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel.:+86 24 23986330;fax: +86 24 23986330.
E-mail address:cundongmei@163.com(D.Cun).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.06.001
1818-0876/©2015 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
猜你喜欢
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Montmorillonite/Poly(L-Lactide)microcomposite spheres as reservoirs of antidepressant drugs and their controlled release property
- Microsponge based drug delivery system for augmented gastroparesis therapy:Formulation development and evaluation
- Development,characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug
- Solid lipid microparticles:An approach for improving oral bioavailability of aspirin
- Transdermal delivery of fluorescein isothiocyanate-dextrans using the combination of microneedles and low-frequency sonophoresis
