3-苯氧基甲基-4-(4-甲基苯基)-5-(2-吡啶基)-1,2,4-三唑的铜(Ⅱ)、镉(Ⅱ)配合物的合成、晶体结构和光学性质
2015-04-01盛俊峰王宁蔡良英宋菲仝玉柱瞿志荣王作祥
盛俊峰 王宁 蔡良英 宋菲 仝玉柱 瞿志荣 王作祥*,
3-苯氧基甲基-4-(4-甲基苯基)-5-(2-吡啶基)-1,2,4-三唑的铜(Ⅱ)、镉(Ⅱ)配合物的合成、晶体结构和光学性质
盛俊峰1王宁1蔡良英1宋菲1仝玉柱1瞿志荣2王作祥*,1
(1东南大学化学化工学院,南京211189)
(2杭州师范大学有机硅化学及材料技术教育部重点实验室,杭州311121)
以3-苯氧基甲基-4-(4-甲基苯基)-5-(2-吡啶基)-1,2,4-三唑(L)为配体分别合成了2个金属配合物即[Cu2L2Cl4]·2H2O(1)和[Cd3L2(μ2-Cl)6]n·2nCH3CN(2),对其进行了红外、紫外、热重、粉末衍射、元素分析和晶体结构等表征。配合物1和2都属于三斜晶系,空间群都为P1,单晶结构分析表明,在配合物1中,中心铜(Ⅱ)原子具有畸变三角双锥构型[CuN3Cl2];配合物2是配位聚合物,每个重复单元有3个Cd(Ⅱ)原子和2个不同的Cd(Ⅱ)配位中心,Cd1(Ⅱ)原子具有中心对称的畸变八面体构型CdCl4N2,Cd2(Ⅱ)原子具有畸变的八面体构型CdCl4N2。
铜(Ⅱ)配合物;镉(Ⅱ)配合物;晶体结构;三唑
1,2,4-triazole and its derivatives have attracted considerable attention in recent years due to their specificmagneticproperties,interestingcatalytic activity,various structures[1-7]and a large number of biological activities such as anticonvulsant,antidepressant,antibacterial,anticancer and antipsychotic[8-10]. In addition,substituted 1,2,4-triazoles play a prominent role in coordination chemistry since they exhibit versatile and various coordination modes[11-13].Some iron(Ⅱ)complexes with 1,2,4-triazoles show attractive spin-crossover properties which can be applied in molecular-based memory devices as switching material and information storage[14-17].1,2,4-triazoles represent a classofheterocycliccompoundsofsignificant importance in wide areas[18].
Somemetalcomplexescontainingsome4-substituted 3,5-di(2-pyridyl)-1,2,4-triazole have been prepared and characterized in the recent decade[12,16,19-21]. However,complexes with 3-phenoxymethyl-4-(4-methy -phenyl)-5-(2-pyridyl)-1,2,4-triazolhavenotbeen studied until now.As a continuation of our investigation of the asymmetrical substituted 1,2,4-triazoles[22-26], herein we report the syntheses,crystal structures and spectroscopic properties of a triazole ligand of 3-phenoxymethy-4-(methyphenyl)-5-(2-pyridy)-1,2,4-triazole(L)and its two complexes,Copper(Ⅱ)complex 1 and Cadmium(Ⅱ)complex 2.
1 Experimental
1.1 Materials and measurements
All chemicals were analytical grade.Melting points were determined using an X4 digital microscopic melting point apparatus and are uncorrected.C,H,N elemental analyses were performed on a Perkin-Elmer 240 analyzer.1H NMR spectra were measured with a BrukerAvance300spectrometeratambienttemperature in CDCl3using TMS as an internal reference.IR spectra were recorded from 4 000 to 400 cm-1using KBr pellets on a Vector22 Bruker spectrophotometer. UV/Vis spectra were recorded on a Hitachi-4100 UVVis absorption spectrophotometer at room temperature in CH3CN solution.Fluorescence spetra data for 2 and L were measured on a Fluoromax-4 spectrofluorometer at room temperature.Thermogravimetric analysis(TGA) measurememtswereobtainedwithaNETZSCH STA49F3 thermal analyzer in a nitrogen atmosphere at a heating rate of 10 K·min-1.Powder XRD diffractograms were obtained with a Bruker D8X diffractmeter equipped with monochromated Cu Kα(λ=0.154 18 nm)radiation at room temperature.
1.2 Synthesis of L
The ligand,3-phenoxymethy-4-(methyphenyl)-5-(2-pyridy)-1,2,4-triazole(L)was synthesized by the following reaction.To a solution of di(p-methyphenyl) phosphazoanilide(4.0 g,16.5 mmol)in anhydrous N, N-dimethylaniline(30 mL)was added N-phenoxyacety -N′-(2-pyridyl)hydraizine(4.1 g,15.0 mmol).The mixture was refluxed at 190~200℃for 3 hours,and then the solvent was removed under reduced pressure. Concentrated hydrochloric acid(4.0 mL)was added to the residue and refluxed for 1 hour.After cooling to room temperature,the solution was filtered,and the filtrate was neutralized to pH=8~9 with K2CO3.A precipitate was formed and collected.The crude product was recrystallized from EtOH to give a white solid.Yield:1.9 g(38.4%based on di(p-methyphenyl) phosphazoanilide),m.p.146~147℃.Anal.Calcd.for C21H18N4O(%):C 73.67,H 5.30,N 16.36;Found(%):C 73.48,H 5.59,N 15.97.1H NMR(300 MHz,CDCl3, 25C,TMS):δ 2.317(s,3H),5.105(s,2H),6.992~7.128 (m,5H),7.230~7.260(m,4H),7.718~8.343(m,4H). UV-Vis(CH3CN):λmax=221.0,269.5 nm.IR(KBr pellet, cm-1):3 075(m),3 051(w),2 949(w),2 870(w),1 587 (s),1 518(s),1 460(s),1 238(s),863(m),827(s),758 (s),690(w).ESI-MS:m/z=343.3.
1.3 Synthesis of complex 1
A solution of CuCl2·2H2O(0.341 g,2 mmol)in 10 mL ethanol was added to a warm solution of L (0.342 g,1 mmol)in 10 mL ethanol.The mixture turned green immediately and a green precipitate formed.The mixture was filtered and the precipitate was collected.Green crystals was obtained by recrystallization from acetonitrile.Yield:0.371g(75.2% based on L).Anal.Calcd.for C42H40Cl4Cu2N8O4(%):C 50.97,H 4.07,N 11.32;Found(%):C 50.89,H 4.21, N 11.07.UV-Vis(CH3CN):λmax=246.0,276.5 nm.IR(KBr pellet,cm-1):3 448(w),3 085(m),1 602(s),1 523 (s),1 496(s),1 245(s),869(m),784(s),707(w).
1.4 Synthesis of complex 2
A solution of CdCl2·2.5H2O(0.457 g,2 mmol) in 10 mL acetonitrile was add to a warm solution of L (0.342 g,1 mmol)in 20 mL acetonitrile.A colorless mixture formed and was filtered.The filtrate was left to stand at room temperature for evaporation.Several days later a colorless crystals was collected.Yield: 0.439 g(66.8%based on L)Anal.Calcd.for C46H42Cl6Cd3N10O2(%):C 41.96,H 3.21,N 10.64;Found(%):C 42.37,H 3.48,N 10.23.UV-Vis(CH3CN):λmax=244.5, 282.5 nm.IR(KBr pellet,cm-1):3 447(w),3 091(m), 1 607(s),1 518(s),1 464(s),1 242(s),868(w),770(s), 702(m).
1.5 Crystal structure determination
Well-shaped single crystals of L,1 and 2 were selected for X-ray diffraction study.The unit cell parameters and the intensity data were collected at 296(2)K on a Bruker Smart APEXⅡCCD diffractometer with a detector distance of 5 cm and frame exposure time of 10 s using graphite-monochromated Mo Kα(λ=0.071 073 nm)radiation.The structures were solved by direct methods and refined on F2by full-matrix least squares procedures using SHELXTL software[27].All nonhydrogen atoms were anisotropically refined and hydrogens were generated geometrically and allowed to ride on their respective parent atoms, but not refined.Crystal data and structure refinement for L,1 and 2 are listed in Table 1.Selected bond lengths and angles for L,1 and 2 are listed in Table 2.Relevant hydrogen-bonding parameters of L,1 and 2 are summarized in Table 3.
CCDC:1015366,L;1015367,1;1015368,2.
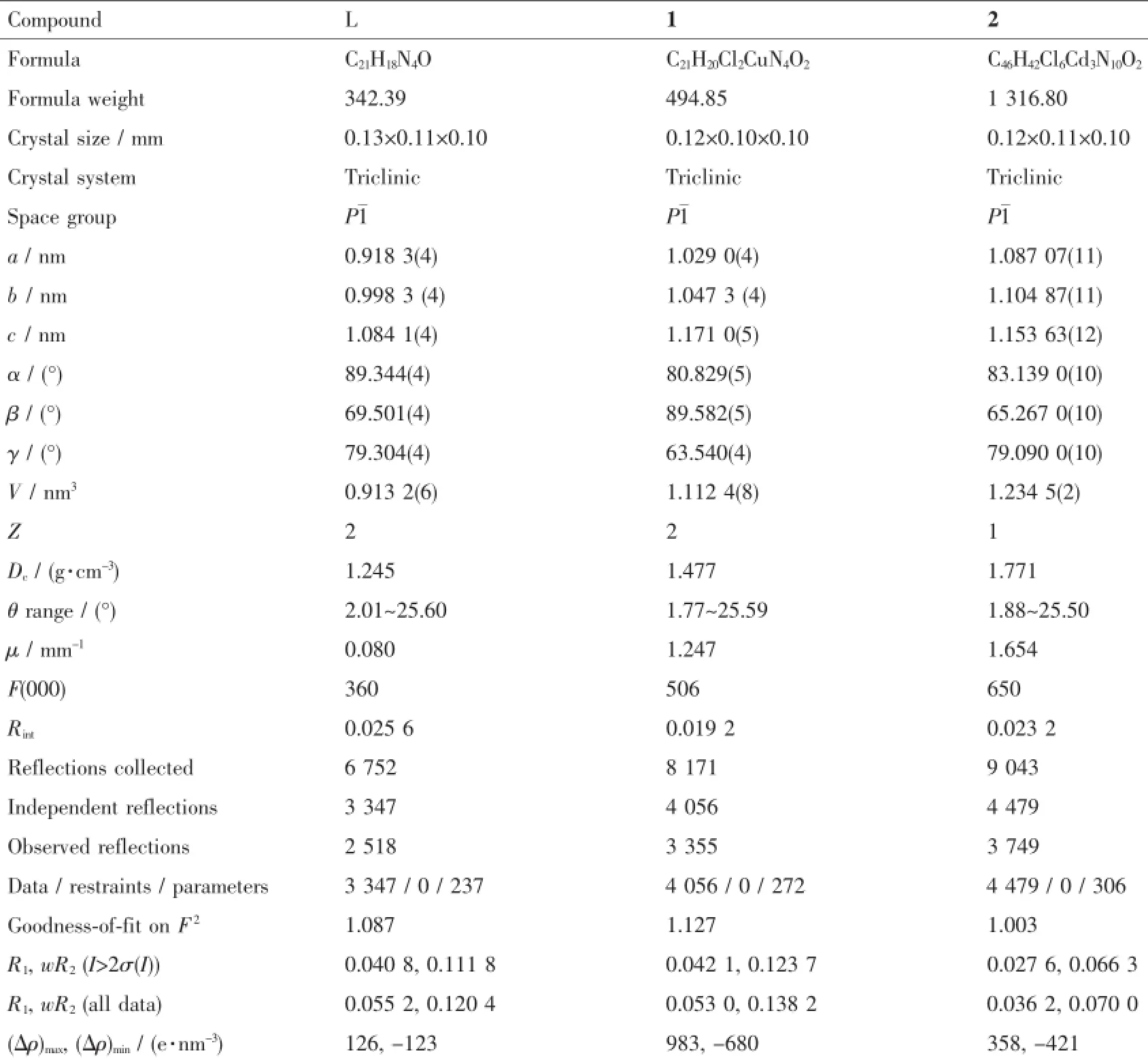
Table 1Crystal data and structure refinement for L,1 and 2
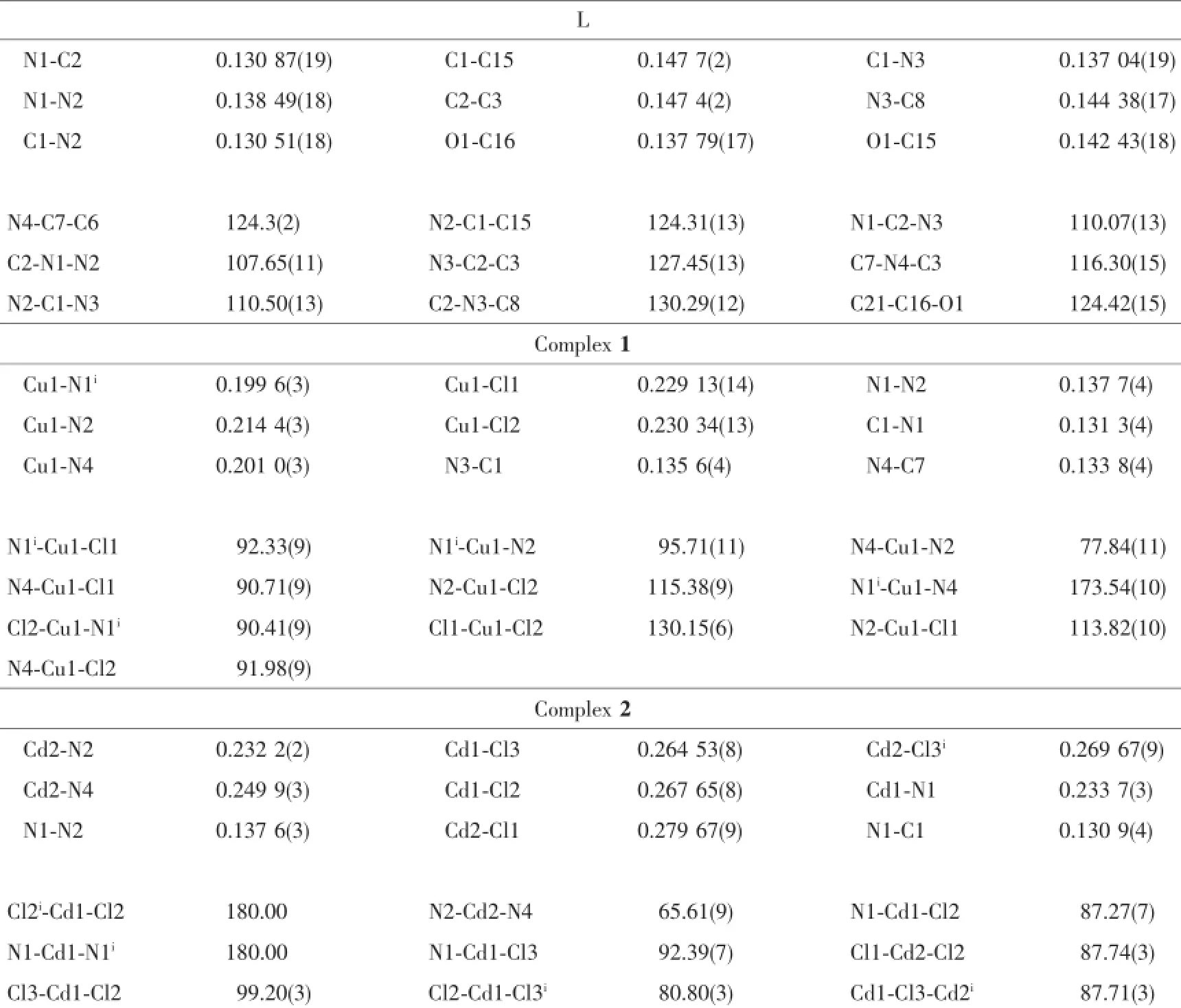
Table 2Selected bond lengths(nm)and bond angles(°)for L,1 and 2
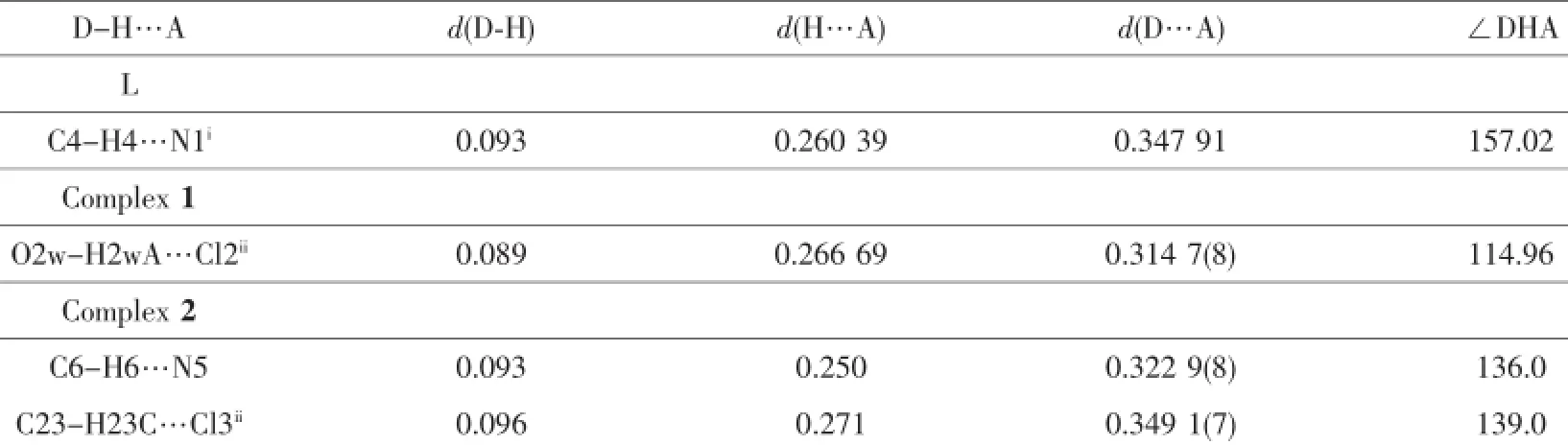
Table 3Hydrogen bonding lengths(nm)and angles(°)for L,1 and 2
2 Results and discussion
2.1 Description of crystal structures
Crystal structure of L:X-ray structure analyses reveals that L crystallizes in the triclinic space group P1.Three aromatic rings of 1,2,4-triazole ring,pyridyl ring and phenyl ring(C8,C9,C10,C11,C12,C13) have planar structures(Fig.1),but they are noncoplanar.The central 1,2,4-triazole ring is oriented at dihedral angles of 41.82(7)°and 73.84(6)°with the pyridyl ring and phenyl ring(C8,C9,C10,C11,C12, C13),respectively.There are a weak intermolecular hydrogen bond of C4-H4…N1 and three C-H…π interactions in the L.The C-H…π interactionsinvolve C6-H6…Cg3,C18-H18…Cg1 and C20-H20…Cg3.Three face-to-face π…π stacking interaction (Fig.2)exists between the two parallel triazole rings. All these interactions assemble the three-dimensional structure of L.
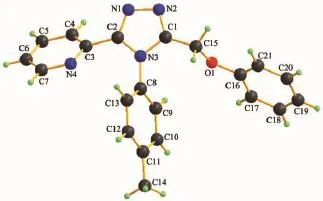
Fig.1Structure of L with the atomic labeling
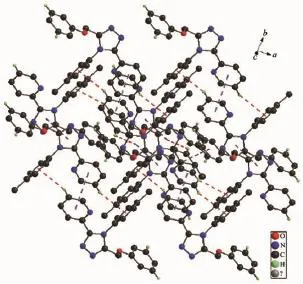
Fig.2Packing diagram of L showing the π…π and C-H…π interactions
Crystal Structure of 1:Complex 1 is a doublebridged binuclear copper(Ⅱ)complex(Fig.3)crystallizing in the triclinic space group P1.Two Cu(Ⅱ)atoms are double-bridged together by four nitrogen atoms (N1,N2)of two triazole rings in L,and the bond lengths of Cu1-N1iand Cu1-N2 are 0.199 6(3)and 0.214 4(3)nm,respectively.Each Cu(Ⅱ)atom is also coordinated by another nitrogen atom(N4)of pyridyl ring and two chloride atoms(Cl1,Cl2),and the bond lengths of Cu1-N4,Cu1-Cl1 and Cu1-Cl2 are 0.201 0(3), 0.229 13(14)and 0.230 34(13)nm,respectively.All these nitrogen atoms(N1,N2,N4)and chloride atoms (Cl1,Cl2)make the center Cu(Ⅱ)atoms form distorted trigonal-bipyramidal geometries[CuN3Cl2].
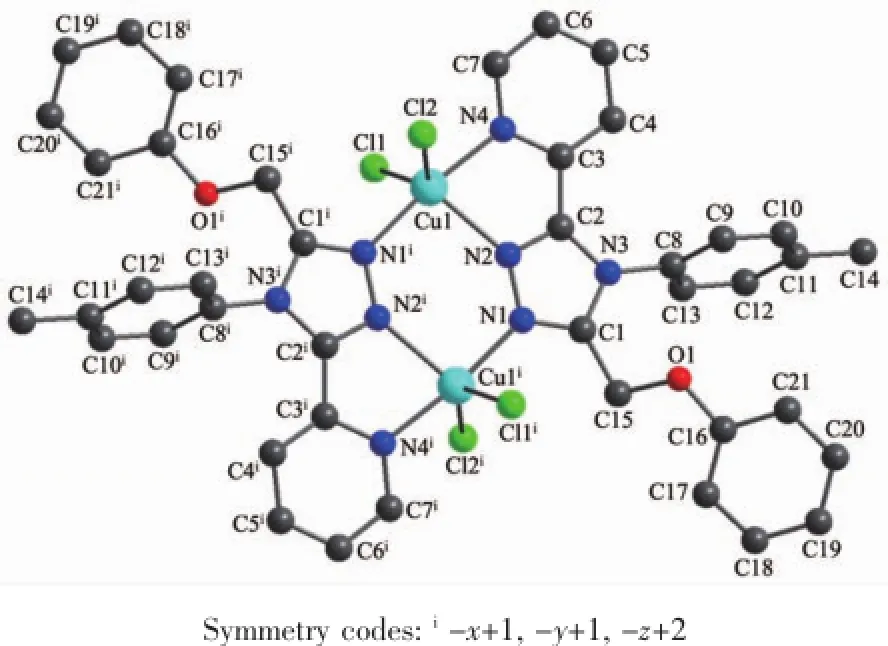
Fig.3Structure of 1 with the atomic labeling
As the nitrogen atom(N4)of pyridyl ring coordinated to the center Cu1(Ⅱ)atom,it makes the 1,2, 4-triazole ring and pyridyl ring almost coplanar with an dihedral angle of 5.97(19)°.There are a weak intermolecular hydrogen bond of O2W-H2WB…Cl2 and several C-H…π interactions in complex 1.The C-H…π interactions involve O2W-H2WA…Cg2,O2WH2WB…Cg2,C9-H9…Cg6,C12-H12…Cg6 and C20-H20…Cg2.An offset face-to-face π…π interaction(Fig.4)exists between the two parallel pyridyl rings with a centroid-centroid distance of 0.370 8(3) nm.There is also another π…π interaction between the triazole ring and phenyl rings(C16,C17,C18,C19, C20,C21).All these interactions assemble the complex1 into a one-dimensional chain structure(Fig.5).
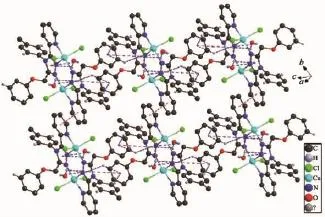
Fig.4Packing diagram of complex 1 showing the π…π and C-H…π interactions

Fig.5One-dimensional network of complex 1
Crystal Structure of 2:Complex 2 is a cadmium(Ⅱ)polymeric complex(Fig.6)crystallizing in the triclinic space group P1.The Cd(Ⅱ)atoms are bridged by the nitrogenatoms(N1,N2)of triazole rings in L and chloride atoms.Every repeat unit has three Cd(Ⅱ)atoms and two different Cd(Ⅱ)centers(Cd2-Cd1-Cd2i)(Fig.7). Two Cd2(Ⅱ)atoms(Cd2,Cd2i)are bridged together by two chloride atoms(Cl1,Cl1i)between every repeat units.The distance of two adjacent Cd(Ⅱ)atoms(Cd1, Cd2)in the repeat units is 0.370 13(4)nm,and that of two adjacent Cd2(Ⅱ)atoms between every repeat units is 0.394 13(5)nm.The Cd1(Ⅱ)atom possesses centre-symmetricaldistorted-octahedralgeometries [CdCl2Cl′2N2],and the bond lengths of Cd1-N1,Cd1-Cl2 and Cd1-Cl3 are 0.233 7(3),0.267 65(8)and 0.264 53(8)nm,respectively.The Cd2(Ⅱ)atoms have asymmetricaldistorted-octahedralgeometries [CdCl2Cl′Cl″NN′].
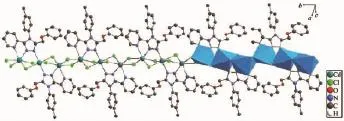
Fig.6A partial chain of complex 2
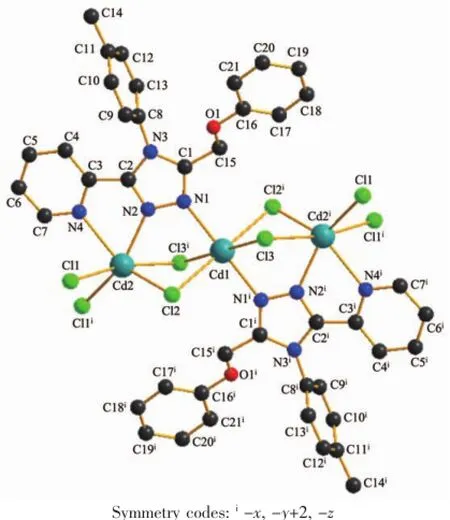
Fig.7Structure of 2 with the atomic labeling
In complex 2 there are two weak hydrogen bond interactions(C6-H6…N5 and C23-H23C…Cl3)and several C-H…π interactions(Fig.8).The C-H…π interactions involve C4-H4…Cg11,C9-H9…Cg12, C18-H18…Cg10,C20-H20…Cg9 and C23-H23B…Cg12.A face-to-face π…π interaction exists between the two parallel pyridyl rings with a centroid-centroid distance of 0.368 0(2)nm.Another π…π interaction involves the triazole ring and phenyl rings(C16,C17, C18,C19,C20,C21).Complex 2 forms a threedimensional structure through these interactions.
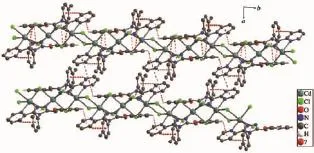
Fig.8Packing diagram of complex 2 showing the π…π and C-H…π interactions
2.2 Spectral characterization
The IR spectrum of the free L shows two medium intensity bands at 1 587 and 1 566 cm-1,which are attributed to pyridine ring vibrations.As the nitrogen atoms of pyridyl rings coordinate to metal atoms,the higher band shifted about 15 cm-1[28].So we can find a band at 1 601 cm-1(or 1 606 cm-1)in the spectrum of 1(or 2),which can be assigned to the coordinated pryidyl rings,indicating that the ligands of L in complexs 1 and 2 are chelating to metal atoms.In addition,the bands at 1 238,1 245 and 1 242 cm-1are attributed to the C-O stretching vibrations in L, complex 1 and 2,respectively.These features are in consistent with the results of X-ray analyses.
The UV-Vis spectra of ligand L,complex 1 and 2 were performed in dilute acetonitrile solution.The ligand L exhibits two strong bands at 221.0 and 269.5 nm.The complex 1(or 2)shows two bands at 246.0 and 276.5 nm(or 244.5 and 282.5 nm),which are attributedtotheLπ-π*andn-π*electronic transitions.
The photoluminescent properties of complex 2 and the free ligand L have been studied in the solid state at room temperature(Fig.9).The complex 2exhibits an emission band at 403 nm with excitation at 314 nm,while free ligand L displays an emission at 422 nm upon excitation at 327 nm.Compared with the free ligand L,the emission band of complex 2 is blue-shifted about 19 nm.As the Cd2+has d10configuration,the luminescence emission of complex 2 is contributed to the intra-ligand fluorescent emission of the ligand L[29].This performance indicates that complex 2 could be potential photoluminescent materials and electron-transport materials.
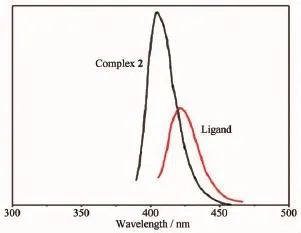
Fig.9Solid-state emission spectra of free Ligand L and 2 at room temperature
2.3 Thermogravimetric analysis(TGA)and PXRD
Thermogravimetric analysis(TGA)of complex 1 and 2 were performed in the temperature range of 40~800℃in nitrogen atmosphere with a heating rate of 10 K·min-1to investigate their thermal stabilities(Fig. 10).For complex 1,the first weight loss of 3.52% between 40 and 95℃is observed,due to the loss of two lattice water molecules(Calcd.3.64%).Practically no weight loss was found between 95 and 245℃,A rapid weight loss was observed above 245℃with the residue of CuCl2(Obsd.26.20%,Calcd.27.28%). For complex 2,the TGA curve shows that no obvious weight loss is found until the abrupt decomposition of the framework occurs at about 260℃.Powder X-ray diffraction(PXRD)experiments were carried out on the ligand L,complex 1 and 2.The experimental and simulated XRD patterns generated from single-crystal diffraction data of ligand L,complex 1 and 2 are shown in Fig.11.The experimental peak position are in agreement with the corresponding simulated XRD patterns,which indicate the single phase purity of ligand L,complex 1 and 2.
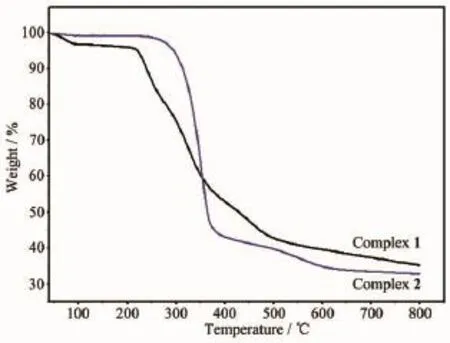
Fig.10TGA curves of the complexes 1 and 2
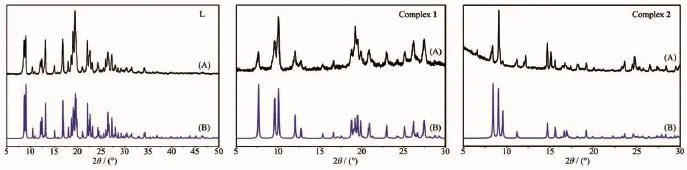
Fig.11Experimental(A)and simulated(B)X-ray powder diffraction patterns of L,1 and 2
3 Conclusions
Two complexes,[Cu2L2Cl4]·2H2O(1)and[Cd3L2(μ2-Cl)6]n·2nCH3CN(2),with 3-phenoxymethyl-4-(4-methyphenyl)-5-(2-pyridyl)-1,2,4-triazole(L)have been synthesized,andtheirmolecularstructureswere determined by X-ray crystallography,element analysis, IR,UV/Vis,fluorescence measurement,TGA and powder XRD.Crystallographic studies reveal that complex1hasadistortedtrigonalbipyramidal geometrey[CuN3Cl2].However,complex2isa polymeric,and every repeat unit has three Cd(Ⅱ)atoms and two different Cd(Ⅱ)centers(Cd2-Cd1-Cd2i). The centre Cd(Ⅱ)atoms possess distorted-octahedral geometries.
[1]Verschoor G C,Reedtjk J.Inorg.Chem.,1985,24:4128-4133
[2]Klingele M H,Boyd P D W,Moubaraki B,et al.Eur.J. Inorg.Chem.,2005,5:910-918
[3]Klingele M H,Moubaraki B,Murray K S,et al.Chem.Eur. J.,2005,11:6962-6973
[4]Koomen-van Oudenniel W M E,de Graaff R A G,Haasnoot J G,et al.Inorg.Chem.,1989,28:1128-1133
[5]Karayel A,Oezbey S,Capan G.J.Mol.Struct.,2007,841: 118-124
[6]Liu K,Shi W,Cheng P.Dalton Trans.,2011,40:8475-8490
[7]Singh N K,Bharty M K,Dulare R,et al.Polyhedron,2009, 28:2443-2449
[8]Walczak K,Gondela A,Suwiński J.Eur.J.Med.Chem., 2004,39:849-853
[9]Abdel-Aal M T,El-Sayed W A,El-Kosy S M,et al.Arch. Pharm.Chem.Life Sci.,2008,341:307-313
[10]Romagnoli R,Baraldi P G,Cruz-Lopez O,et al.Med.Chem., 2010,53:4248-4258
[11]Haasnoot J G.Coord.Chem.Rev.,2000,200/201/202:131-185
[12]Klingele M H,Brooker S.Coord.Chem.Rev.,2003,241:119 -132
[13]Beckmann U,Brooker S.Coord.Chem.Rev.,2003,245:17-29
[14]Kahn O,Martinez C J.Science,1998,279:44-48
[15]Zhu D R,Xu Y,Yu Z,et al.Chem.Mater.,2002,14:838-843
[16]Kitchen J A,Brooker S.Coord.Chem.Rev.,2008,252:2072 -2092
[17]Bao X,Guo P H,Liu W,et al.Chem.Sci.,2012,3:1629-1633
[18]Al-Masoudi I A,Al-Soud Y A,Al-Salihi N J,et al.Chem. Heterocycl.Compd.,2006,42:1377-1404
[19]QI Li(齐丽),ZHU Dun-Ru(朱敦如),XIE Da-Jing(谢大景), et al.Chinese J.Inorg.Chem.(无机化学学报),2008,24: 868-872
[20]Yang J,Bao W W,Ren X M,et al.J.Coord.Chem.,2009, 62:1809-1816
[21]Matouzenko G S,Bousseksou A,Borshch S A,et al.Inorg. Chem.,2004,43:227-236
[22]Xie D J,Lu W,Wang Z X,et al.Acta Crystallogr.,2009, E65:o1177-o1178
[23]WANG Zuo-Xiang(王作祥),XU Hai-Jun(徐海军),GONG Xiao-Ning(宫晓宁),et al.Chinese J.Inorg.Chem.(无机化学学报),2009,25:1492-1496
[24]LU Wei(卢伟),XIE Da-Jing(谢大景),WANG Zuo-Xiang(王作祥),et al.Chinese J.Inorg.Chem.(无机化学学报),2010, 25:717-720
[25]Li Y,Zhang C G,Cai L Y,et al.Coord.Chem.,2013,66: 3100-3112
[26]Li Y,Wang Z X,Zhang C G,et al.Inorg.Chem.Commun., 2013,38:58-61
[27]Sheldrick G M.SHELXTL,Structure Determination Software Programs,Version 5.10,Bruker Analytical X-ray Systems Inc.,Madison,WI,USA,1997.
[28]Zhu D R,Xu Y,Mei Y H,et al.J.Mol.Struct.,2001,559: 119-125
[29](a)Gao Z G,Cao R,Li X J,et al.Eur.J.Inorg.Chem.,2007, 5:742-748 (b)Jing X M,Li G H,Yu Y,et al.Cryst.Growth Des.,2010, 10:3489-3495 (c)Wang H L,Wang K,Sun D F,et al.CrystEngComm, 2011,13:279-286
Syntheses,Crystal Structures,and Spectral Properties of Copper(Ⅱ)and Cadmium(Ⅱ)Complexes with 3-Phenoxymethyl-4-(4-methyphenyl)-5-(2-pyridyl)-1,2,4-triazole
SHENG Jun-Feng1WANG Ning2CAI LIANG-Ying1SONG Fei1TONG Yu-Zhu1QU Zhi-Rong2WANG Zuo-Xiang*,1
(1School of Chemistry and Chemical Engineering,Southeast University,Nanjing 211189,China)
(2Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education,Hangzhou Normal University,Hangzhou 311121,China)
Two complexes[Cu2L2Cl4]·2H2O(1)and[Cd3L2(μ2-Cl)6]n·2nCH3CN(2)were obtained by the reaction of 3-phenoxymethyl-4-(4-methyphenyl)-5-(2-pyridyl)-1,2,4-triazole(L)with Copper(Ⅱ)chloride and cadmium(Ⅱ)chloride respectively.The complexes have been characterized by IR,UV,TGA,powder XRD,elemental analyses and crystal structures.The complexes 1 and 2 crystallize in the triclinic space group P1.Crystallographic studies reveal that 1 has a distorted trigonal bipyramidal[CuN3Cl2]coordination.2 is a polymeric complex.every repeat unit has three cadmium(Ⅱ)atoms and two different cadmium(Ⅱ)centers.Cd1(Ⅱ)atom possesses a centrosymmetric distorted-octahedral geometry with[CdCl2Cl′2N2],and Cd2(Ⅱ)atom has a distorted-octahedral geometries with [CdCl2Cl′Cl″NN′].CCDC:1015366,L;1015367,1;1015368,2.
copper(Ⅱ)complex;cadmium(Ⅱ))complex;crystal structures;triazole
O614.121;O614.24+2
A
1001-4861(2015)02-0405-08
10.11862/CJIC.2015.062
2014-09-29。收修改稿日期:2014-12-07。
企业合作基金(No.8507040052)资助项目。
*通讯联系人。E-mail:wangzx0908@aliyun.com
