Fe2BiTaO7纳米催化剂的组织结构及光催化性能
2015-04-01栾景飞胡文华陈标杭裴冬华
栾景飞 胡文华 陈标杭 裴冬华
(南京大学环境学院污染控制与资源化研究国家重点实验室,南京210023)
Fe2BiTaO7纳米催化剂的组织结构及光催化性能
栾景飞*胡文华 陈标杭 裴冬华
(南京大学环境学院污染控制与资源化研究国家重点实验室,南京210023)
用固相反应合成法合成了光催化剂Fe2BiTaO7,通过XRD、SEM、TEM、紫外-可见漫反射等表征方法对其组织结构及光催化性能进行了研究。结果表明Fe2BiTaO7为立方晶系烧绿石结构,空间群为Fd3m,禁带宽度为1.72 eV。通过比较Fe2BiTaO7、P25 TiO2、掺氮TiO2和Bi2InTaO7的可见光光催化降解罗丹明B,发现Fe2BiTaO7降解效果及催化活性均高于其它催化剂,并且Fe2BiTaO7降解罗丹明B效率是掺氮二氧化钛的1.5倍。Fe2BiTaO7降解罗丹明B的曲线符合一级动力学,一级动力学常数为0.022 93 min-1。研究了罗丹明B可能的降解路径和Fe2BiTaO7在可见光下降解苯酚的效果。Fe2BiTaO7(可见光)光催化剂系统适用于纺织工业废水处理。
催化作用;Fe2BiTaO7;光学性能;可见光;光催化降解;罗丹明B
0 Introduction
Dyes in the effluents of the textile,leather,food processing,dyeing,paper,andmanufacturing industries have become one of the most notorious organic pollutants in aquatic environments in recent years[1-6].There is huge volume of dye wastewater releasing to the ecosystem each year,which causes serious environmental pollutions[7].The presence of dye in water is not only aesthetically displeasing but alsoadversetowatertransparency,resultingin reduction of sunlight penetration,gas solubility and reducing the photosynthetic reaction[3-4,8].Some dyes also exhibit toxic effects toward microbial populations and some are even carcinogenic to mankind[9-10]. Rhodamine B(RhB)is one of the most important representatives of xanthene dyes widely utilized as a photosensitizer,a quantum counter and an active medium in dye lasers,etc.Most dyes are resistant to biodegradation and direct photolysis.As a N-containing dye,RhBundergoesnaturalreductiveanaerobic degradation to yield potentially carcinogenic aromatic amines[11-12].Therefore,the removal of RhB from wastewater is necessary and should be highly concerned.
Conventionalmethodsincludingphysical, chemical and biological processes are used for the removal of dyes[13-16].However,it is usually inefficient to use biological oxidation or multi-step physicalchemicaltreatmentsforremovingdyecolors[17]. Photocatalysis has emerged as an efficient approach for purifying water[18-20].In recent years,photosensitive degradation of colored contaminants in wastewater on semiconductor surface has attracted a great deal of attentions[21-25].Zhao et al.[26]reported that some dyes could be degraded under visible light irradiation over TiO2by a self-photosensitized process.Some dyes are often utilized as a probe contaminant to evaluate the activity of a photocatalyst under irradiation of both ultraviolet light and visible light[27-28].Asthe most commonly used photocatalyst,TiO2has shown effective photocatalytic activity for RhB under ultraviolet light irradiation accounted for 4%of sunlight.However, TiO2cannot be used in the visible light region which accounts for 43%of sunlight,and this problem limits itsapplicationsinthefieldofphotocatalysis. Therefore,some efficient catalysts,which can generate electron-hole pairs under visible light irradiation, should be developed.Fortunately,there are some oxides such as BiVO4,Bi12TiO20,K6Nb10.8O30,Bi38ZnO58which show photocatalytic activity under visible light irradiation[29-37].Because of the controllability in composition, the diversity in structure and the difference in property, transitionmetaloxidesnano-compositehasbeen further developed in the field of photocatalysis.A series of A2B2O7inorganic compounds with different structures have been resulted from the wide-range chemicalreplacementinA,BandOsite.In particular,A2B2O7compounds are considered to own betterphotocatalyticpropertyundervisiblelight irradiation[38-42],they have not only been the important part of the nano-materials field but also have a good application in real life.In our previous work[43],we found that Bi2InTaO7crystallized with the pyrochloretype structure which could be used as a photocatalyst under visible light irradiation.It seems that Bi2InTaO7has a potential for improvement of photocatalytic activity by modification of its structure.According to above analysis,we guess that the substitution of Bi3+by Fe5+and substitution of In3+by Bi3+in Bi2InTaO7may increase carriers concentration.A change and improvementoftheelectricaltransportationand photophysicalpropertiescanbefoundinthe Fe2BiTaO7compound.
To the best of our knowledge,there have no reports on preparation and structural,photophysical andphotocatalyticpropertycharacterizationsfor Fe2BiTaO7.The molecular composition of Fe2BiTaO7is very similar to other A2B2O7compounds.The resemblance suggests that Fe2BiTaO7may possess photocatalytic property under visible light irradiation,similar to that of other members in A2B2O7family.Fe2BiTaO7also seemstohavepotentialforimprovingthe photocatalytic activity by modification of its structure.
We report here the synthesized semiconductor compound Fe2BiTaO7and its photocatalytic property for photosensitized removal of colored contaminants inwastewaterundervisiblelightirradiation.The structural,photophysical and photocatalytic property of Fe2BiTaO7were investigated.A comparison among the photocatalytic property of Fe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7was performed in order to reveal the structure-photocatalytic activity relationship for the title compound.
1 Experimental
1.1 Preparation of Fe2BiTaO7powder photocatalyst
The photocatalysts were synthesized by a solidstate reaction method[36].Fe2O3,In2O3,Bi2O3and Ta2O5with purity of 99.99%(Sinopharm Group Chemical Reagent Co.,Ltd.,Shanghai,China)were utilized as precursors.All powders were dried at 200℃for 4 h before synthesis.The precursors were stoichiometrically mixed prior to the synthesis of Fe2BiTaO7and the mixture was then pressed into small columns and put into an alumina crucible(Shenyang Crucible Co.,Ltd., China).Finally,calcination was carried out at 1 060℃for 30 h in an electric furnace(KSL 1700X,Hefei KejingMaterialsTechnologyCO.,Ltd.,China). Similarly,Bi2InTaO7was prepared by calcination at 1 050℃for 46 h.
1.2 Preparation of nitrogen-doped titania photocatalyst
Nitrogen-doped titania(N-doped TiO2)catalyst with tetrabutyl titanate as a titanium precursor was preparedbyusingthesol-gelmethodatroom temperature.17 mL tetrabutyl titanate and 40 mL absolute ethyl alcohol were mixed as solution a,which was then added dropwise under vigorous stirring into a mixture(solution b)of 40 mL absolute ethyl alcohol, 10 mL glacial acetic acid and 5 mL double distilled waterto form transparent colloidal suspension c. Subsequently aqua ammonia with nN/nTiof 8%was added into c under vigorous stirring and stirred for 1 h.Finally,the xerogel was formed after being aged for 2 d.The xerogel was ground into powder and was then calcined at 500℃for 2 h,subsequently was ground in agate mortar and screened by shaker to obtain N-doped TiO2powder.
1.3 Characterization of photocatalysts
The crystal structures of the photocatalysts were analyzed by the powder X-ray diffraction method(D/ MAX-rB,Rigaku Corporation,Japan)with Cu Kα radiation(λ=1.54 18 nm).The data were collected at 295 K with a step-scan procedure in the range of 2θ= 10°~95°.The step interval was 0.02°and the time per step was 1.2 s.The chemical composition of Fe2BiTaO7was estimated by scanning electron microscope energy dispersiveX-rayspectroscopy(SEM-EDS,LEO 1530VP,LEO Corporation,Germany).The contents of surface O2-,Fe3+,Bi3+and Ta5+in Fe2BiTaO7were determined by X-ray photoelectron spectroscopy(XPS, ESCALABMK-2,VGScientificLtd.,U.K.).The chemical composition within the depth profile of Fe2BiTaO7was examined by the argon ion denudation method when X-ray photoelectron spectroscopy was utilized.The optical absorption of Fe2BiTaO7was analyzedwithanUV-Visiblespectrophotometer (Lambda 40,Perkin-Elmer Corporation,USA).The surface area of Fe2BiTaO7was determined by BET model(MS-21,Quantachrome Instruments Corporation, USA)with N2adsorption at liquid nitrogen temperature. The particle sizes of the photocatalysts were measured by malvern′s mastersize-2000 particle size analyzer (Malvern Instruments Ltd.,United Kingdom).The particle morphology was measured by transmission electronmicroscope(TecnalF20S-Twin,FEI Corporation,USA).
1.4 Photocatalytic reaction
The photocatalytic degradation of RhB(Tianjin Kermel Chemical Reagent Co.,Ltd.)was performed with 0.8 g photocatalyst(Fe2BiTaO7,P25 TiO2,N-doped TiO2or Bi2InTaO7)powder suspended in 300 mL of 0.029 3 mmol·L-1RhB solution within a pyrex glass cell(Jiangsu Yancheng Huaou Industry,China). Before visible light irradiation,the suspension was magnetically stirred in the dark for 45 min to ensure establishment of an adsorption/desorption equilibrium among the photocatalysts,RhB dye and atmospheric oxygen.Thephotocatalyticreactionsystemis consisted of a 300 W Xe arc lamp with the main emission wavelength at 436 nm(Nanjing JYZCPSTCO.,Ltd.),a magnetic stirrer and a cut-off filter(λ>400 nm,Jiangsu Nantong JSOL Corporation,China). The Xe arc lamp was surrounded by a quartz jacket andwaspositionedwithintheinnerpartofa photoreactor quartz vessel(5.8 cm in diameter and 68 cm in length),through which a suspension of RhB and photocatalyst was circulated.An outer recycling water glass jacket maintained a near constant reaction temperature(22℃),and the solution was continuously stirred and aerated.2 mL aliquots were sampled at various time intervals.The incident photon flux Iomeasured by a radiometer(Model FZ-A,Photoelectric Instrument Factory Beijing Normal University,China) was 4.76×10-6Einstein·L-1·s-1under visible light irradiation(wavelength range of 400~700 nm).The incident photon flux on the photoreactor was varied by adjusting the distance between the photoreactor and the Xe arc lamp.The adjustment of pH value was not carried out and the initial pH value was 7.0.The concentration of RhB was determined according to the absorption at 554 nm by an UV-Vis spectrophotometer (Lambda 40,Perkin-Elmer Corporation,USA).The inorganic products obtained from RhB degradation were analyzed by ion chromatograph(DX-300,Dionex Corporation,USA).
The identification of RhB and the degradation intermediate products of RhB were performed by gas chromatograph-mass spectrometer(HP 6890 Series Gas Chromatograph,HP-Innowax column,30 m×0.32 mm×0.25 μm)operated at 320℃and connected to HP 5973 mass selective detector and a flame ionization detector with He as the carrier gas.The split ratio was 40∶1,the injection and detector temperature were 250℃and 300℃respectively.Intermediate products of RhB were also identified by liquid chromatographmass spectrometer(LC-MS,Thermo Quest LCQ Duo, USA,HPLC column:Beta Basic-C18(150 mm×2.1 mm×5 μm),Finnigan,Thermo,USA).Here,20 μL of post-photocatalysis solution was injected automatically into the LC-MS system.The eluent contained 60% methanol and 40%water,and the flow rate was 0.2 mL·min-1.MS conditions included an electrospray ionization interface and a capillary temperature of 27℃with a voltage of 19.00 V,a spray voltage of 5 000 V and a constant sheath gas flow rate.The spectrum was acquired in the negative ion scan mode,sweeping the m/z range from 50 to 600.Evolution of CO2was analyzedwithanintersmatTMIGC120-MBgas chromatograph equipped with a porapack Q column (30 m×0.32 mm×20 μm),which was connected to a catharometer detector.
The total organic carbon(TOC)concentration was determined with a TOC analyzer(TOC-5000,Shimadzu Corporation,Japan).Thephotonicefficiencywas calculated according to the following equation[44-45]:
φ=R/Io
where φ is the photonic efficiency(%),and R is the rate of RhB degradation(mol·L-1·s-1),and Iois the incident photon flux(Einstein·L-1·s-1).
The catalyst recycling experiment was taken to prove that the Fe2BiTaO7catalyst was still active.The Fe2BiTaO7catalyst was washed by anhydrous ethanol and distilled water for 6 times after recycling from the previous experiment,then it was dried in the 60℃oven and put it into the photocatalytic reactor for the degradation of rhodamine B again,the catalyst was recycled for three times.
2 Results and discussion
2.1 Structure analysis
Fig.1showstheTEMimageofFe2BiTaO7nanoscale particles and regular shapes.The diameter of Fe2BiTaO7particles is 400~600 nm,indicating a small mean particle size.Fig.2(a)and(b)present SEM image and EDS spectrum of Fe2BiTaO7,respectively. SEM-EDSspectrumtakenfromthepreparedFe2BiTaO7indicates the presence of iron,bismuth, tantalum and oxygen.Other elements can not be identified from Fe2BiTaO7.
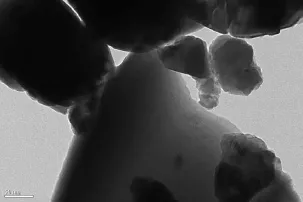
Fig.1TEM image of Fe2BiTaO7
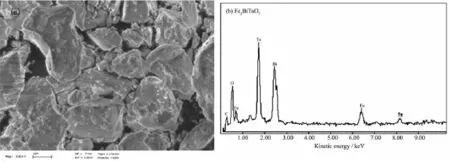
Fig.2SEM image and EDS spectrum of Fe2BiTaO7
Fig.3 shows the XRD pattern of Fe2BiTaO7.The full-profile structure refinements of the collected data are obtained by the RIETANTM[46]program based on Rietveld analysis.The results of the final refinements for Fe2BiTaO7indicate a good agreement between the observed intensities and calculated intensities for the pyrochlore-type structure,a cubic crystal system and a space group Fd3m(O atoms are included in the model).The lattice parameter α for Fe2BiTaO7is 1.048 734 4 nm.All the diffraction peaks for Fe2BiTaO7can be indexed according to the lattice constant and the space group above.The atomic coordinates and structural parameters of Fe2BiTaO7are listed in Table 1.It can be seen from Fig.3 that Fe2BiTaO7is a single phase.In addition,the XRD results show that Fe2BiTaO7crystallizes by the pyrochlore-type structure,a cubic crystal system and a space group Fd3m.The 2θ angles for each diffraction of Bi2InTaO7change with Bi3+substitution by Fe3+and In3+substitution by Bi3+. The lattice parameter decreases from α=1.074 641 0 nm for Bi2InTaO7to α=1.048 734 4 nm for Fe2BiTaO7, indicating a decrease in lattice parameter of the photocatalyst with a decrease of corresponding ionic radii,Fe3+(0.078 nm)<Bi3+(0.117 nm)and Fe3+(0.078 nm)<In3+(0.092 nm).
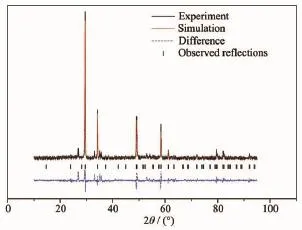
Fig.3XRD patterns and Rietveld refinements for Fe2BiTaO7prepared by a solid-state reaction method at 1 060℃
Fe2BiTaO7and Bi2InTaO7crystallize with the same pyrochlore-type structure according to the X-ray diffraction results.The cubic system structure with space group Fd3m for Bi2InTaO7keep unchanged upon substituting Fe3+by Bi3+and substituting Bi3+by In3+. The outcome of refinements for Fe2BiTaO7generates the unweighted R factors,RP=18.64%with spacegroup Fd3m.The crystal structure of Bi2InNbO7was refined by Zou et al.[47]and R factor obtained was large due to a slightly modified structure model for Bi2InNbO7.Accordingtothehighpurityofthe precursors utilized in this study and no impurity elements observed from EDS results,it is unlikely that the observed space groups originate from the impurities. Therefore,it is suggested that the slightly high RPfactor for Fe2BiTaO7is due to a slightly modified structure model for Fe2BiTaO7.It should be emphasized that the defects or the disorder/order of a fraction of the atoms can result in the change of structures, including different bond-distance distributions,thermal displacement parameters and occupation factors for some of the atoms.
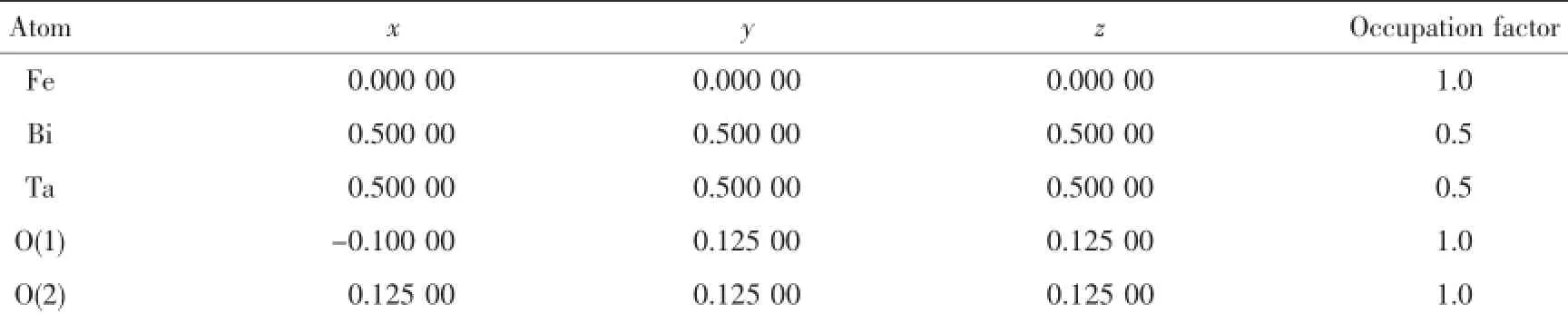
Table 1Atomic coordinates and structural parameters of Fe2BiTaO7prepared by the solid state reaction method
ThevariouselementalXPSpeaksandthe corresponding specific binding energies of Fe2BiTaO7, i.e.Bi4f7/2,Ta4f7/2,Fe2p3/2,O1s,are 155.9,26.6,708.2, 529.0 eV,respectively.The results further suggest that the oxidation states of Fe,Bi,Ta and O ions from Fe2BiTaO7are+3,+3,+5 and-2 respectively.For Fe2BiTaO7,the average atomic ratios of Fe∶Bi∶Ta∶O are 2.00∶0.97∶1.01∶6.96 according to the average results of XPS,SEM-EDS.Similarly,the oxidation states of Bi, In,Ta and O ions from Bi2InTaO7are+3,+3,+5 and -2 respectively.It is obvious that the observed XPS spectra of Fe2BiTaO7show neither shoulders nor widening peaks,implying(albeit not proving)the absence of any other phases.Hence,it can be deduced that the obtained material is of high purity under our preparation conditions.

Fig.4Selected area electron diffraction pattern of Fe2BiTaO7
Fig.4presentstheselectedareaelectron diffraction pattern of Fe2BiTaO7.It can be seen from Fig.4 that Fe2BiTaO7is a single phase.As shown in Fig.4,Fe2BiTaO7crystallizes with the pyrochlore-type structure,cubic crystal system and space group Fd3m. The lattice parameter for Fe2BiTaO7is α=1.048 734 4 nm.According to the calculation results from Fig.4, the(hkl)value for the main peaks of Fe2BiTaO7can be found and indexed.
2.2 UV-Vis diffuse reflectance spectroscopy
TheabsorptionspectrumofFe2BiTaO7is presented in Fig.5.Compared with the well-known TiO2whose absorption edge is less than 380 nm,the absorption edge of newly synthesized Fe2BiTaO7is at 710 nm,which is in the visible region of the spectrum.It is noteworthy that the apparent absorption(defined hereby as 1-transmission)can not take reflection and scatteringintoconsideration.Consequently,the apparent absorbance at sub-bandgap wavelengths(600 to 800 nm for Fe2BiTaO7)is higher than zero.
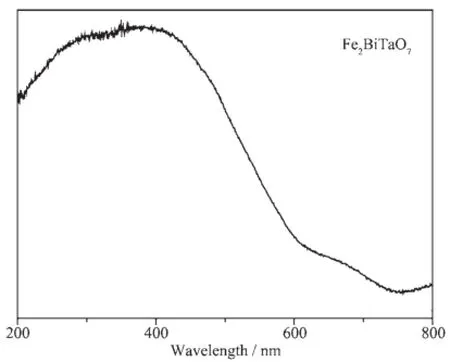
Fig.5Absorption spectrum of Fe2BiTaO7
For a crystalline semiconductor,the optical absorption near the band edge follows the equation:αhν= A(hν-Eg)n[48-49].Here,A,α,Egand ν are proportional constant,absorption coefficient,band gap and light frequency,respectively.Withintheequation,n determinesthecharacterofthetransitionina semiconductor.Egand n can be calculated by the following steps:(i)plotting ln(αhν)versus ln(hν-Eg)by assuming an approximate value of Eg,(ii)deducing the value of n according to the slope in the graph.(iii) refining the value of Egby plotting(αhν)1/nversus hν and extrapolating the plot to(αhν)1/n=0.According tothis method,Fig.6 shows the plot of(αhν)1/nversus hν for Fe2BiTaO7.According to the data in Fig.6,the value of Egfor Fe2BiTaO7is calculated to be 1.72 eV, while the value of n for Fe2BiTaO7is 0.5.The results above indicate that Fe2BiTaO7possesses a narrower band gap compared with Bi2InTaO7.At the same time, the optical transition for Fe2BiTaO7is directly allowed.
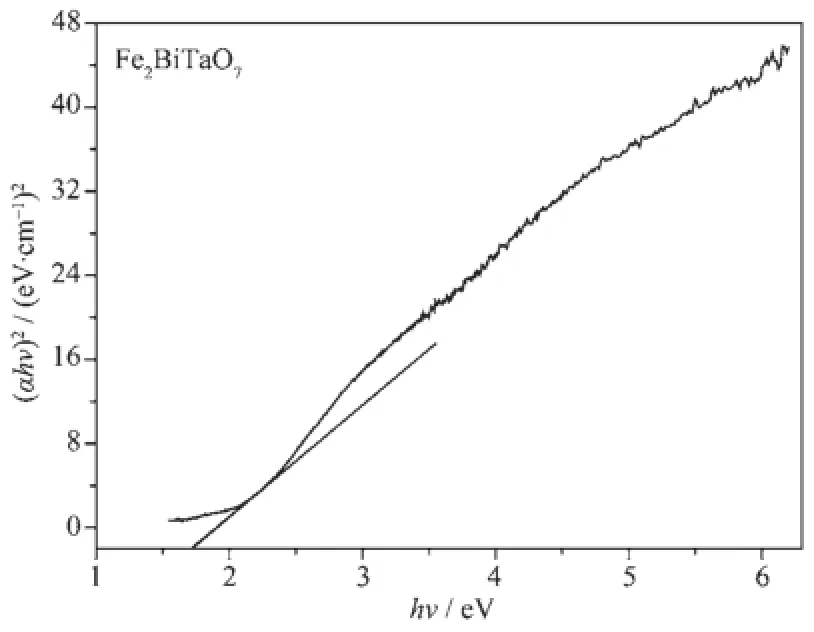
Fig.6Plot of(αhν)2versus hν for Fe2BiTaO7
2.3 Photocatalytic activity
Generally speaking,the semiconductor photocatalysis starts from the direct absorption of suprabandgap photons and the generation of electron-hole pairs in the semiconductor particles.Subsequently, the diffusion of the charge carriers to the surface of the semiconductor particles is followed.Fig.7 presents the concentration changes of RhB during the process ofphotocatalyticdegradationundervisiblelight irradiation(λ>400 nm)with the presence of Fe2BiTaO7, P25 TiO2,N-doped TiO2,Bi2InTaO7as well as with the absence of photocatalyst.Above measurements are performed under oxygen-saturation conditions(cO2sat=1.02 mmol·L-1).Though the photocatalyst/RhB suspension or RhB suspension exists in the experimental system,the degradation of RhB does not happen in dark.It can be clearly noticed from the results that a reduction of typical RhB peaks at 554 nm and 525 nm appear.Table 2 provides the photocatalytic effects with Fe2BiTaO7,P25 TiO2,N-doped TiO2or Bi2InTaO7as the catalyst under visible light irradiation(λ>400 nm).It can be seen from Table 2 that the photocatalytic efficiency is 95%with Fe2BiTaO7,59%with P25 TiO2,63%with N-doped TiO2,37.5%with Bi2InTaO7after 140 min under visible light irradiation.A complete color change from deep pink into colorless solution of the absorption signal is obtained with Fe2BiTaO7within 230 min, which shows a complete degradation.According to the results,fastdegradationrateisobservedwith Fe2BiTaO7as the catalyst,and the photocatalytic degradation activity of Fe2BiTaO7is higher than that of P25 TiO2,N-doped TiO2or Bi2InTaO7.Furthermore, the photocatalytic degradation activity of N-doped TiO2is higher than that of P25 TiO2and Bi2InTaO7. Additionally,somedecreasefortheUV-Vis absorbance signal of RhB is obtained under visible lightirradiationevenintheabsenceofa photocatalyst.The initial rate of RhB degradation isestimated to be 0.071 nmol·L-1·s-1and the photonic efficiency is 0.00149%(λ=420 nm)after visible light irradiation for 200 min with the absence of a photocatalyst.It suggests that the observed disappearance of RhB in the absence of a photocatalyst is due to direct dye-sensitization,and the dye-sensitization mechanism is similar to the observation from Liu et al.[50].
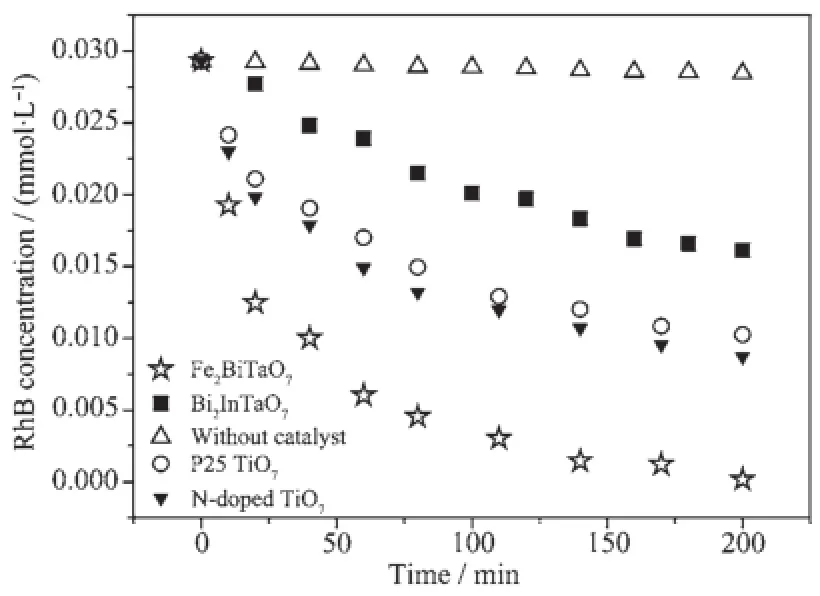
Fig.7Photocatalytic degradation of rhodamine B under visible light irradiation with the presence of Fe2BiTaO7,P25 TiO2,N-doped TiO2,Bi2InTaO7as well as with the absence of a photocatalyst
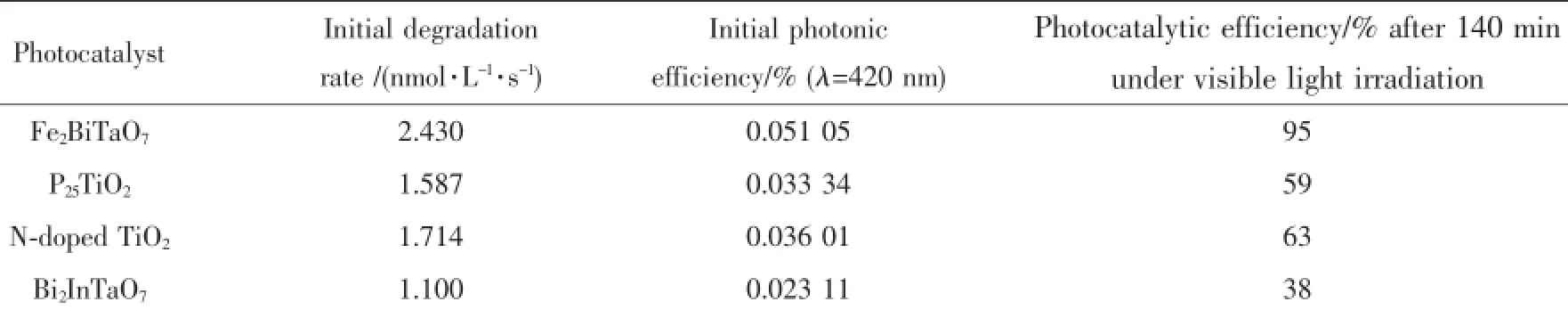
Table 2Photocatalytic effects with Fe2BiTaO7,P25 TiO2,N-doped TiO2or Bi2InTaO7as the catalyst under visible light irradiation
The degradation rate is almost 100%after visible light irradiation for 200 min with Fe2BiTaO7as the catalyst,the catalyst is not in deactivation at this time.Accordingtotheexperimentaldata,the degradation rate of the recycling experiment is 96%, 92%,90%,respectively.Although the degradation rate drops each time,the change is not great,thus the results above can prove that the Fe2BiTaO7catalyst is still active after the previous experiment,the property of Fe2BiTaO7catalyst is stable and it can be recycled for photocatalysis many times.
Thefirstordernatureofthephotocatalytic degradation kinetics with Fe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7as catalysts is clearly demonstratedinFig.8,whichpresentsalinear correlation between ln(C/C0)(or ln(TOC/TOC0))and the visible light irradiation time for the photocatalytic degradationofRhBwiththepresenceofthe photocatalysts.In above equation,C represents the RhB concentration at time t,and C0represents the initial RhB concentration,and TOC represents the total organic carbon concentration at time t and TOC0represents the initial total organic carbon concentration.According to the relationship between ln(C/C0) and the irradiation time,the apparent first order rate constant k is 0.022 93 min-1withFe2BiTaO7,0.006 27 min-1with P25 TiO2,0.007 15 min-1with N-doped TiO2and 0.003 29 min-1with Bi2InTaO7,indicating that Fe2BiTaO7is more efficient than P25 TiO2,N-doped TiO2or Bi2InTaO7for the photocatalytic degradation of RhB under visible light irradiation.In addition,N-doped TiO2is more efficient than P25 TiO2and Bi2InTaO7for the photocatalytic degradation of RhB undervisiblelightirradiation.Accordingtothe relationship between ln(TOC/TOC0)and the irradiation time,the apparent first order rate constantkTOCis estimated to be 0.019 22 min-1with Fe2BiTaO7, 0.006 22 min-1with P25 TiO2,0.006 73 min-1with N-dopedTiO2and0.00317min-1with Bi2InTaO7, indicatingthatthephotodegradationintermediate productsofRhBprobablyappearduringthe photocatalytic degradation of RhB under visible light irradiation.
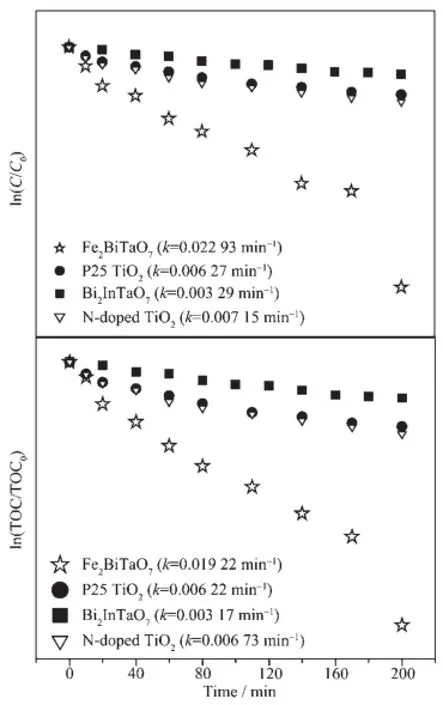
Fig.8Observed first order kinetic plots for the photocatalytic degradation of rhodamine B with Fe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7as catalysts under visible light irradiation
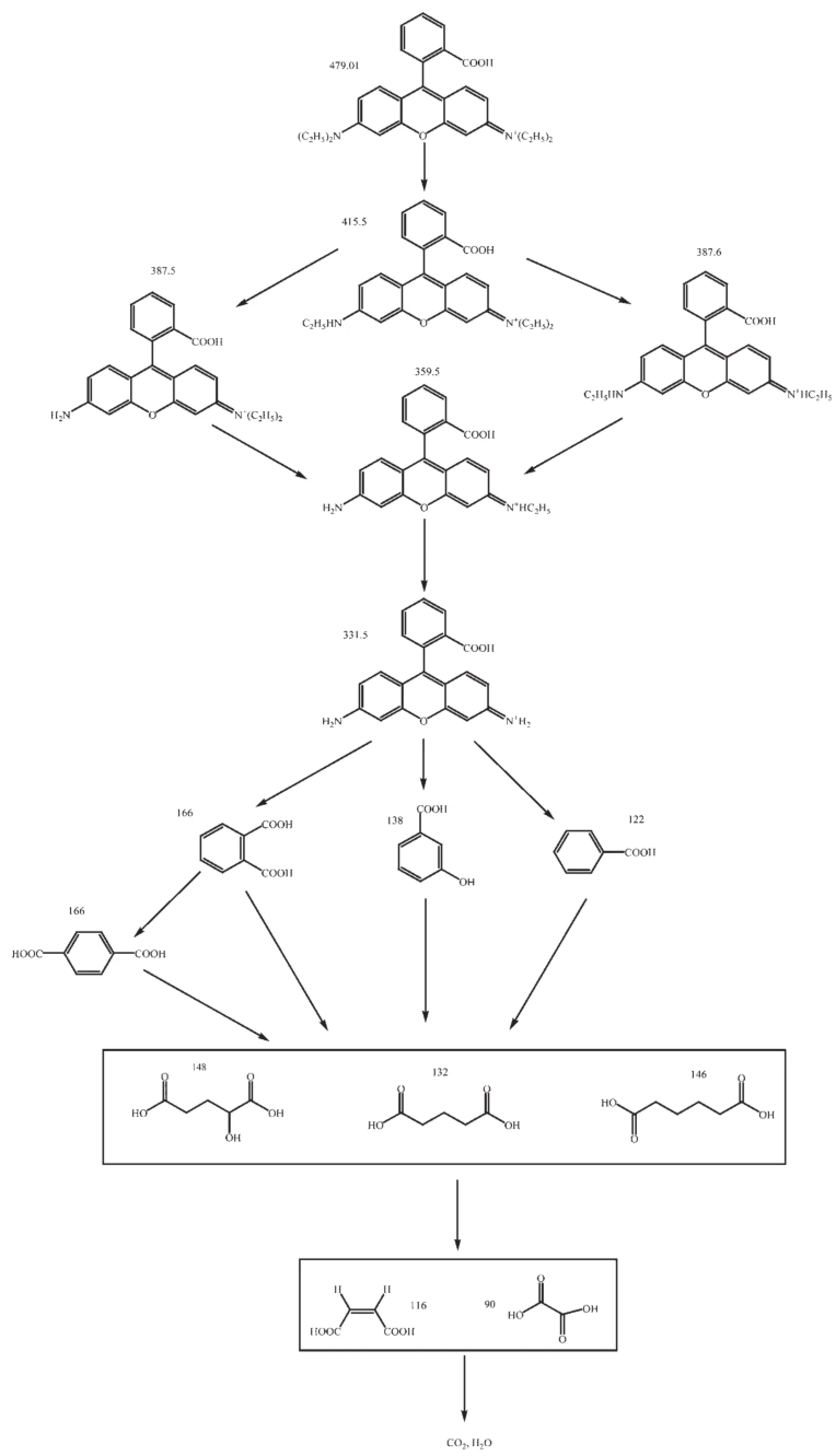
Fig.9Suggested photocatalytic degradation pathway scheme for rhodamine B under visible light irradiation with the presence of Fe2BiTaO7
Inourexperiments,thephotodegradation intermediate products of RhB with Fe2BiTaO7as the catalyst under visible light irradiation are identified as N,N-diethyl-N′-ethylrhodamine(m/z:415.5),N,N-diethylrhodamine(m/z:387.5),N-ethyl-N′-ethylrhodamine(m/z:387.6),N-ethylrhodamine(m/z:359.5)and rhodamine(m/z:331.5),benzoic acid(m/z:122), terephthalic acid(m/z:166),pentanedioic acid(m/z: 132),3-Hydroxybenzoic acid(m/z:138),1,2-benzenedicarboxylic acid(m/z:166)and maleic acid(m/z: 116),oxalic acid(m/z:90),2-hydroxypentanedioic acid(m/z:148)and adipic acid(m/z:146).Fig.9 suggests a possible photocatalytic degradation pathwayforRhBaccordingtotheintermediateproducts identified in this work.The main identified intermediates are the same as the results from Li et al.[51]for the TiO2-assisted photodegradation of RhB under visible light irradiation.However,Zhong et al.[52]reported that the major intermediates of RhB during microwaveenhanced photocatalysis also include malonic acid, succinic acid,phthalic acid and 3-nitrobenzoic acid. In addition to benzoic acid,2-hydroxypentanedioic acid,adipic acid,3-hydroxybenzoic acid and terephthalic acid,He et al.[53]indentified the presence of succinic acid and phthalic acid as well in the process of photocatalytic degradation of RhB by Bi2WO6with electron accepting agent under microwave irradiation.
The pathway was similar,but not identical to the pathway proposed by Horikoshi et al.[54]for the photodegradationofRhBunderultravioletlight irradiation and visible light irradiation assisted by microwave radiation with TiO2as the photocatalyst. According to the results of Li et al.[33],the RhB photodegradation occurs via two competitive processes: one process is N-demethylation,and the other process is the destruction of the conjugated structure.Thus, we consider that chromophore cleavage,ring-opening and mineralization should be the main photocatalytic degradation pathway of RhB in our work.RhB is convertedtosmallerorganicspecies,andthen mineralizes together with other organic groups to inorganic products such as CO2and water ultimately. Fig.10 presents the CO2yield during the photocatalytic degradation of RhB with Fe2BiTaO7,P25 TiO2,N-doped TiO2or Bi2InTaO7as the catalyst under visible light irradiation.The results show that the CO2yield increases gradually with increasing reaction time.The production rate of CO2with Fe2BiTaO7is higher than that with P25 TiO2,N-doped TiO2or Bi2InTaO7,which is in accordance with the absorption curve(Fig.5)of Fe2BiTaO7.The production amount of CO2is 0.241 03 mmol with Fe2BiTaO7as the catalyst,0.159 02 mmol with P25 TiO2,0.166 73 mmol with N-doped TiO2and 0.108 03 mmol with Bi2InTaO7in 300 mL reaction system after visible light irradiation for 200 min.
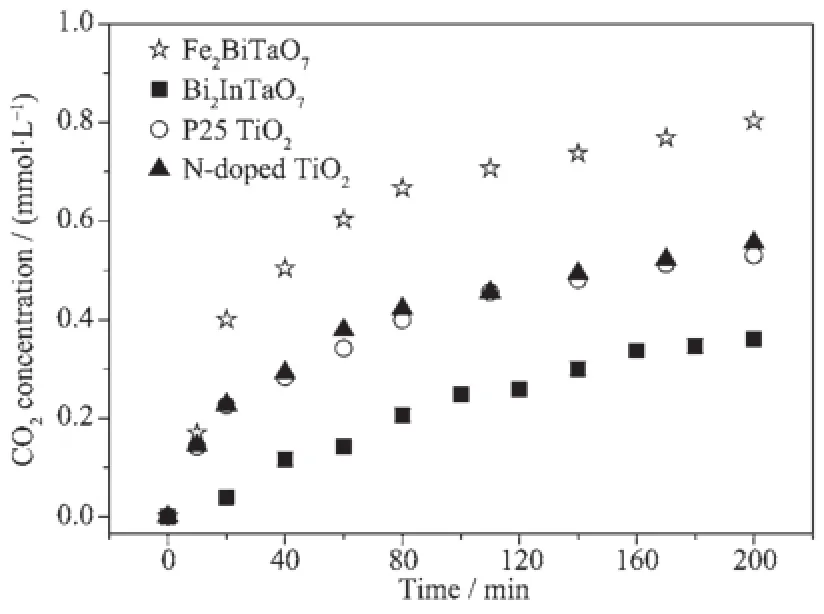
Fig.10CO2production kinetics during the photocatalytic degradation of rhodamine B with Fe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7as catalysts under visible light irradiation
Fig.11 demonstrates the results of total organic carbon(TOC)measurements.The results reveal that the total disappearance of organic carbon occurs after visible light irradiation for 230 min with Fe2BiTaO7as the catalyst.The results show that after visible light irradiation for 140 min,91%of TOC decrease is obtained with Fe2BiTaO7as the photocatalyst,59% with P25 TiO2,61%with N-doped TiO2and 37%with Bi2InTaO7.The turnover number(the ratio between the total amount of evolved gas and dissipative catalyst)is 0.185 for Fe2BiTaO7after visible light irradiation for 200min,suggestingthatthereactionsoccur catalytically.The reactions stop when the light is turned off.
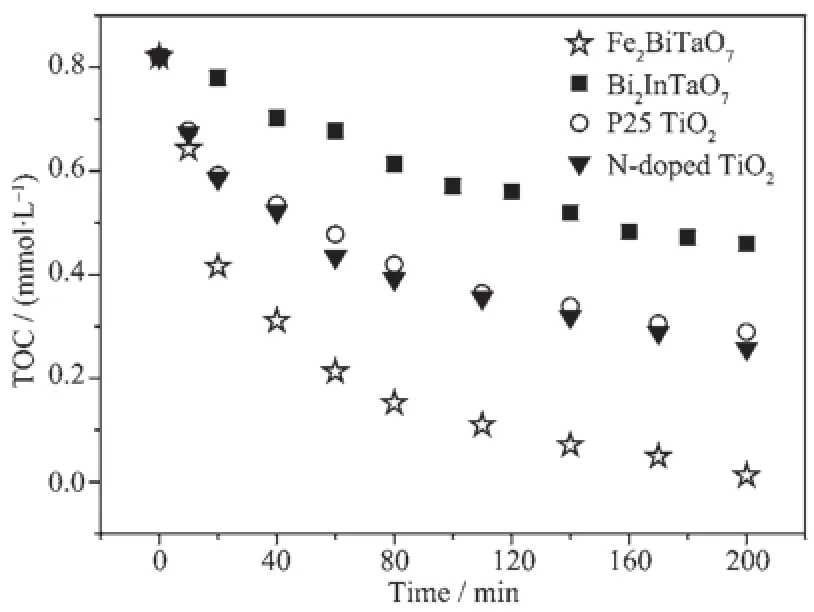
Fig.11Disappearance of total organic carbon(TOC) during the photocatalytic degradation of rhodamine B with Fe2BiTaO7,P25 TiO2, N-doped TiO2and Bi2InTaO7as catalysts under visible light irradiation
The photocatalytic property of the new compound Fe2BiTaO7is notable under visible light irradiation.This superior quality can be even more appreciated if we consider the fact that the specific surface area of Fe2BiTaO7is apparently smaller than that of titanium dioxide.In our work,the BET specific surface area is 46 m2·g-1for P25 TiO2,46 m2·g-1for N-doped TiO2,2 m2·g-1for Fe2BiTaO7and 1 m2·g-1for Bi2InTaO7respectively.The specific surface area of Fe2BiTaO7is almost 20 times smaller than that of P25 TiO2.
The action spectra of RhB degradation with the presence of Fe2BiTaO7under visible light irradiation show a clear photonic efficiency(0.03722%at its maximal point)at wavelengths corresponding to sub-Eg energies of Fe2BiTaO7(λ from 710 to 800 nm). The existence of photonic efficiency at energies where photons are not absorbed by Fe2BiTaO7,in particular the correlation between the low-energy action spectrum and the absorption spectrum of RhB,demonstrates clearlythatanyphotodegradationatwavelengths above 710 nm should be attributed to photosensitization by RhB itself(SchemeⅠ).
SchemeⅠ:
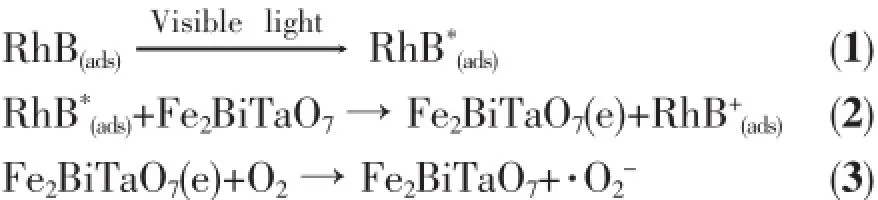
Accordingtothemechanismabove,RhB adsorbed on Fe2BiTaO7is excited by visible light irradiation.Subsequently an electron is injected from the excited RhB to the conduction band of Fe2BiTaO7where the electron is scavenged by molecular oxygen. SchemeⅠmay explain the results obtained with Fe2BiTaO7asthecatalystundervisiblelight irradiation,where Fe2BiTaO7may reduce recombination ofphotoinducedelectronsandholesviathe scavenging of electrons[56].
However,thesituationforphotocatalytic degradation mechanism of RhB is different below 710 nm,where the photonic efficiency correlates well with the absorption spectrum of Fe2BiTaO7.It evidently shows that the photocatalytic degradation mechanism of RhB is responsible for the photodegradation of RhB viabandgapexcitationofFe2BiTaO7.Although detailed experiments about the effects of oxygen and water on the degradation mechanism of RhB are not performed,it is sensible to assume that the degradation mechanism of RhB in the first step is similar to the degradation mechanism of RhB observed for Fe2BiTaO7under supra-bandgap irradiation,namely SchemeⅡ:
SchemeⅡ:
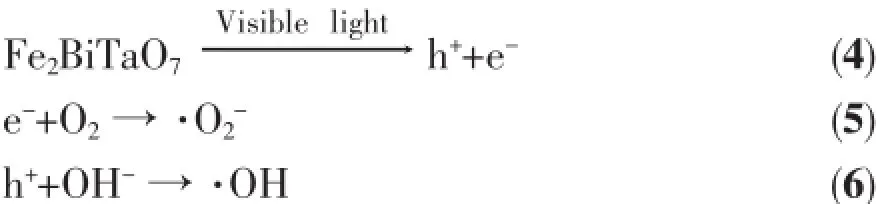
The M-O-M bond angle is closer to 180°,and the excited state is more delocalized as shown by previous study[57],thus the charge carriers can move easily in the matrix.High diffusivity due to the mobility of the photoinduced electrons and holes helps impel more electrons and holes to reach the reactive sites on the catalyst surface,resulting in the improvement of the photonic efficiency of Fe2BiTaO7.The lattice parameter a=1.048 734 4 nm for Fe2BiTaO7is smaller than the lattice parameter a=1.074 641 0 nm for Bi2InTaO7. Therefore,thephotoinducedelectronsandholes inside the particle of Fe2BiTaO7are easier and faster to reach the reactive sites on the catalyst surface compared with those of Bi2InTaO7.As a result,the photocatalytic degradation activity of Fe2BiTaO7is higher than that of Bi2InTaO7.The Bi-O-Ta bond angle of Fe2BiTaO7is 121°,close to 180°.Thus,the photocatalytic activity of Fe2BiTaO7is accordingly higher.Inaddition,theBi-O-Tabondangleof Fe2BiTaO7is larger than the Bi-O-Ta bond angle of Bi2InTaO7,resulting in an increase of photocatalytic activity for Fe2BiTaO7compared with Bi2InTaO7.The crystal structure of Fe2BiTaO7is similar to that of Bi2InTaO7,but the crystal structures of Fe2BiTaO7and P25 TiO2are different,and the electronic structures of them are also different.For Fe2BiTaO7,Fe is 3d-block metal element,and Bi is 6p-block metal element,and Ta is 5d-block metal element.But for Bi2InTaO7,Bi is 6p-block metal element,and In is 5p-block metal element,and Ta is 5d-block metal element.Moreover, for P25 TiO2,Ti is 3d-block metal element,indicating that the photocatalytic activity may be affected by not only the crystal structure but also the electronic structure of the photocatalysts.The difference of the photocatalytic degradation activity of RhB amongFe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7can be attributed mainly to the difference of their crystalline and electronic structures.
Fig.12 shows the suggested band structures of Fe2BiTaO7.Recently,the electronic structures of InMO4(M=V,Nb and Ta)and BiVO4have been reported by Oshikirietal.accordingtothefirstprinciples calculations[58].The conduction bands of InMO4(M=V, Nb and Ta)are mainly composed of a dominant orbital component from V3d,Nb4d and Ta5d orbitals, respectively.The valence bands of BiVO4are composed of a small Bi6s orbital component and a dominant O2porbitalcomponent.Thebandstructuresof Fe2BiTaO7should be similar to those of InMO4(M=V, NbandTa)andBiVO4.Therefore,itcanbe concluded that the conduction band of Fe2BiTaO7is composed of Ta5d,Fe3d and Bi6p orbitals,and the valence band of Fe2BiTaO7is composed of a small dominant O2p orbital component and a small Bi6s orbital component.Direct absorption of photons by Fe2BiTaO7can produce electron-hole pairs in the catalyst,showingthatthenecessaryenergyfor decomposing RhB by photocatalysis should be larger than the band gap energy.
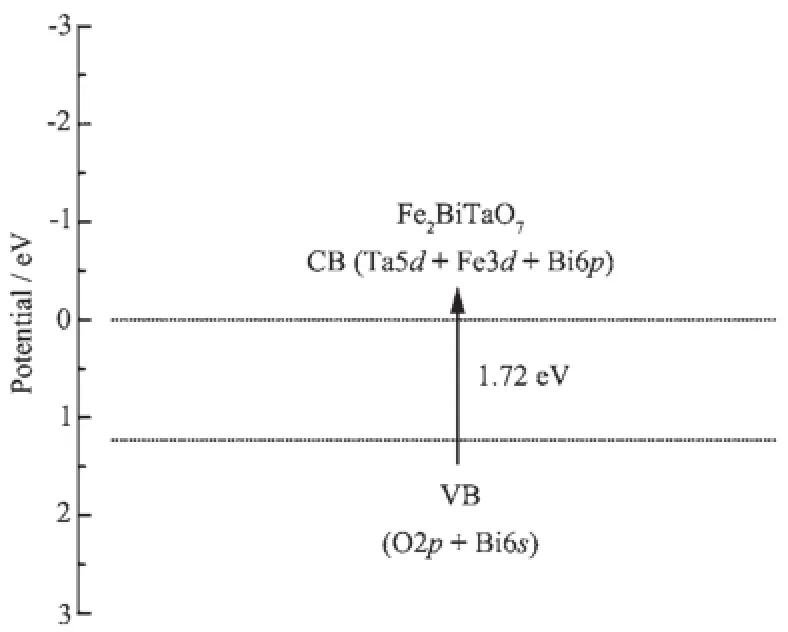
Fig.12Suggested band structure of Fe2BiTaO7
In order to see the effect of light wavelength on the degradation efficiency of rhodamine B,rhodamine B is degraded with Fe2BiTaO7,P25 TiO2,N-doped TiO2or Bi2InTaO7as the catalyst under visible light irradiation(λ>500 nm).The results show that the photocatalytic efficiency is 42%with Fe2BiTaO7,26% with P25 TiO2,28%with N-doped TiO2,17%with Bi2InTaO7after 140 min under visible light irradiation.
RhB has a certain absorption in visible light area.In order to eliminate the influence of photosensitization,we have substituted phenol for RhB as the reaction substrate.The process of the experiment is as follows:The photocatalytic degradation of phenol is performed with 0.8 g photocatalyst(Fe2BiTaO7or N-doped TiO2)powder suspended in 300 mL of 0.029 3 mmol·L-1phenol wastewater.The photocatalytic reaction system and initial experimental conditions are performed as the same as the previous experiment with RhB as the reaction substrate.The results show thatthephotocatalyticefficiencyis88%with Fe2BiTaO7,62%with N-doped TiO2after 200 min under visible light irradiation.As phenol has no absorption effect in visible light area,the degradation of phenol can only be caused by photocatalysis.Thus, it can be deduced that Fe2BiTaO7has a strong photocatalytic activity under visible light.
3 Conclusions
Fe2BiTaO7was prepared by a solid-state reaction method.The structural,optical absorption and photocatalytic properties of Fe2BiTaO7were investigated and compared with that of P25 TiO2,N-doped TiO2and Bi2InTaO7.XRD results demonstrate that Fe2BiTaO7crystallizes with the pyrochlore-type structure,cubic crystal system and space group Fd3m.The lattice parameter of Fe2BiTaO7is found to be 1.048 734 4 nm. The band gap of Fe2BiTaO7is estimated to be about 1.72 eV,indicating that Fe2BiTaO7shows a strong optical absorption during the visible light region(λ>400 nm).Photocatalytic degradation of aqueous RhB solutions is observed under visible light irradiation with the presence of Fe2BiTaO7accompanied with the formation of end products such as carbon dioxide and water.Therefore,it can be concluded that Fe2BiTaO7/ (Visible light)system may be regarded as an effective way for removing colored contaminants from waste water.Fe2BiTaO7also shows higher photocatalytic activity for photocatalytic degradation of RhB under visible light irradiation compared with P25 TiO2,N-dopedTiO2andBi2InTaO7.Thephotocatalytic degradation of RhB follows the first order reactionkinetics.The apparent first order rate constant k is 0.022 93 min-1withFe2BiTaO7,0.006 27 min-1with P25 TiO2,0.007 15 min-1with N-doped TiO2and 0.003 29 min-1with Bi2InTaO7.The possible photocatalytic degradation pathway of RhB is provided in this paper. The results in our work prove that Fe2BiTaO7/(visible light)photocatalysis may be regarded as a method for practical treatment of diluted colored waste water.The Fe2BiTaO7/(visible light)photocatalysis system without demanding chemical reagents or using high pressure of oxygen or heating can be utilized for decolorization, purification and detoxification in textile industries, and printing and dyeing industries.In conclusion,the Fe2BiTaO7/(visible light)photocatalysis system may provide a valuable treatment for purifying and reusing colored aqueous effluents.
Acknowledgements:This work was supported by the National Natural Science Foundation of China(No.21277067), by a grant from China-Israel Joint Research Program in Water Technology and Renewable Energy(No.5).by a grant from the NaturalScienceFoundationofJiangsuProvince(No. BK20141312),byaprojectofscienceandTechnology Development Plan of Suzhou City of China from 2014(No. ZXG201440).
[1]Annadurai G,Juang R S,Lee D J.J.Hazard.Mater.,2002, 92(3):263-274
[2]Bhatnagar A,Jain A K.J.Colloid Interface Sci.,2005,281 (1):49-55
[3]Su L,GanY X.Composites Part B,2012,43(2):170-182
[4]Wang S B,Boyjoo Y,Choueib A.Chemosphere,2005,60 (10):1401-1407
[5]Shakir K,Elkafrawy A F,Ghoneimy H F,et al.Water Res., 2010,44(5):1449-1461
[6]Shen C S,Shen Y,Wen Y Z,et al.Water Res.,2011,45(16): 5200-5210
[7]Zhang F L,Zhao J C,Zang L,et al.J.Mol.Catal.A:Chem., 1997,120(1/2/3):173-178
[8]Brustein V P,Cavalcanti C L B,de Melo M R,et al.Appl. Biochem.Biotechnol.,2012,166(2):268-275
[9]Wang S B,Li H,Xu L Y.J.Colloid Interface Sci.,2006, 295(1):71-78
[10]Guo Y P,Zhao J Z,Zhang H,et al.Dyes Pigm.,2005,66 (2):123-128
[11]Fu H B,Pan C S,Yao W Q,et al.J.Phys.Chem.B,2005, 109(47):22432-22439
[12]Ashraf U,Chat O A,Dar A A.Chemosphere,2014,99:199-206
[13]Parida K M,Sahu N,Biswal N R,et al.J.Colloid Interface Sci.,2008,318(2):231-237
[14]Mahmoodi N M,Najafi F.Microporous Mesoporous Mater., 2012,156:153-160
[15]Park H O,Oh S,Bade R,et al.KSCE J.Civ.Eng.,2011,15 (3):453-461
[16]Chatha S A S,Asgher M,Ali S,et al.Carbohydr.Polym., 2012,87(2):1476-1481
[17]Xie Y B,Yuan C W,Li X Z.Colloid Surf.A,2005,252 (1):87-94
[18]Pan H Q,Li X K,Zhuang Z J,et al.J.Mol.Catal.A:Chem., 2011,345(1/2):90-95
[19]Luan J F,Wang S,Ma K,et al.J.Phys.Chem.C,2010,114 (20):9398-9407
[20]Rauf M A,Ashraf S S.Chem.Eng.J.,2009,151(1/2/3):10-18
[21]Chatterjee D,Mahata A.J.Photochem.Photobiol.A-Chem., 2002,153(1/2/3):199-204
[22]Kyung H,Lee J,Choi W Y.Environ.Sci.Technol.,2005,39 (7):2376-2382
[23]Su L S,Gan Y X.Composities Part B,2012,43(2):170-182
[24]Dubal D P,Dhawale D S,More A M,et al.J.Mater.Sci., 2011,46(7):2288-2293
[25]Bao N,Li Y,Yu X H,et al.Environ.Sci.Pollut.Res.Int., 2013,20(2):897-906
[26]Qu P,Zhao J C,Shen T,et al.J.Mol.Catal.A:Chem., 1998,129(2-3):257-268
[27]Ghosh J P,Sui R H,Langford C H,et al.Water Res.,2009, 43(18):4499-4506
[28]Adhikari R,Gyawali G,Sekino T,et al.J.Solid State Chem., 2013,197:560-565
[29]Zhang X,Ai Z H,Jia F L,et al.Mater.Chem.Phys.,2007, 103(1):162-167
[30]Zhou J K,Zou Z G,Ray A K,et al.Ind.Eng.Chem.Res., 2007,46(3):745-749
[31]Zhang G K,Zou X,Gong J,et al.J.Alloys Compd.,2006, 425(1/2):76-80
[32]Feng P,Chen C L,Hao Y,et al.Mater.Chem.Phys.,2009, 116(1):294-299
[33]Li J P,Zhang X,Ai Z H,et al.J.Phys.Chem.C,2007,111 (18):6832-6836
[34]Li X K,Kako T,Ye J H.Appl.Catal.A:Gen.,2007,326(1): 1-7
[35]Hou L R,Yuan C Z,Peng Y.J.Mol.Catal.A:Chem.,2006, 252(1/2):132-135
[36]Tang X D,Ye H Q,Liu H,et al.Chem.Phys.Lett.,2009, 484(1/2/3):48-53
[37]Dong H J,Chen G,Sun J X,et al.Appl.Catal.B:Environ., 2013,134:46-54
[38]Li K W,Wang H,Yan H.J.Mol.Catal.A:Chem.,2006,249 (1/2):65-70
[39]Luan J F,Pan B C,Paz Y,et al.Phys.Chem.Chem.Phys., 2009,11(29):6289-6298
[40]Luan J F,Li M,Ma K,et al.Chem.Eng.J.,2011,167(1): 162-171
[41]Yang H,Li J,Wang L Y,et al.Catal.Commun.,2013,35: 101-104
[42]Nashim A,Parida K M.Chem.Eng.J.,2013,215:608-615
[43]Luan J F,Zhao W,Feng J W,et al.J.Hazard.Mater.,2009, 164(2/3):781-789
[44]Marugan J,Hufschmidt D,Sagawe G,et al.Water Res., 2006,40(4):833-839
[45]Sakthivel S,Shankar M V,Palanichamy M,et al.Water Res.,2004,38(13):3001-3008
[46]Fazey P G,Oconnor B H,Hammond L C.Clays Clay Miner., 1991,39(3):248-253
[47]Zou Z,Ye J,Arakawa H.J.Mater.Sci.Lett.,2000,19(21): 1909-1911
[48]Tauc J,Grigorov R,Vancu A.Phys.Status Solidi,1966,15 (2):627-637
[49]Butler M A.J.Appl.Phys.,1977,48(5):1914-1920
[50]Liu G M,Wu T X,Zhao J C,et al.Environ.Sci.Technol., 1999,33(12):2081-2087
[51]Li J Y,Ma W H,Lei P X,et al.J.Environ.Sci.,2007,19 (7):892-896
[52]He Z,Yang S G,Ju Y M,et al.J.Environ.Sci.,2009,21(2): 268-272
[53]He Z,Sun C,Yang S G,et al.J.Hazard.Mater.,2009,162 (2/3):1477-1486
[54]Oshikiri M,Boero M,Ye J H,et al.J.Chem.Phys.,2002, 117(15):7313-7318
Structural and Photocatalytic Properties of Fe2BiTaO7Nanocatalyst
LUAN Jing-Fei*HU Wen-HuaCHEN Biao-HangPEI Dong-Hua
(State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment,Nanjing University,Nanjing 210023,China)
Fe2BiTaO7powder photocatalyst was synthesized by a solid state reaction method.The structural and photocatalytic properties of Fe2BiTaO7were characterized by XRD,SEM,TEM and UV-Vis diffuse reflectance spectroscopy.The results show that Fe2BiTaO7crystallizes with the pyrochlore-type structure,cubic crystal system and space group Fd3m.The estimated band gap of Fe2BiTaO7is 1.72 eV.The photocatalytic degradation of rhodamine B over Fe2BiTaO7,P25 TiO2,N-doped TiO2and Bi2InTaO7was investigated under visible light irradiation.The photocatalytic efficiency with Fe2BiTaO7catalyst is 1.5 times of N-doped TiO2catalyst after 140 minutes under visible light irradiation.Fe2BiTaO7has higher visible-light photocatalytic performance and shows much better activity than that of other photocatalysts.The photocatalytic degradation of rhodamine B follows the first-order reaction kinetics,and the first-order rate constant is 0.022 93 min-1for Fe2BiTaO7.The possible photocatalytic degradation pathway of rhodamine B under visible light irradiation is suggested.In addition,the photocatalytic degradation of phenol over Fe2BiTaO7catalyst was investigated under visible light irradiation. Fe2BiTaO7(visible light)photocatalysis system is confirmed to be suitable for textile industry wastewater treatment.
catalysis;Fe2BiTaO7;optical properties;visible light;photocatalytic degradation;rhodamine B
O643.36
A
1001-4861(2015)02-0385-14
10.11862/CJIC.2015.046
2014-09-17。收修改稿日期:2014-11-13。
国家自然科学基金(No.21277067);中以科学与战略研究开发专项资金(No.5)资助项目。*
。E-mail:jfluan@nju.edu.cn
