基于联苯四羧酸的Zn(Ⅱ)配合物的合成、结构和性质
2015-04-01李欣周尚永田丽
李欣 周尚永 田丽
基于联苯四羧酸的Zn(Ⅱ)配合物的合成、结构和性质
李欣 周尚永 田丽*
(天津师范大学化学学院,天津市功能分子结构与性能重点实验室,无机-有机杂化功能材料化学省部共建教育部重点实验室,天津300387)
利用联苯四羧酸(H4bptc)和双三唑乙烷(bte)合成了2个配位聚合物{[Zn2(bptc)(DMF)2(H2O)]·DMF·H2O}n(1)和{[Zn(bte)(bptc)0.5]·DMF· 0.5H2O}n(2)。化合物1为三维PtS-拓扑结构,在该配合物中每个双核次级构筑单元(SBU){Zn2(O2CR)4}与4个联苯四酸配体相连,从而将配合物构筑成了三维MOF结构。化合物2为二维梯形结构,并通过弱氢键O…H-C将二维层连接为三维结构。同时对配合物的热稳定性和荧光特征进行了讨论。
双三唑乙烷;联苯四羧酸;三维框架结构;超分子结构
0 Introduction
Owning to the potential applications of metalorganic frameworks(MOFs)in areas such as catalysis, adsorption(gas storage),luminescence and magnetism, designingandsynthesizingofnovelpolymersis becoming an increasingly popular field of research[1-7]. Because of the diversity of the coordination modes and high structural stability,multi-carboxylic ligands with suitable spacers,especially benzoic acid based ligands have been extensively explored to design and prepare a variety of intriguing coordination frameworks with promising applications[8-10].
Such ligands with multi-carboxyl groups locatedat different positions of the aromatic backbones may display the distinct spatial effects and deprotonated degrees of carboxyl groups,which results in their diverse binding modes upon metal complexation and regulating the structural assembly[11-13].Besides,these supramolecular tectons can also affect the network structuresvianoncovalentinteractionssuchas hydrogen bonding and aromatic stacking.In addition, aromatic multi-carboxylates can exhibit luminescence in coordination polymers with metal nodes such as CuI,AgI,ZnIIand CdIIions[14-15].Whats more,a lot of porousmetal-organicframeworksorporous coordinationpolymersbasedonaromaticmulticarboxylates ligands has raised intense interest for their promising applications in gas storage[16-20].
Biphenyl tetracarboxylic acid have a rich variety of coordination modes and deprotonated forms with four carboxylic groups,which can help to construct novel and various coordination polymers[21-24].On the otherhand,thebridgingN-containingauxiliary ligandssuchasbis-triazolealkaneshavebeen frequently used in the preparation of coordination polymers because they are very strong N-ligating donors for d-metal ions and can bridge metal ions into extendedhigherdimensional(2Dand3D) coordination polymers[25-28].
In particular,the reported research demonstrated that the multidentate organic ligands with N and/or O-donors as coordination sites are effective building blocks in the construction of MOFs,thus combining thetriazole-containingligandwithmulticarboxylic acids will be a useful strategy for construction of novel MOFs[29-31].In this regard,our recent study has been mainly focused on the construction of new MOFs from accessibletriazolecontainingligandsandmulticarboxylic acids,which show rich structural features, gas storage and luminescence properties[32-36].As an extension of our work,we choose bte and H4bptc as the building blocks.The ZnII-H4bptc and ZnII-H4bptc/ bte mixed-ligand metal-organic frameworks have been obtained.Thethermalstabilityandfluorescent emission of the two new materials have also been discussed.
1 Experimental
1.1 General considerations
Thereagentsandsolventsemployedwere commercially available and used as received without further purification.Biphenyl-3,3′,5,5′-tetracarboxylic acid(H4bptc)was purchased from Aldrich.Ligand bte wassynthesizedasreportedpreviously[37].The elemental analyses(C,H,and N)were carried out on a Perkin-Elmer elemental analyzer.The fluorescent spectra were measured on a Varian Cary Eclipse Fluorescence spectrophotometer.TG experiments were performed on a NETZSCH TG 209 instrument with a heating rate of 10℃·min-1under nitrogen conditions. PowderX-raydiffraction(PXRD)patternswere obtained from a Rigaku D/max-2500 diffractometer at 60 kV and 300 mA for Cu Kα radiation(λ=0.154 06 nm),with a scan speed of 2°·min-1and a step size of 0.02°in 2θ.
1.2 Preparation
{[Zn2(bptc)(DMF)2(H2O)]·DMF·H2O}n(1):A mixture of Zn(NO3)2·6H2O(14.8 mg,0.05 mmol), H4bptc(13.3 mg,0.05 mmol),DMF(4 mL)and H2O(1 mL)was added into a Parr Teflon-lined stainless steel vessel(15 mL),and then the vessel was sealed and heated to 90℃,kept for 3 days.After that the autoclave was cooled to room temperature at a rate of 1.5℃·h-1,a colorless crystalline product 1 was filtered off,washed with distilled water and dried in air(12.5 mg,yield 70%based on Zn).Anal.Calcd. for C25H31N3O13Zn2(712.27)(%):C,42.15;H,4.39;N, 5.90.Found(%):C,42.52;H,4.10;N,5.42.
{[Zn(bte)(bptc)0.5]·DMF·0.5H2O}n(2).Colorless crystals of 2 were obtained by adopting the similar synthetic procedure as 1 except that bte(8.2 mg,0.05 mmol)was added in the reaction mixture.Yield:15.4 mg,65%(basedonZn).Anal.Calcd.for C17H19N7O5.5Zn(474.78)(%):C,43.00;H,4.03;N, 20.65.Found(%):C,43.21;H,3.63;N,20.28.
1.3 X-ray crystallography
Single-crystal X-ray diffraction measurements of 1 and 2 were carried out with a Bruker Smart CCD diffractometer and a graphite crystal monochromatorsituated in the incident beam for data collection at 150(2)K.Lorentzpolarizationand absorption corrections were applied.The structures were solved by direct methods and refined by full-matrix leastsquarestechniquesusingtheSHELXS-97and SHELXL-97 programs[38-39].All the non-hydrogen atoms wererefinedwithanisotropicdisplacement parameters.Hydrogenatomswereincludedin calculated position and refined with isotropic thermal parameters riding on the parent atoms.H atoms bonded toOwerelocatedindifferenceFouriermaps. Crystallographic data for 1 and 2 are summarized in Table 1.Selected bond distances and angles of 1 and 2 are listed in Table 2.
CCDC:1018691,1;1018790,2.
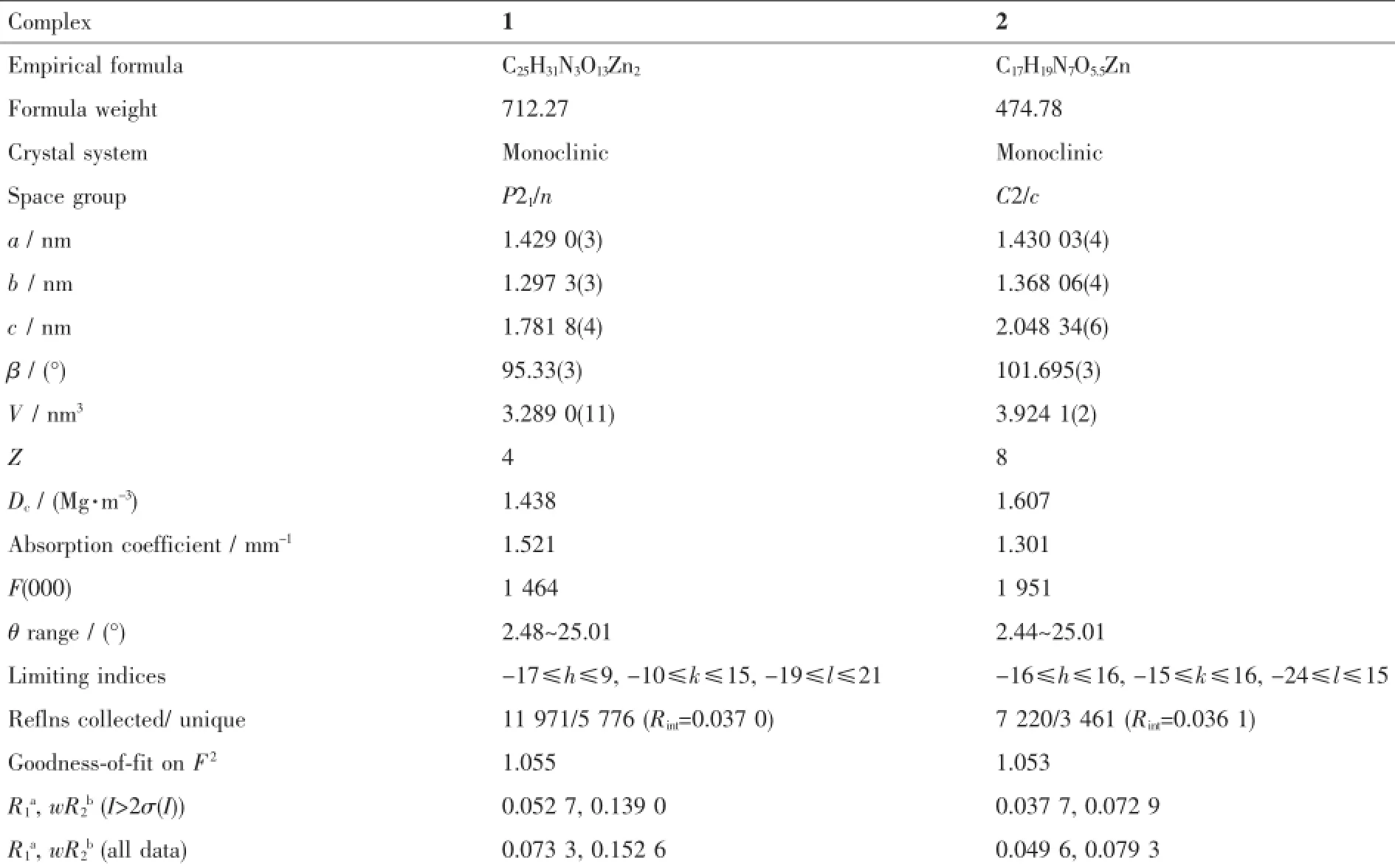
Table 1Crystallographic data and structure refinement details for complexes 1 and 2

Table 2Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
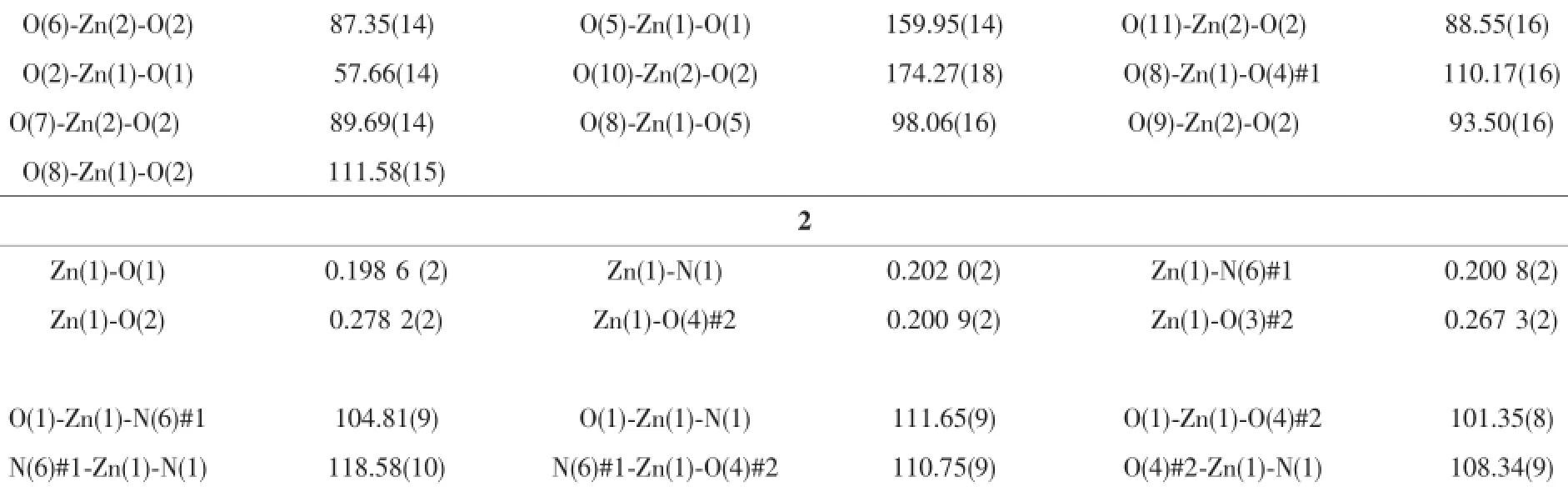
Continued Table 2
2 Results and discussion
2.1 Description of crystal structure of{[Zn2(bptc) (DMF)2(H2O)]·DMF·H2O}n(1)
The X-ray structural analysis reveals that 1 crystallizes in monoclinic crystal system,space group P21/n.Therepeatedunitin1consistsoftwo crystallographically independent zinc atoms(Zn1 and Zn2).As shown in Fig.1a,Zn1 is six-coordinated in a distortedoctahedralcoordinationspherethatis defined by six carboxylic oxygen atoms from four different bptc4-ligands.The three carboxylic oxygen atoms,two DMF oxygen atoms and one water molecule complete the hexadentate sphere of Zn2,which is in a highly distorted octahedral coordination sphere.The Zn1-O1(0.246 5(4)nm)and Zn1-O3(0.268 5(4)nm) (Table 2)bond distances are slightly longer than normal Zn-O distances,which can be described as asemi-chelating coordination mode.All other Zn-O and Zn-N bond lengths fall in the normal range of 0.195~0.219 nm.
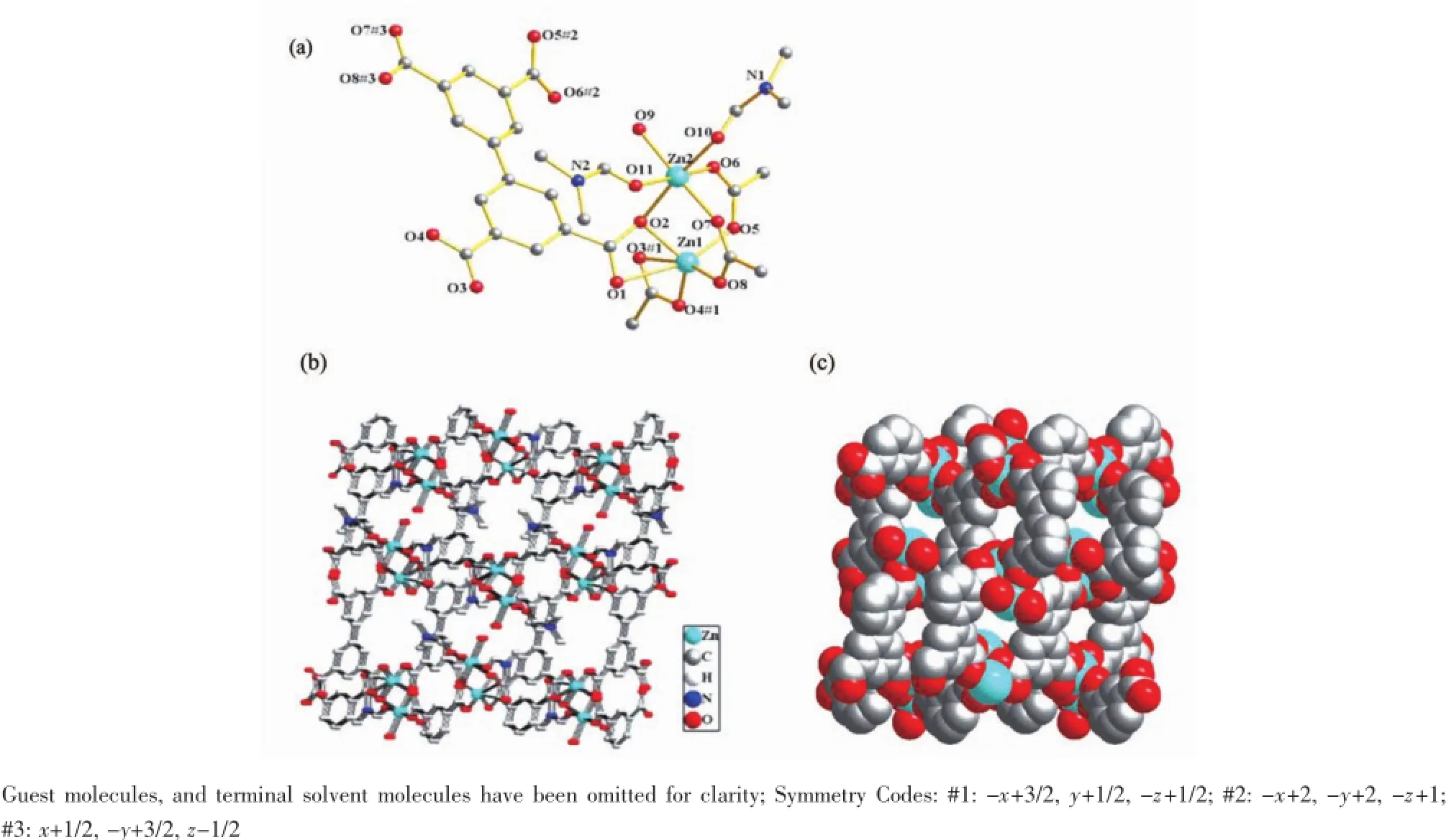
Fig.1(a)Molecular structure of 1,showing the coordination environments of ZnIIand bptc ligands;(b)The 3D framework of 1 viewed along the b direction;(c)Space-filling views of the framework of 1 viewed along the b direction
Pairs of Zn1 and Zn2 centers are bridged by three carboxylate groups,forming binuclear secondbuilding-unit(SBU){Zn2(O2CR)4}.The chelating modes of the four carboxylate groups are two 1,3-bridge,one chelating μ2-O bridging tridentate and one bidentate (Scheme 1).Each SBU{Zn2(O2CR)4}is linked to four biphenyl connectors(and vice versa),to give 3D frameworks with PtS-type topologies(Fig.1b),which is very like the framework of Co[40]and Cu[19]complexes. In Co complex,binuclear Co2(CO2)4(H2O)(DMF)2units connect with BPTC4-to give the similar frameworks as compound 1.For Cu complex,each{Cu2(O2CR)4} paddle wheel is linked to four biphenyl connectors (and vice versa),to give frameworks with NbO-type topologies.
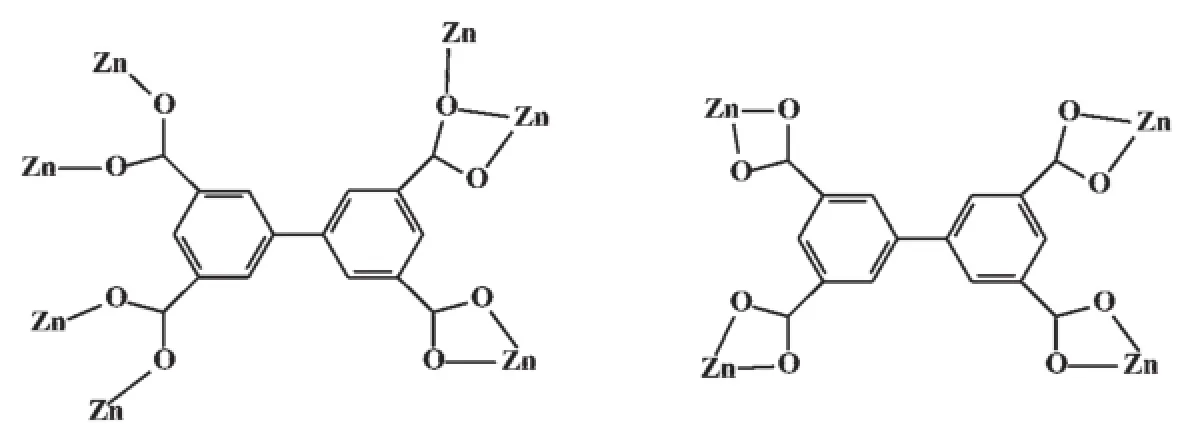
Scheme 1Coordination modes of the bptc4-anion in 1 and 2
By using PLATON/SOLV,the accessible voids in the desolvated structures of 1 retains an effective volume 2.015 6 nm3per unit cell(Fig.1c),which is estimated to correspond to 61.3%of the total volumes. The free DMF and H2O are trapped in the pores as guest molecules.
2.2 Description of crystal structure{[Zn(bte) (bptc)0.5]·DMF·0.5H2O}n(2)
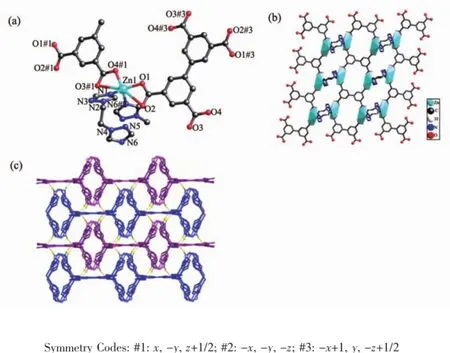
Fig.2(a)Molecular structure of 2,showing the coordination environments of ZnII,bptc and bte ligands;(b)The 2D layer of compound 2 along b direction;(c)The 3D supramolecular structure of 2 viewed from c direction
When bte was introduced in the above system,a 2D layer is obtained.The coordination environment of ZnIIions in 2 is presented in Fig.2a.ZnIIion is sixcoordinated by four carboxylic oxygen atoms from twobptc4-ligands,two nitrogen atoms from two different bte ligands,which is in a distorted octahedral coordination sphere.All the Zn-O and Zn-N bond lengths fall in the normal range of 0.198~0.202 nm except that the Zn1-O2 and Zn1-O3 distances are 0.278 2(4)nm and 0.267 3(4)nm,respectively(Table 2).So there is a nonnegligible interaction between the uncoordinated carboxylate oxygen atom and Zn1,which can be described as a semi-chelating coordination mode.
It is noteworthy that every bptc4-ligand bridge four Zn(Ⅱ)ions with its four carboxylic group,which forms one dimensional ladder chain running along the c axis as depicted in Fig.2b.The four carboxylate groups in very BPTC4-take the same bidentate chelating mode (Scheme 1).Along the a direction,the 1D ladders are connected into a 2D layer by the bidentate bte ligand with Zn…Zn separations of 0.635 8 nm.The adjacent 2D layers repeat in an…ABAB…stacking sequence along the[001]direction with an interlayer distance(A…A or B…B)of 1.368 nm(the separations of Zn…Zn). The neighboring two dimensional AB layers are stacked in an offset way(Fig.2c).Two neighboring planes have weak C-H…O(C12-H12A…O1,0.336 7(4)nm) interactions,whichmakethemforma3D supramolecular framework with channels filled by free DMF and solvated water molecules(Fig.2c).The solvated water is connected with the 2D layer through H-bonding interaction(O6-H6A…O3,0.277 2(4)nm). Computation of the channel space using PLATON suggests a value of 1.004 nm3,corresponding to 25.6% of the unit-cell volume.
2.3 TGA and XRPD Analyses
TGA and Powder X-ray diffraction(PXRD)were used to investigate the framework stability and phase purity,respectively.The TGA result of 1 reveals that the lattice and coordinated water molecules and the lattice DMF molecules in the channels can be easily removed at 110℃(Obsd.16.5%;Calcd.15.3%) under a flow of N2at room pressure,while the coordinated DMF molecules can be removed when the temperature up to 180℃(Obsd.35.8%;Calcd. 35.8%).The guest-free framework is stable up to 430℃,above which it decomposes rapidly to produce ZnO(Fig.3).The TGA curve of 2(Fig.3)shows that the lattice water and DMF molecules are removed till 200℃(Obsd.16.4%;Calcd.17.3%),and the framework rapidly decomposes when the temperature is above 280℃to give ZnO.PXRD has been carried out to confirm phase purity of compound 1 and 2(Fig. S1 and Fig.S2,Supporting Information).The peak positions of the experimental PXRD patterns are well in agreement with the simulated data,demonstrating the phase purity of the compounds.
2.4 Luminescent properties
The emission spectra of 1 and 2 in the solid state at room temperature are investigated,as depicted in Fig.4.Complexes 1 and 2 have emission bands with the maximum at ca.430,422 nm,respectively,upon excitation at ca.360 nm.Free H4bptc ligand is also luminescent at 390 nm,whereas the bte ligand is nonluminescent.Thus,it can be presumed that the emission of 1 and 2 originates from intraligand transitions,and the presence of ZnIIions shows theelectron withdrawing effect owing to the+2 charge of the cations.The red shift of the emission peaks can be ascribed to metal-ligand coordinative interactions.
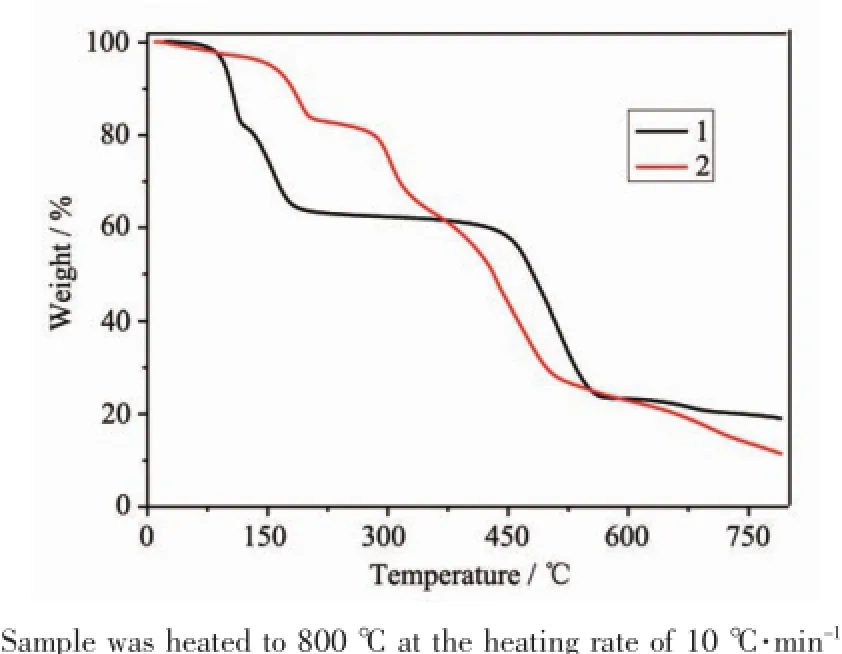
Fig.3TGA curves for 1 and 2
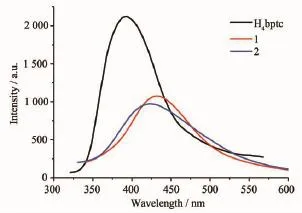
Fig.4Solid-state emission spectra of 1,2 and H4bptc at room temperature
3 Conclusions
In summary,two metal-organic frameworks have been constructed by two ligands and ZnIIsalt.By reaction of biphenyl tetracarboxylic acid(H4bptc)and Zn(NO3)2·6H2O,a 3D framework with PtS-type topology wasobtained,inwhicheachbinuclearSBUs {Zn2(O2CR)4}is linked to four biphenyl connectors(and vice versa).When flexible bte spacers is added in the above reaction,a 2D layer is obtained,in which 1D Zn-bptc ladder are connected into 2D layer through bte bridge.Both of the complexes exhibit fluorescent emission bands at room temperature.
Acknowledgments:This work was financially supported bytheNationalNaturalScienceFoundationofChina (21371133),and the Natural Science Fund of Tianjin,China (12JCZDJC27600).
[1]Savaki T,Dewa T,Aoyama Y.J.Am.Chem.Soc.,1998,120: 8539-8540
[2]Ikkala O,Brinke G.Science,2002,295:2407-2409
[3]Ishikava N,Sugita M,Ishikawa T,et al.J.Am.Chem.Soc., 2003,125:8694-8695
[4]Zhang J P,Lin Y Y,Zhang W X,et al.J.Am.Chem Soc., 2005,127:14162-14163
[5]WuCD,LinWB.Angew.Chem.Int.Ed.,2007,46:1075-1078 [6]Zhang J,Chen S M,Valle H,et al.J.Am.Chem.Soc., 2007,129:14168-14169
[7]Panella B,Hones K,Muller U,et al.Angew.Chem.Int.Ed., 2008,47:2138-2142
[8]Morris W,Doonan C J,Furukawa H,et al.J.Am.Chem. Soc.,2008,130:12626-12627
[9]Doonan C J,Morris W,Furukawa H,et al.J.Am.Chem.Soc., 2009,131:9492-9493
[10]Chen Q,Chang Z,Song W C,et al.Angew.Chem.Int.Ed., 2013,52:11550-11553
[11]Moulton B,Abourahma H,Bradner M W,et al.Chem. Commun.,2003,39:1342-1343
[12]Zhang J,Kang Y,Zhang J,et al.Eur.J.Inorg.Chem., 2006,2253-2258
[13]Maspoch D,Molina D R,Veciana J.Chem.Soc.Rev., 2007,36:770-818
[14]Li C P,Chen J,Du M.CrystEngComm,2010,12:4392-4402
[15]Hao H J,Sun D,Li Y H,et al.Cryst.Growth Des., 2011,11:3564-3578
[16]Zhang S M,Chang Z,Hu T L,et al.Inorg.Chem.,2010,49: 11581-11586
[17]Zhao D,Yuan D Q,Sun D F,et al.J.Am.Chem.Soc., 2009,131:9186-9487
[18]Glover T G,Peterson G W,Schindler B J,et al.Chem.Eng. Sci.,2011,66:163-170
[19]Lin X,Jia J H,Zhao X B,et al.Angew.Chem.Int.Ed., 2006,45:7358-7364
[20]Lin X,Telepeni I,Blake A J,et al.J.Am.Chem.Soc., 2009,131:2159-2171
[21]Chen B L,Ockwig N W,Millward A R,et al.Angew.Chem. Int.Ed.,2005,44:4745-4749
[22]Weng D F,Zheng X J,Li L C,et al.Dalton Trans., 2007,36:4822-4828
[23]Chang Z,Zhang D S,Hu T L,et al.Inorg.Chem.Commun., 2011,14:1082-1085
[24]Tian D,Pang Y,Zhou Y H,et al.CrystEngComm,2011,13: 957-966
[25]Huang Y Q,Ding B,Song H B,et al.Chem.Commun., 2006,11:4906-4908
[26]ZhuX,GeH,ZhangY,etal.Polyhedron,2006,25:1875-1883
[27]Tian L,Chen Z,Yu A,et al.CrystEngComm,2012,14:2032 -2039
[28]Habib H A,Sanchiz J,Janiak C.Dalton Trans.,2008,20: 1734-1744
[29]Tian A X,Ying J,Peng J,et al.Cryst.Growth Des.,2008,8: 3717-3724
[30]Habib H A,Hoffmann A,Höppe H A,et al.Dalton Trans., 2009,21:1742-1751
[31]Marchettia F,Masciocchib N,Albisettib A F,et al.Inorg. Chim.Acta,2011,373:32-39
[32]Tian L,Yan L,Liu S Y.J.Coord.Chem.,2011,64:2945-2952
[33]Tian L,Zhang Z J,Yu A,et al.Cryst.Growth Des.,2010, 10:3847-3849
[34]Tian L,Niu Z,Yang N,Zou J Y.Inorg.Chim.Acta,2011, 370:230-235
[35]Tian L,Chen Z.Inorg.Chem.Commun.,2011,14:1302-1305
[36]Tian L,Yan L,Wang L P,et al.J.Mol.Struct.,2011,998: 30-36
[37]Torres J,Lavandera J L,Cabildo P,et al.J.Heterocyc. Chem.,1988,25:771-782
[38]Sheldrick G M.SHELXS 97,Program for Crystal Structure solution,University of Göttingen,Germany,1997.
[39]Sheldrick G M.SHELXS 97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[40]Chen B L,Ockwig N W,Fronczek F R,et al.Inorg.Chem., 2005,44:181-183
Zinc(Ⅱ)Coordination Polymers Based on Aromatic Tetracarboxylate:Syntheses, Structures,and Properties
LI XinZHOU Shang-YongTIAN Li
(Tianjin Key Laboratory of Structure and Performance for Functional Molecules,Key Laboratory of Inorganic-Organic Hybrid Functional Material Chemistry,Ministry of Education,College of Chemistry,Tianjin Normal University,Tianjin 300387,China)
Two coordination compounds,namely{[Zn2(bptc)(DMF)2(H2O)]·DMF·H2O}n(1)and{[Zn(bte)(bptc)0.5]· DMF·0.5H2O}n(2)(bte=bis(1,2,4-triazol-1-yl)ethane,H4bptc=biphenyl-3,3′,5,5′-tetracarboxylic acid),have been synthesized and structurally characterized.1 features a 3D framework with PtS-type topology,in which each binuclear second-building-unit(SBU){Zn2(O2CR)4}is linked to four biphenyl connectors(and vice versa).2 is 2D layers,which are interconnected by weak C-H…O interactions to lead to 3D supramolecular framework.The thermal stability,and fluorescent emission of the two complexes have also been discussed.CCDC:1018691,1; 1018790,2.
bis(1,2,4-triazol-1-yl)ethane;biphenyl-3,3,5,5-tetracarboxylic acid;3-D framework;supramolecular structure
O614.24+1
A
1001-4861(2015)02-0338-07
10.11862/CJIC.2015.057
2014-08-11。收修改稿日期:2014-11-25。
国家自然科学基金(No.21371133)和天津市自然科学基金资助项目(No.12JCZDJC27600)资助项目。*
。E-mail:lilytianli@hotmail.com;Tel:+86-22-23766515;会员登记号:S06N549M1304。
