CuII and CuI Complexes of 1,1΄-(Pyridin-2-ylmethylene)-bis[3-(pyridin-2-yl)imidazo[1,5-a]pyridine]:in situ Generation of the Ligand via Acetic Acid-controlled Metal-ligand Reactions①
2015-03-25ZHANGHiFengCHENYnMeiQINRuLIHongLIWu
ZHANG Hi-Feng CHEN Yn-Mei QIN Y-Ru LI Y-Hong② LI Wu
a(College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China)
b (Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China)
1 INTRODUCTION
Imidazo[1,5-a]pyridine and its derivatives, as a class of important ligand, have attracted considerable attention of coordination chemists because these ligands and their coordination complexes have extensive applications in medicine[1-3], OLEDs[4,5]and photoluminescent materials[6-8]. Despite all these interests, only few complexes have been reported due to the strict reaction conditions and length sequence required for the synthesis of imidazo[1,5-a]pyridine and its derivatives. Hence, the development of efficient access to the ligands as well as compounds is of great significance.
In our previous work[9], we report an efficient strategy for the preparation of coordination complexes of imidazo[1,5-a]pyridine and its derivatives.By employing this strategy, a variety of derivatives of imidazo[1,5-a]pyridine were generated via metalligand reactions among picolinaldehyde, ammonium acetate and metal salts. The mechanism investigation revealed that the free radicals were formed in the reaction process. We envisioned that there might be a number of new complexes available by adding acetic acid in the reaction system because acetic acid could sever as a radical inhibitor in some reactions[10].
To this end, we conducted the reactions of HPIP(HPIP = 3-(pyridin-2-yl)-imidazo[1,5-a]pyridine),picolinaldehyde and CuCl2·2H2O (or CuCl) in the presence of acetic acid under solvothermal conditions. Four complexes of formulas [LCuCl][Cu2Cl3](1), [HLCuCl]2[CuCl2]2[CuCl3]·2H2O (2), [Cu3L2Cl2][CuCl2]·2H2O] (3) and [Cu3L2][CuCl2]3(4) were prepared. Complexes 1 and 2 are CuII/CuImixedvalence compounds. Herein, we report the syntheses and structures of these complexes.
2 EXPERIMENTAL
All solvents and reagents were purchased from commercial suppliers and used without further purification. Crystal determinations of complexes 1~4 were performed with a Bruker SMART APEXⅡ CCD diffractometer equipped with a graphite-monochromatized MoKa radiation (λ =0.71073 Å). Elemental analyses (C, N, H) were performed with a Carlo-Erba EA1110 CHNO-S elemental analyzer. Powder X-ray diffraction(PXRD) was recorded on a Rigaku D/Max-2500 diffractiometer. IR spectra (4000~400 cm-1) were recorded by using KBr pellets with a Nicolet MagNa-TR500 FT-IR spectrometer.
2.1 Syntheses of complexes 1 and 2
A mixture of HPIP (0.0390 g, 0.2 mmol),picolinaldehyde (0.0214 g, 0.2 mmol), CuCl2·2H2O(0.0524 g, 0.3 mmol), CH3COOH (1 mL) and EtOH(2 mL) was sealed in an 8 mL Pyrex tube and heated for 72 h at 125 ℃ under autogenous pressure. Darkgreen block crystals [LCuCl][Cu2Cl3] (1) and green rod crystals [HLCuCl]2[CuCl2]2[CuCl3]·2H2O (2)were obtained simultaneously. The crystals were collected by filtration, washed with Et2O (2×3 mL),and dried in air. Yield: 19% (based on CuCl2·2H2O).
Elemental Anal. Calcd. for C30H21Cl4Cu3N7(1): C,44.38; H, 2.61; N, 12.07%. Found: C, 43.85; H, 2.44;N 11.97%. Selected IR data (KBr, cm-1): 2361 (w),1639 (w), 1602 (m), 1481 (s), 1371 (m), 1311 (m),1255 (m), 1146 (m), 1053 (w), 1005 (w), 983 (w),781 (m), 738 (m), 678 (m).
Elemental Anal. Calcd. for C60H48Cl9Cu5N14O2(2): C, 44.11; H, 2.96; N, 12.00%. Found: C, 43.34;H, 2.67; N, 11.73%. Selected IR data (KBr, cm-1):1606 (m), 1519 (w), 1495 (s), 1367 (m), 1315 (m), 1252(m), 1071 (w), 1008 (w), 774 (s), 750 (m), 697 (m).
2.2 Synthesis of complex 3
A mixture of HPIP (0.0390 g, 0.2 mmol), picolinaldehyde (0.0214 g, 0.1 mmol), CuCl (0.0099 g,0.1 mmol), CH3COOH (1 mL) and EtOH (2 mL)was sealed in an 8 mL Pyrex tube. The tube was heated for three days at 125 ℃ under autogenous pressure. Slow cooling of the resultant solution to room temperature over 24 h gave orange-block crystals. The crystals were collected by filtration,washed with Et2O (23 mL), and dried in air. Yield:34% (based on CuCl).
Elemental anal. calcd. for C60H46Cl4Cu4N14O2: C,51.80; H, 3.33; N, 14.10%. Found: C, 51.34; H, 3.41;N 13.95%. Selected IR data (KBr, cm-1): 3463 (s),3423 (s), 2361 (w), 1637 (w), 1594 (w), 1473 (w),1438 (w), 1150 (w), 774 (m), 723 (w), 618 (w).
2.3 Synthesis of complex 4
A mixture of HPIP (0.0390 g, 0.2 mmol), picolinaldehyde (0.0214 g, 0.1 mmol), CuCl (0.0297 g,0.3 mmol), CH3COOH (1 mL) and EtOH (2 mL)was sealed in an 8 mL Pyrex tube. The tube was heated for three days at 125 ℃ under autogenous pressure. Slow cooling of the resultant solution to room temperature over 24 h gave red-platy crystals.The crystals were collected by filtration, washed with Et2O (23 mL), and dried in air. Yield: 29%(based on CuCl). Elemental anal. calcd. for C60H42Cl6Cu6N14: C, 46.40; H, 2.73; N, 12.63%.Found: C, 46.72; H, 2.67; N, 12.21%. Selected IR data (KBr, cm-1): 3446 (s), 1635 (w), 1590 (m), 1478(s), 1359 (w), 1312 (w), 1236 (w), 1070 (w), 1002(w), 778 (m), 735 (w), 619 (w).
2.4 Single-crystal X-ray crystallography
Data were collected at 296(2) K on a Bruker SMART APEX CCD area-detector diffractometer equipped with a graphite-monochromated MoKα radiation (λ = 0.71073 Å) for 1~4 using the ω-φ scan technique. The structures were solved by direct methods using SHELXS-97[11]and refined on F2using full-matrix least-squares with SHELXL-97[12].Crystallographic data together with refinement details for the new complexes reported in this work are summarized in Table 1. Selected bond lengths and bond angles of 1~4 are shown in Table 2, and hydrogen bond lengths and bond angles are listed in Table 3.
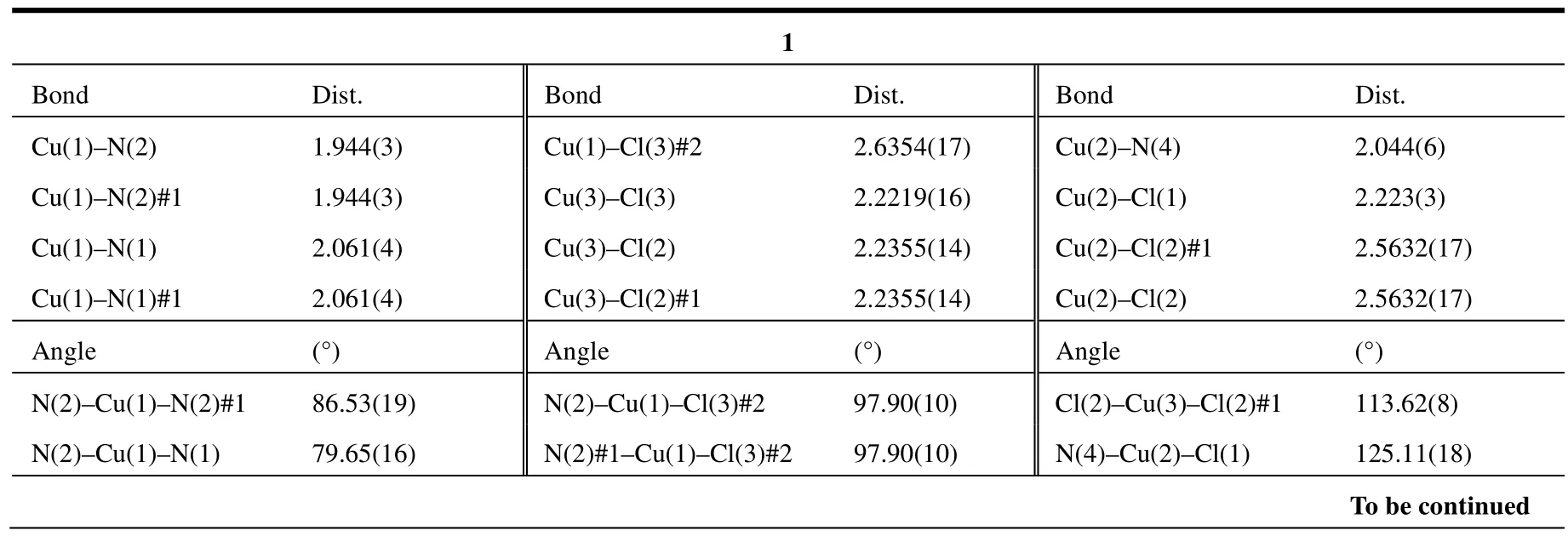
Table 2. Selected Bond Lengths (Å) and Bond Angles (°) for 1~4

Symmetry transformation: (1) #1: x, –y+1/2, z; #2: x–1, y, z; (2) #1: –x+1, –y, z
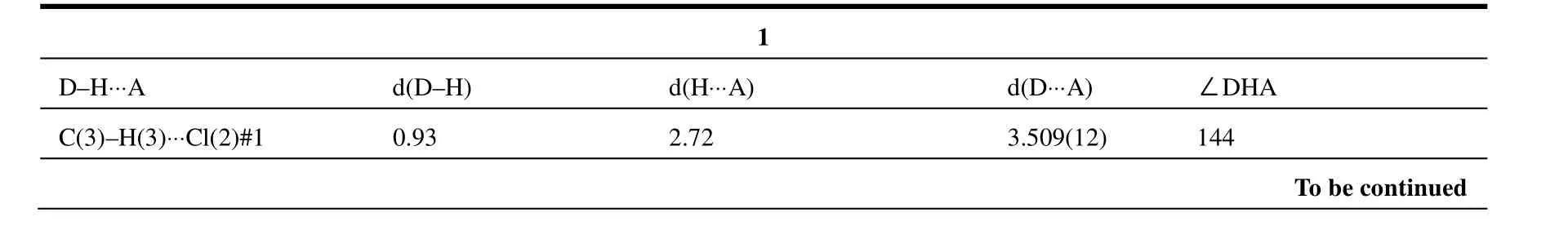
Table 3. Selected Hydrogen Bond Lengths (Å) and Bond Angles (°) for 1~4
Symmetry codes: (1) #1: –x, 1/2+y, 1–z; #2: –1+x, y, z; #3: 1–x, 1/2+y, 1–z; #4: –1/2+x, 1/2–y, 1/2–z; (2) #1: 3/4–x, 1/4+y, 3/4+z;#2: –1/4+x, 1/4–y, –1/4+z; #3: –x, –y, z; #4: x, y, –1+z; #5: 1/4+x, 1/4–y, 1/4+z; #6: 1/4+x, –1/4–y, –1/4+z; (3) #1: x, 1+y, z;#2: 1–x,1–y,–z; #3: –1+x,1+y,z; #4: 1–x,–y,1–z; (4) #1: x, 1+y, z; #2: 1–x, 1–y, 1–z; #3: –x, 1–y, 1–z; #4: 1–x, 1–y, –z
3 RESULTS AND DISCUSSION
3.1 Synthesis
In our previous work[9], we have conducted the reactions of HPIP, picolinaldehyde and CuCl2·2H2O in EtOH. Three complexes have been produced via solvothermal in situ metal-ligand reactions. The radical mechanisms are proved for these reactions. It is well known that acetic acid could serve as an inhibitor for radical reactions[10]. We were curious if the in situ generation of the ligand through metalligand reaction could occur again in the presence of acetic acid. To our delight, the solvothermal metalligand reaction was determined, and the ligand L (L= 1,1΄-(pyridin-2-ylmethylene)bis[3-(pyridin-2-yl)imidazo[1,5-a]pyridine) was generated again (Scheme 1).The reaction of HPIP, picolinaldehyde and CuCl2·2H2O gave crystals 1 and 2 simultaneously.The color of crystal 1 is dark green, while that of crystal 2 is green-rod. They are easy to distinguish.
Intrigued by the above work, we are eager to know if other new complexes could be obtained when CuCl2·2H2O is replaced by CuCl. Two reactions were examined. The 2:1:1 (HPIP:picolinaldehyde:CuCl) reaction in the mixture of EtOH and acetic acid gave orange-block crystals 3. If the ratio of HPIP, picolinaldehyde and CuCl is 2:3:1,red-platy crystals of 4 were generated.
It is possible that acetic acid plays two roles in the reactions. First, it acts as a radical inhibitor, preventing the further reduction of aldehyde group and the formation of tertiary carbon centers[10]. Secondly,it serves as an acid to facilitate the condensation reaction between HPIP and picoli-naldehyde.
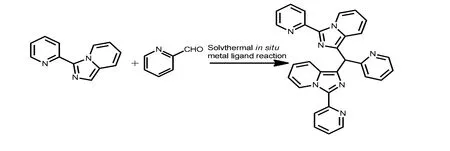
Scheme 1. Synthesis of the L ligand
3.2 Structure description
The collected crystal data for the four structures are shown in Table 1. Selected bond lengths and bond angles of complexes 1~4 are listed in Table 2.Selected hydrogen bond lengths and bond angles for 1~4 are summarized in Table 3.
Single-crystal X-ray diffraction reveals that 1 crystallizes in the monoclinic crystal system of Pnma space group. It displays a 1D chain structure.The asymmetric unit of 1 is shown in Fig. 1. The 1D and 2D networks of 1 are displayed in Figs. 2 and 3,respectively. The asymmetric unit of 1 consists of one CuIIion (Cu(1)), two CuIions (Cu(2),Cu(3)),one L ligand and four Cl-ions. The in situ formed L ligand coordinates to two Cu ions by five nitrogen atoms. The Cu(1) ion is five-coordinated by four nitrogen atoms (N(1), N(2), N(1A), N(2A)) originating from two PIP units of the L ligand and one Clion (Cl(3B)). The Cu(2) ion is bound to one nitrogen atom (N(4)) from pyridine unit of the L ligand and three Cl-anions (Cl(1), Cl(2), Cl(2A)), and Cu(3) is coordinated by three Cl-anions (Cl(2), Cl(3),Cl(2A)). The two adjacent Cu(2) and Cu(3) ions were doubly bridged by two Cl-anions (Cl(2),Cl(2A)), and Cu(1) and Cu(3) were bridged by one Cl-anion (Cl(3B)), forming a 1-D chain structure.

Fig. 1. Partially labeled plot of the asymmetric unit of 1 with H atoms being omitted for clarity
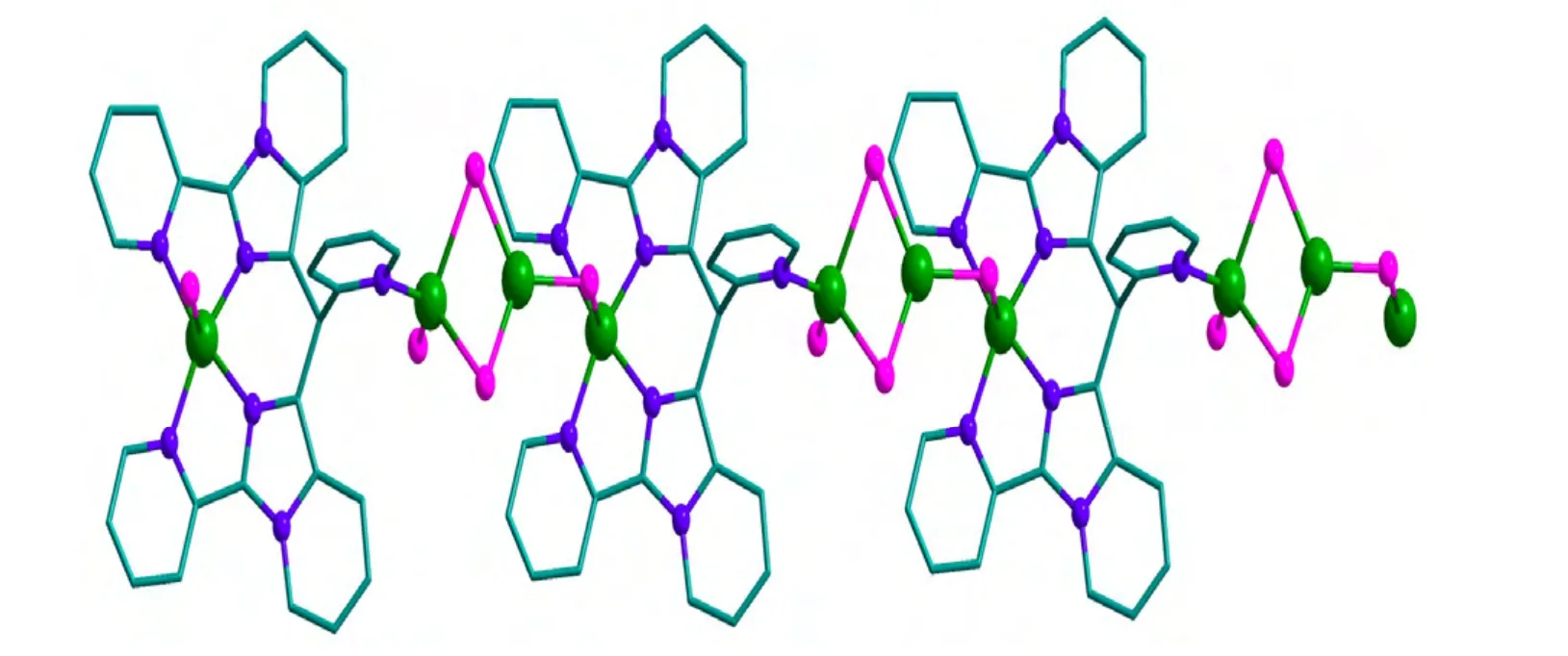
Fig. 2. 1D chain structure of 1 with H atoms being omitted for clarity

Fig. 3. 2D structure created by intermolecular hydrogen bonding interactions.Hydrogen atoms which are not involved in hydrogen bonds are omitted for clarity
Complex 1 features intermolecular C–H···Cl hydrogen bonding contacts between the C–H of pyridine rings as hydrogen atom donors, and Cl atoms coordinated to Cu ions as acceptors (C(8)–H(8)···Cl(3), C(3)–H(3)···Cl(2)). These intermolecular hydrogen bonds connect the 1-D chains to generate an infinite 2-D network.
Complex 1 joins a big family of Cu chain complexes[13-15]. However, such examples that use Clions as single or double bridges are very rare[16].
Compound 2 crystallizes in the orthorhombic crystal system of Fdd2 space group. Fig. 4 gives a perspective view of 2. Compound 2 consists of one CuIIion (Cu(1)), two CuIions (Cu(2), Cu(3)), one in situ formed L ligand, five Cl-ions and one solvent water molecule. The central copper(II) ion is coordinated by four N atoms from an in situ formed L ligand and one Cl-ion. Interestingly, there is one hydrogen atom that bonds to N(6) to balance the negative charge. The [CuCl3]2-unit and [CuCl2]-moiety are located around the [Cu(HL)Cl]2+unit to balance the charge of the molecule.

Fig. 4. Molecular structure of 2. Hydrogen atoms and water molecules are omitted for clarity
Strong intermolecular π-π stacking interactions exist in the structure (Fig. 5a). Beside the π-π interaction, the molecules are also linked to each other by three different hydrogen bonding interactions, namely, O(1)–H(1A)…Cl(1) (the oxygen atom O(1) from water molecule as a donor and the chloride atom Cl(1) as an acceptor), C(3)–H(3)…Cl(3) (the carbon atom C(3) as a donor and chloride atom Cl(3) from the [CuCl2] unit as an acceptor), and the other five C–H…Cl interactions(Table 3). An infinite 3-D structure is generated by these hydrogen bonding interactions (Fig. 5b).

Fig. 5. (a) Intermolecular π···π stacking interactions found in 2. (b) 3D structure created by intermolecular hydrogen bonding interactions. Hydrogen atoms involved in hydrogen bonds are omitted for clarity
X-ray single-crystal diffraction determination indicates that complex 3 crystallizes in the triclinic crystal system of P1 space group. Its structure is comprised of one [Cu3L2Cl2]+unit, one [CuCl2]-unit and two water molecules. The coordination environments of three Cu atoms are shown in Fig. 6. Cu(1)atom is coordinated by two N atoms from one L ligand and another two N atoms from another L ligand to generate a distorted tetrahedral coordination environment. Cu(2) and Cu(3) ions have similar coordination environments. They are coordinated by two N atoms from one L ligand and one Cl-ion to form a triangular geometry. The [CuCl2]-unit is located around the [Cu3L2Cl2]+unit to balance the charge of the molecule.
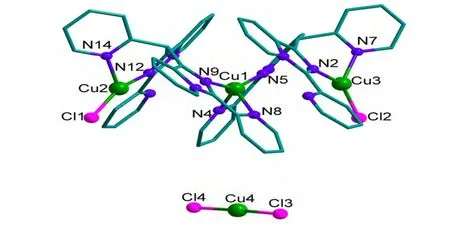
Fig. 6. Structure of 3. Hydrogen atoms and water molecules are omitted for clarity
The hydrogen bond interactions are found between the adjacent units and solvent water molecules (Fig. 7). These hydrogen bonds connect the units to afford a 2D framework structure.
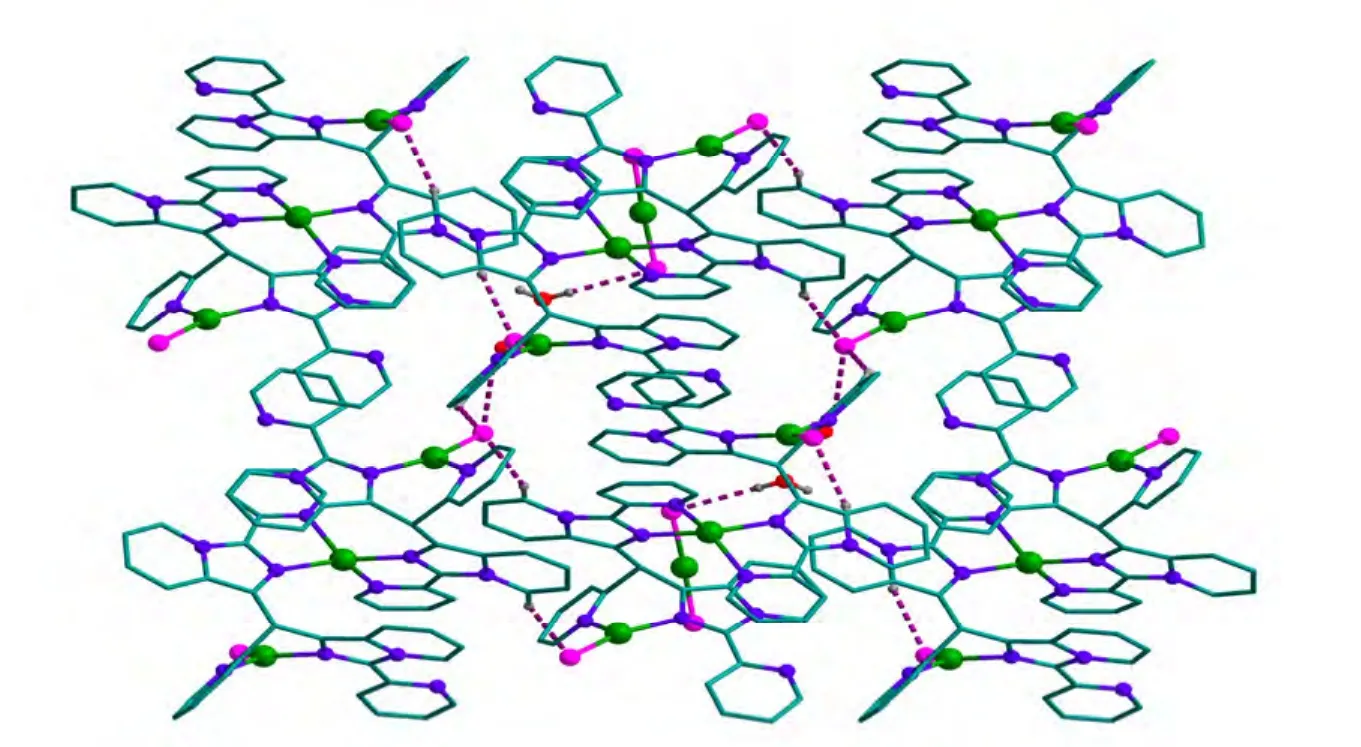
Fig. 7. 2D structure created by intermolecular hydrogen bonding interactions.Hydrogen atoms which are not involved in hydrogen bonds are omitted for clarity
Complex 4 crystallizes in the triclinic system,space group P 1. Its structure consists of three crystallographically independent Cu+cations, two in situ formed L ligand, and three [CuCl2]-units. The copper atoms in [Cu3L2]3+unit exhibit two different coordination geometries. The Cu(1) ion is coordinated by four nitrogen atoms from two PIP units to give rise to a tetrahedral coordination environment,as shown in Fig. 8. The coordination environments of Cu(2) and Cu(3) are the same. They are both three coordinated by three nitrogen atoms from the L ligand.
The intermolecular π-π stacking interactions are found between the adjacent units (Fig. 9). Beside these π-π stacking interactions, hydrogen bonds are also found between the adjacent units and [CuCl2]-moiety (Fig. 9). All these weak interactions connect the asymmetric units to give a 3D framework structure.

Fig. 8. Structure of 4. Hydrogen atoms are omitted for clarity

Fig. 9. (a) Intermolecular π···π stacking interactions found in 4. (b) 3D structure created by intermolecular hydrogen bonding interactions. Hydrogen atoms which are not involved in hydrogen bonds are omitted for clarity
Complex 4 was formed by slightly adjusting the ratio of the reactants of 3. However, the structures of complexes 3 and 4 were totally different, demonstrating the synthetic novelty of this work.
Complexes 3 and 4 are the members of a great family of trinuclear copper complexes[17-19]. The triangle topology is a common conformation of trinuclear copper complexes, whereas the linear configuration of Cu3 complexes is not common.
4 CONCLUSION
In summary, we have prepared four new complexes via solvothermal in situ metal ligand reactions in the presence of CH3COOH. It is believed CH3COOH plays a key role in forming complexes 1~4. This work demonstrates that the structures of 1~4 could be rationally tuned via the proper selection of different starting materials and dexterous adjustment of the ratio of the starting materials. We are currently studying the coordination chemistry of imidazo[1,5-a]pyridine and its derivatives with other metals in the presence of CH3COOH. This work is on-going and will be reported soon.
(1) Siamaki, A. R.; Aradtsen, B. A. A direct, one step synthesis of imidazoles from imines and acid chlorides: a palladium catalyzed multicomponent coupling approach. J. Am. Chem. Soc. 2006, 128, 6050–6051.
(2) Kanazawa, C.; Kamijo, S.; Yamamoto, Y. Synthesis of imidazoles through the copper-catalyzed cross-cycloaddition between two different isocyanides. J. Am. Chem. Soc. 2006, 128, 10662–10663.
(3) Sharma, G. V. M.; Jyothi, Y.; Sree Lakshmi, P. Efficient room-temperature synthesis of tri- and tetrasubstituted imidazoles catalyzed by ZrCl4. Synth.Commun. 2006, 36, 2991–3000.
(4) Garino, C.; Ruiu, T.; Salassa, L.; Albertino, A.; Volpi, G.; Nervi, C.; Gobetto, R.; Hardcastle, K. I. Spectroscopic and computational study on new blue emitting ReL(CO)3Cl complexes containing pyridylimidazo[1,5-a]pyridine ligands. Eur. J. Inorg. Chem. 2008, 2008, 3587–3591.
(5) Salassa, L.; Garino, C.; Albertino, A.; Volpi, G.; Nervi, C.; Gobetto, R.; Hardcastle, K. I. Computational and spectroscopic studies of new rhenium(I)complexes containing pyridylimidazo[1,5-a]pyridine ligands: charge transfer and dual emission by fine-tuning of excited states. Organometallics 2008, 27, 1427–1435.
(6) Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernández, R.; Brown, J. M.; Lassaletta, J. M. Imidazo[1,5-a]pyridine: a versatile architecture for stable N-heterocyclic carbenes. J. Am. Chem. Soc. 2005, 127, 3290–3291.
(7) Tong, Y. P.; Zheng, S. L.; Chen, X. M. Structures, photoluminescence and theoretical studies of two ZnIIcomplexes with substituted 2-(2-hydroxyphenyl)benzimidazoles. Eur. J. Inorg. Chem. 2005, 2005, 3734–3741.
(8) Eseola, A. O.; Li, W.; Adeyemi, O. G.; Obi-Egbedi, N. O.; Woods, J. A. O. Hemilability of 2-(1H-imidazol-2-yl)pyridine and 2-(oxazol-2-yl)pyridine ligands: imidazole and oxazole ring Lewis basicity, Ni(II)/Pd(II) complex structures and spectra. Polyhedron 2010, 29, 1891–1901.
(9) Chen, Y. M.; Li, L.; Chen, Z.; Liu, Y. L.; Hu, H. L.; Chen, W. Q.; Liu, W.; Li, Y. H.; Lei, T.; Cao, Y. Y.; Kang, Z. H.; Lin, M. S.; Li, W.Metal-mediated controllable creation of secondary, tertiary, and quaternary carbon centers: a powerful strategy for the synthesis of iron, cobalt, and copper complexes with in situ generated substituted 1-pyridineimidazo[1,5-a]pyridine ligands. Inorg. Chem. 2012, 51, 9705–9713.
(10) Roy, M.; Chakravarthi, B. V. S. K.; Jayabaskaran, C.; Karande, A. A.; Chakravarty, A. R. Impact of metal binding on the antitumor activity and cellular imaging of a metal chelator cationic imidazopyridine derivative. Dalton Trans. 2011, 40, 4855–4864.
(11) Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution. University of Göttingen, Germany 1997.
(12) Sheldrick, G. M. SHELXL-97, Program for the Refinement of Crystal Structures from Diあraction Data. University of Göttingen, Germany 1997.
(13) Escuer, A.; Vicente, R.; EI Fallah, M. S.; Goher, M. A. S.; Mautner, F. A. Synthesis and structural characterization of the one-dimensional[Cu(3-Clpy)2(N3)2]ncomplex (3-Clpy = 3-Chloropyridine): a singular ferrimagnetic chain with local SA= SB. Inorg. Chem. 1998, 37, 4466–4469.
(14) Li, B. L.; Xu, Z.; Cao, Z. B.; Zhu, L. M.; Yu, K. B. One-dimensional chain copper complexes [Cu(dien)(btrz)(ClO4)2] and [Cu(en)2(btrz)(ClO4)2]based on the bridging ligand 1,2-bis(1,2,4-triazole-1-yl)ethane (btrz). Transit. Metal. Chem. 1999, 24, 622–627.
(15) Li, L. C.; Liao, D. Z.; Liu, S. Y.; Jiang, Z. H.; Yan, S. P. A one-dimensional chain compound [Cu(im2-py)(SCN)2]n exhibiting strong ferromagnetic coupling. Inorg. Chem. Commun. 2003, 6, 225–228.
(16) Song, J. L.; Dong, Z. C.; Zeng, H. Y.; Zhou, W. B.; Naka, T.; Wei, Q.; Mao, J. G.; Guo, G. C.; Huang, J. S. [Cu(H4C3N2S)Cl2]n, an unprecedented diazole-bridged one-dimensional copper halide: synthesis, structure, and magnetic properties. Inorg. Chem. 2003, 42, 2136–2140.
(17) Joshi, S. A.; Kulkarni, N. D. A new trinuclear Cu(II) complex of inositol as a hydrogelator. Chem. Commun. 2009, 17, 2341–2343.
(18) Driessen, W. L.; Chang, L.; Finazzo, C.; Gorter, S.; Rehorst, D.; Reedijk, J.; Lutz, M.; Spek, A. L. Two pyrazolato-bridged, linear trinuclear Cu(II)complexes. Crystal structures and magnetic properties. Inorg. Chim. Acta 2003, 350, 25–31.
(19) Tercero, J.; Diaz, C.; Ribas, J.; Mahia, J.; Maestro, M. A. New oxamato-bridged trinuclear CuII-CuII-CuIIcomplexes with hydrogen-bond supramolecular structures: synthesis and magneto-structural studies. Inorg. Chem. 2002, 41, 5373–5381.
杂志排行
结构化学的其它文章
- A New Pleuromutilin Derivative: Synthesis,Crystal Structure and Antibacterial Evaluation①
- Hydrogen Abstraction Reaction Mechanisms and Rate Constants for Isoflurane with a Cl Atom at 200~2000 K: A Theoretical Investigation①
- Synthesis, Crystal Structure and Analysis of 6-Methoxycarbonylmethyl-7-methyl-6H-dibenzopyran①
- Molecular Structure and Theoretical Thermodynamic Study of Folic Acid Based on the Computational Approach
- Synthesis, Crystal Structure and Biological Activity of N-cyanosulfoximine Derivative Containing 1,2,3-Thiadiazole①
- A 2D Brickwall-like Copper(II) Coordination Polymer Based on Phenyliminodiacetate and 4,4΄-Bipyridine:Synthesis, Crystal Structure and Magnetic Property①
