白光荧光粉Ba1.3Ca0.7-y-z(AlxSi1-x)O4:yEu2+,zMn2+发光性能研究
2015-03-22陈永杰吴胜男耿秀娟
陈 琳, 陈永杰, 吴胜男, 杨 英, 耿秀娟
(沈阳化工大学 辽宁省稀土化学及应用重点实验室, 辽宁 沈阳 110142)
白光荧光粉Ba1.3Ca0.7-y-z(AlxSi1-x)O4:yEu2+,zMn2+发光性能研究
陈 琳, 陈永杰, 吴胜男, 杨 英, 耿秀娟
(沈阳化工大学 辽宁省稀土化学及应用重点实验室, 辽宁 沈阳 110142)
采用高温固相反应在还原气氛中合成Ba1.3Ca0.7-y-z(AlxSi1-x)O4:yEu2+,zMn2+白光荧光粉.研究硅铝摩尔比变化对荧光粉晶体结构和光谱性能的影响.XRD结果表明:改变硅铝摩尔比对荧光粉晶体结构基本无影响,晶相结构为Ba1.3Ca0.7SiO4;荧光光谱显示在277 nm紫外光激发下,Eu2+,Mn2+共掺杂的荧光粉的发射光谱覆盖425~550 nm蓝绿光波带和550~650 nm橙红光波带,最大发射峰位于454、593 nm,这两个发射宽带组合形成白光.
发光二级管; 白色荧光粉; 高温固相反应; Ba1.3Ca0.7(AlxSi1-x)O4
白光LED是一类新型光源,其中以半导体化合物InGaN为基础的白光LED有许多优点:发光效率高、节能、稳定性好、绿色环保和寿命长等,逐渐取代白炽灯和汞灯成为第四代照明光源[1-2].近年来,由于稀土离子和过渡金属激活的碱土铝硅酸盐具有作为荧光材料的潜在利益而被广泛研究[3-6].利用高温固相反应,紫外芯片激发的硅铝酸盐荧光粉具有较稳定的晶体结构、简单的制备工艺、良好的物理化学稳定性和原料丰富等优点[7],被认为是稀土发光材料中一类有潜力的基质材料.蓝色荧光粉是合成白光LED不可缺少的成分,因此对蓝色荧光粉的研发也成为热点之一.通常情况下,Eu2+在Ba1.3Ca0.7SiO4基质中的发光是由4f→5d能级跃迁引起的,以发射蓝光为主,其中部分能量能够传递给 Mn2+,使其发射红光[8-9],因此蓝光发射强度的强弱限制着白光荧光粉的发光强度.不同基质对荧光粉发光的影响很大,所以基质组成的任何变化都可能改变能量传递过程、晶场强度和共价性,进而最终影响发光材料的发光效率和发光特性.本文采用高温固相反应合成六方晶系碱土硅铝酸盐白光LED用荧光粉Ba1.3Ca0.7-y-z(AlxSi1-x)O4:yEu2+,zMn2+.探讨了Al3+取代Si4+的最佳摩尔比,以及共掺杂Eu2+-Mn2+对此体系荧光粉晶体结构、荧光性能的影响.
1 实 验
1.1 样品的制备
采用高温固相反应法制备白荧光粉Ba1.3Ca0.7-y-zAlxSi1-xO4:yEu2+,zMn2+,所用原料为BaCO3(A.R.),CaCO3(A.R.),Al2O3(A.R.),SiO2(A.R.),Eu2O3(4N),MnCO3(A.R.),BaCl2·2H2O(A.R.)作为助熔剂.按照所设计的化学式的化学计量比称取原料,Al2O3掺杂的摩尔分数为0.01~0.06,Eu2O3和MnCO3掺杂的摩尔分数为0.01~0.08.将称取的原料置于玛瑙研钵中,用无水乙醇作为研磨介质充分研磨15 min,在60 ℃真空干燥箱中干燥30 min后装入瓷舟,在V(H2)/V(N2)=1∶9的氮氢还原气体下,于1 000 ℃下保温1.5 h,程序结束后令其自然冷却至室温,研磨即得样品.
1.2 样品的表征
采用Bruker D8型X射线衍射(XRD)仪测定样品的粉末衍射图(辐射源为Cu靶,管电压40 kV,工作电流40 mA,λ=0.154 06 nm).样品的荧光光谱采用Hitachi F-4600型荧光分光光度计(Fluoroscence Spectrophotometer)测定(狭缝2.5 nm,工作电压400 V,扫描速度1 200 nm/min).使用PMS-50型紫外-可见-近红外光谱(Ultraviolet-visible-near Infrared Spectroscopy)分析系统对样品进行色温(Tc)、显色指数(Ra)和色坐标(CIE)测试(扫描步长为5 nm,激发波长为365 nm).所有测量均在室温下进行.
2 结果与讨论
2.1 晶相分析
制备的Ba1.3Ca0.68Al0.025Si0.975O4:0.02Eu2+和Ba1.3Ca0.65Al0.025Si0.975O4:0.02Eu2+,0.03Mn2+荧光粉的XRD图谱如图1所示.与X射线衍射标准数据库《Powder Diffraction File》的标准化合物Ba1.3Ca0.7SiO4(JCPDS#48-0210)数据对比发现,所制样品的XRD衍射峰的数据与标准卡片数据基本一致,说明少量Al3+,Eu2+,Mn2+的掺杂没有产生杂相.

图1 Ba1.3Ca0.68Al0.025Si0.975O4:0.02Eu2+和Ba1.3Ca0.65Al0.025Si0.975O4:0.02Eu2+,0.03Mn2+的XRD图谱
2.2 光谱分析
2.2.1 硅铝摩尔比对Ba1.3Ca0.68AlxSi1-xO4:0.02Eu2+荧光粉光谱性能的影响
为探讨Ba1.3Ca0.68AlxSi1-xO4:0.02Eu2+荧光粉中硅铝摩尔比对其发光性质的影响及找出合适的硅铝摩尔比,合成一系列硅铝摩尔比为0.99/0.01,0.975/0.025,0.96/0.04,0.94/0.06,0.92/0.08的Ba1.3Ca0.68AlxSi1-xO4:0.02Eu2+荧光粉,其发射光谱如图2所示,插图为发射峰(456 nm)强度与掺杂离子掺杂量关系图.由图2可以看出:发射光谱覆盖430~590 nm的宽带,最大峰值位于456 nm处,随着增加Al3+取代Si4+的含量,荧光粉发射光谱形状没有明显的改变,但属于Eu2+的特征发射峰(456 nm)强度逐渐增大,这可能是因为Al3+取代Si4+在体系中造成局部缺陷结构,在某种程度上这些缺陷结构能够提高发光中心离子的跃迁几率,从而增强体系的发光性能[10],它们的增强机理有待于进一步研究.当硅铝摩尔比为0.975/0.025时,荧光粉的发射强度最大.因此选用0.025/0.975的铝硅配比较为合理,确定荧光粉的组成是Ba1.3Ca0.68Al0.025Si0.975O4:0.02Eu2+.

图2 Ba1.3Ca0.68AlxSi1-xO4:0.02Eu2+(x=0,0.01,0.025,0.04,0.06)的发射光谱
2.2.2 Ba1.3Ca0.7-y-zAl0.025Si0.975O4:yEu2+,zMn2+的光谱性能
图3为荧光粉Ba1.3Ca0.68-yAl0.025Si0.975O4:yEu2+,0.02Mn2+(y=0,0.01,0.02,0.03,0.04,0.06,0.08)的发射光谱(277 nm激发).当y=0时,Ba1.3Ca0.68Al0.025Si0.975O4:0.02Mn2+发射带没有发射峰,说明单掺杂Mn2+的荧光粉不发光;当y=0.01~0.08时,在590 nm附近出现了红光发射峰,这是因为Eu2+的一部分能量传递给Mn2+的结果,从而形成Mn2+的能级跃迁.发射带由425~550 nm的蓝绿光波带和550~650 nm的橙红光波带组成,最大峰值位于454、590 nm.从图3可以看到:固定Mn2+的摩尔分数为0.02时,Eu2+的变化对发射光谱的形状没有产生明显的影响,但是随着Eu2+掺杂量的增加,一定程度上改变了蓝绿光和红光的发光强度.当Eu2+摩尔分数为0.02时,其蓝绿光和红光的发光强度均较强.其中,蓝绿光波带为Eu2+取代Ca格位的5d→4f能级跃迁的特征发射,红光波带可归属于Mn2+取代与其半径相近的Ca格位的4T1—6A1跃迁发射[11-12].

图3 Ba1.3Ca0.68Al0.025Si0.975O4:yEu2+,0.02Mn2+(y=0,0.01,0.02,0.03,0.04,0.06,0.08)的发射光谱
图4为荧光粉Ba1.3Ca0.68Al0.025Si0.975O4:0.02Eu2+,zMn2+(z=0,0.01,0.02,0.03,0.04,0.06,0.08)的发射光谱(277 nm激发下).由图4可知:不同含量的Mn2+对荧光粉的发射光谱具有较大的影响.当掺杂的Mn2+含量较少时,蓝光带的发光强度较强,红光发射带较弱.随着Mn2+含量的增加,由Eu2+传递给Mn2+的能量增加,使得Eu2+的425~550 nm蓝绿光发射强度逐渐降低,Mn2+的550~700 nm红光强度逐渐增强.当Mn2+的摩尔分数为0.03时,蓝绿光波带较强.
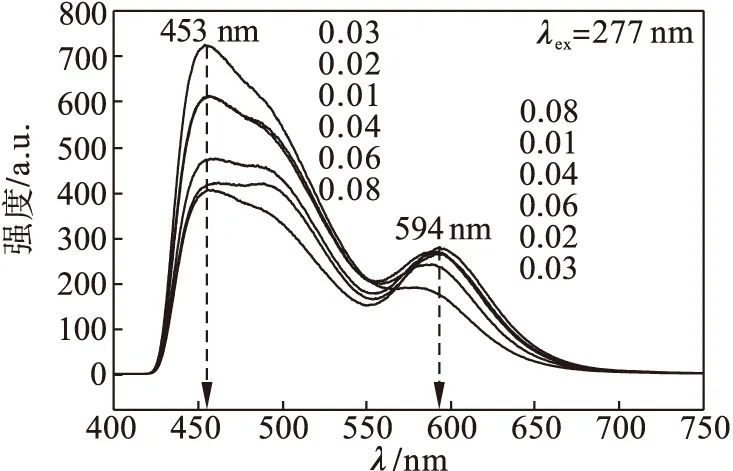
图4 Ba1.3Ca0.68Al0.025Si0.975O4:0.02Eu2+,zMn2+(z=0,0.01,0.02,0.03,0.04,0.06,0.08)的发射光谱
2.3 光色参数分析
Ba1.3Ca0.68-yAl0.025Si0.975O4:yEu2+,0.02Mn2+(y=0.01~0.08)和Ba1.3Ca0.7-yAl0.025Si0.975O4:yEu2+,zMn2+(z=0.01~0.08)荧光粉的光色参数分别如表1、表2所示.
表1 Ba1.3Ca0.68-yAl0.025Si0.975O4:yEu2+,0.02Mn2+(y=0.01~0.08)的光色参数
Table 1 The light and color parameters of Ba1.3Ca0.68-yAl0.025Si0.975O4:yEu2+,0.02Mn2+(y=0.01~0.08)

y(x,y)Tc/KRa0 01(0 2829,0 2991)923685 70 02(0 3009,0 3232)724884 50 03(0 3159,0 3342)630084 10 04(0 3287,0 3629)565182 20 06(0 3533,0 3819)482680 60 08(0 3451,0 3744)506781 6
表2 Ba1.3Ca0.68-zAl0.025Si0.975O4:0.02Eu2+,zMn2+(z=0.01~0.08)的光色参数
Table 2 The light and color parameters of Ba1.3Ca0.68-zAl0.025Si0.975O4:0.02Eu2+,zMn2+(z=0.01~0.08)

z(x,y)Tc/KRa0 01(0 2667,0 3063)1045782 60 02(0 3009,0 3232)724884 50 03(0 3226,0 3290)598483 60 04(0 3379,0 3446)526782 60 06(0 3538,0 3527)468181 50 08(0 3758,0 3480)390079 4
由上可知,Eu2+在Ba1.3Ca0.7-y-zAl0.025Si0.975O4:yEu2+,zMn2+体系中作为发光中心,发射蓝绿光,同时激活Mn2+使其发射红光.当固定Mn2+含量为0.02时,由表1、表2可知:当增加Eu2+和Mn2+的掺杂量时,荧光粉的色温向着低色温规律性变化.已知标准白光的光谱性质参数的色坐标是(0.33,0.33),Tc=6 430 K,Ra=76,当x(Eu2+)=0.02,x(Mn2+)=0.03时,荧光粉的色坐标(x,y)=(0.322 6,0.329 0),Tc= 5 984 K,Ra=83.6,明显优于标准白光的光谱参数,发光效果较好.综合考虑,Ba1.3Ca0.65Al0.025Si0.975O4:0.02Eu2+,0.03Mn2+为此系列最佳荧光粉.
总之,改变Eu2+和Mn2+的掺杂比例能对Ba1.3Ca0.7-y-zAl0.025Si0.975O4:yEu2+,zMn2+系列荧光粉的发光颜色起到调节作用.
3 结 论
采用高温固相反应合成了白光荧光粉Ba1.3Ca0.65(Al0.025Si0.975)O4:0.02Eu2+,0.03Mn2+.XRD结果表明:少量Al2+,Eu2+,Mn2+的掺杂没有使晶体产生杂相,晶体结构为Ba1.3Ca0.7SiO4.荧光光谱表明:在体系中Al3+取代Si4+可能会造成局部的缺陷结构,这些缺陷结构能够提高发光中心离子的跃迁几率,从而增强体系的发光强度;共掺杂Eu2+-Mn2+荧光粉的激发光谱激发主峰位于277 nm左右,在220~450 nm之间均有较强吸收,发射光谱由425~550 nm的蓝绿光波带和550~650 nm的红橙光波带组成,其分别属于Eu2+的5d→4f能级跃迁的特征发射和Mn2+的4T1—6A1跃迁发射.光色参数结果表明:Eu2+-Mn2+比例变化对此体系荧光粉的发光颜色能够起到调节作用.因此,可调节的白光荧光粉Ba1.3Ca0.65(Al0.025Si0.975)O4:0.02Eu2+,0.03Mn2+在白光LED领域具有应用潜力.
[1] Marc D,Nadarajah N,Andrew B,et al.Impact of Dimming White LEDs:Chromaticity Shifts Due to Different Dimming Methods[J].Proceedings of SPIE,2005(5941):291-299.
[2] Narendran N,Deng L,Pysar R M,et al.Performance Characteristics of High-power Light-emitting Diodes[J].Proceedings of SPIE,2004(5187):267-275.
[3] 陈立松,柏朝晖,李金伟,等.白光LED用SrAl2Si2O8:Eu2+荧光粉的制备与发光性能研究[J].无机化学学报,2010,26(8):1409-1414.
[4] Zhang Q,Wang J,Zhang M,et al.Tunable Bluish Green to Yellowish Green Ca2(1-x)Sr2xAl2SiO7:Eu2+Phosphors for Potential LED Application[J].Applied Physics B,2008,92(2):195-198.
[5] Kuang J Y,Liu Y L,Zhang J X.Effects of RE3+as a Co-dopant in Blue-emitting Long-lasting Phosphors,Sr3Al10SiO20:Eu2+[J].Journal of Materials Science,2006,41(17):5500-5503.
[6] Denis G,Rocquefelte X,Deniard P,et al.Site Preference of Eu2+Dopants in the(Ba,Sr)13-xAl22-2xSi10+2xO66Phosphor and Its Effect on the Luminescence Properties:A Density Functional Investigation[J].Journal of Materials Chemistry,2009,19(48):9170-9175.
[7] Jüstel T,Nikol H,Ronda C.New Developments in the Field of Luminescent Materials for Lighting and Displays[J].Angewandte Chemie International Edition,1998,37(22):3084-3103.
[8] Xiao F,Xue Y N,Ma Y Y,et al.Ba2Ca(B3O6)2:Eu2+,Mn2+:A Potential Tunable Blue-white-red Phosphors for White Light-emitting Diodes[J].Physical B:Condensed Matter,2010,405(3):891-895.
[9] 丁振瑞,王凤和,杨志平,等.KNaCa2(PO4)2中Eu2+的发光及Eu2+对Mn2+的能量传递[J].中国稀土学报,2010,28(3):266-269.
[10]徐叙瑢,苏勉曾.发光学与发光材料[M].北京:化学工业出版社,2004:74-79.
[11]Park K,Choi N,Kim J,et al.Temperature and Excitation Power-resistant White-light Emission of the T-phase(Ba,Ca)2SiO4:Eu2+,Mn2+Phosphor[J].Solid State Communications,2010,150(7/8):329-332.
[12]Choi N S,Park K W,Park B W,et al.Eu2+-Mn2+Energy Transfer in White-light-emitting T-phase(Ba,Ca)2SiO4:Eu2+,Mn2+Phosphor[J].Journal of Luminescence,2010,130(4):560-566.
Luminescent Properties of White Light Phosphors Ba1.3Ca0.7-y-z(AlxSi1-x)O4:yEu2+,zMn2+
CHEN Lin, CHEN Yong-jie, WU Sheng-nan, YANG Ying, GENG Xiu-juan
(Shenyang University of Chemical Technology, Shenyang 110142, China)
The white light phosphor Ba1.3Ca0.7(AlxSi1-x)O4:Eu2+,Mn2+were synthesized by high-temperature solid-state reaction under the reducing atmosphere.The effect of silicon/aluminum ratio(the molar ratio)changing on crystal structure and spectral properties of the phosphors were investigated.The XRD result indicates that there is no influence on the crystal structure of sample when we change the silicon/aluminum ratio,the crystal structure is Ba1.3Ca0.7SiO4.The luminescence spectrum shows,under the UV light excitation at 277 nm,the emission spectrum of Eu2+-Mn2+co-doped phosphor covers the blue-green wave band of 420~550 nm and orange-red wave band of 550~650 nm,and the maximum emission peaks are at 454 and 593 nm,and two emitting bands can be combined into a white light.
light-emitting diode; white color phosphor; high-temperature solid-state reaction Ba1.3Ca0.7(AlxSi1-x)O4
2013-12-19
辽宁省自然科学基金项目(LS201102174)
陈琳(1988-),女,辽宁锦州人,硕士研究生在读,国家奖学金获得者,主要从事稀土发光材料方面的研究.
陈永杰(1963-),女,辽宁本溪人,教授,博士,主要从事稀土发光材料和精细化工方面的研究.
2095-2198(2015)04-0289-04
10.3969/j.issn.2095-2198.2015.04.001
TQ422;TQ630.6
A
