脂肪分化相关信号通路及microRNA调节研究进展
2015-03-22贾夏丽潘洋洋乔利英郭云雁胡子乔李宝钧刘文忠
贾夏丽,潘洋洋,乔利英,郭云雁,胡子乔,张 方,李宝钧,刘文忠
(山西农业大学动物科技学院,太谷 030801)
脂肪分化相关信号通路及microRNA调节研究进展
贾夏丽#,潘洋洋#,乔利英,郭云雁,胡子乔,张 方,李宝钧,刘文忠*
(山西农业大学动物科技学院,太谷 030801)
畜禽体内脂肪含量的多少决定了其肉质的好坏,而脂肪分化则是一个由许多因子调控的复杂生物学过程。了解脂肪分化相关的信号通路及其miRNAs的调节作用,可以在一定程度上揭示脂肪分化的分子调控机制。本文在对6个重要的调控脂肪细胞分化的信号通路进行介绍的基础上,分别对8个促进脂肪细胞分化和8个抑制脂肪细胞分化的miRNAs的生物学功能和作用机制进行了探讨,总结了其靶基因在脂肪形成中的作用。有关miRNAs及其靶基因的研究为畜禽肉质性状的遗传改良提供了理论依据。
脂肪细胞;成脂分化;信号通路;microRNA;靶基因
脂肪是动物体内重要的贮能物质,也是机体能量稳态的调节器。当机体需要能量时,贮存在脂肪细胞中的脂肪,通过动员被脂肪酶水解为游离脂肪酸和甘油,释放入血液,被其他组织氧化利用。脂肪也与肥胖相关的一些代谢性疾病如心血管疾病和Ⅱ型糖尿病的发生和发展有关[1-3]。动物脂肪的发育是一个复杂过程,受多种转录因子、细胞信号分子的调控。其中,脂肪分化相关的几个重要信号通路和miRNAs对脂肪发育具有重要的调控作用。
1 调控脂肪细胞分化的信号通路
脂肪的发育过程受许多因子调控,形成了一个复杂的调控网络。脂肪细胞分化的信号通路主要包括:Wnt信号通路、过氧化物酶体增殖物激活受体(Peroxisome proliferator-activated receptors,PPARs)通路、固醇调节组件结合蛋白(Sterol regulatory element-binding proteins,SREBPs)通路、ERK-MAPK信号通路、cAMP/PKA信号通路和Hedgehog信号通路等。
1.1 Wnt信号通路
Wnt信号通路是一种高度保守的信号通路,通过抑制核受体因子过氧化物酶体增殖物激活受体γ(PPARγ)及其转录因子CCAAT/增强子结合蛋白(CCAAT/enhancer binding protein,C/EBP),削弱骨髓基质细胞(Bone marrow stromal cells,BMSCs)向脂肪细胞的分化[4],从而抑制脂肪细胞的分化[5],该通路失控可导致肥胖的发生[6]。此外,细胞因子如白细胞介素-6(Interleukin-6,IL-6)和肿瘤坏死因子α(Tumor necrosis factor,TNF-α)对Wnt通路也有一定的调节作用。IL-6可以促进Wnt蛋白的表达,TNF-α增加了Fz/LRP/Dvl复合体的磷酸化,二者均激活Wnt信号,抑制β联蛋白(β-catenin)的降解,激活的Wnt信号使前体脂肪细胞生长停滞。生长停滞的前体脂肪细胞在MDI(0.5 mmol·L-1甲基异丁基黄嘌呤(Methylisobutylxanthine)、1 μmol·L-1地塞米松(Dexamethasone)和 865 nmol·L-1胰岛素(Insulin))诱导下分化,β-catenin抑制下游水平的C/EBPα和PPARγ,削弱甚至阻止脂肪细胞分化(图1)[7]。Wnt通路中的转录因子7-类似物2(Transcription factor 7-like 2,Tcf7l2)是触发该通路的重要因子,与Ⅱ型糖尿病的发生有着密切的关系[8]。
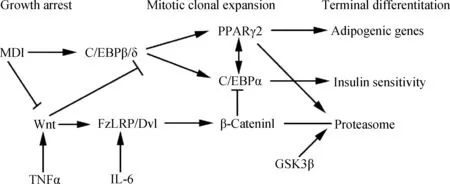
图1 细胞因子诱导影响Wnt信号通路及下游事件导致脂肪细胞分化示意图[7]Fig.1 Schematic representation of the cytokine-induced effect on the Wnt signaling pathway and the downstream events leading to adipocyte differentiation[7]
1.2 PPARs信号通路
PPARs家族是控制目的基因转录的核受体因子[9]。PPARs包括α、β、γ 3种亚型,其中PPARα和PPARγ与高血脂症和动脉粥样硬化等相关疾病有关[10],二者在脂肪分化与代谢中发挥重要作用。PPARα与甘油三酯、低密度胆固醇以及血脂药物应答的炎症标志物有关[11]。PPARγ是一种成脂分化的重要转录因子,可以加快脂肪细胞分化和沉积[12]。林婄婄等[13]发现,PPARα与PPARγ在调节脂肪代谢方面作用相反,但是存在协同作用。
1.3 SREBP信号通路
固醇调节组件结合蛋白(SREBPs)一直是研究鞘脂(Sphingolipids)合成抑制动脉粥样硬化机制的焦点[14],是脂质代谢的重要调控因子。其家族中的3个转录因子SREBP-1a、SREBP-1c和SREBP-2分别由SREBP1和SREBP2两个基因编码,可参与调节胆固醇、脂肪酸、甘油三酯等代谢相关基因的表达。SREBP-1a和SREBP-1c是同一个基因选择性剪接的结果,其差异仅体现在N端序列的不同。前者是成脂分化关键的转录调控因子,可以促进人的前脂肪细胞增殖,并调控细胞周期[15];后者限制血浆甘油三酯和胆固醇水平,还可以调节极低密度脂蛋白(Very low density lipoprotein,VLDL)的大小。由于其表达水平在胰岛素抵抗和Ⅱ型糖尿病中偏高,它可能对糖尿病血脂异常和因血脂异常引起的动脉粥样硬化具有重要作用[16]。SREBP-2是激活胆固醇吸收所需的主要基因,控制胆固醇的合成[17]。
1.4 ERK-MAPK信号通路
丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)信号通路在细胞增殖和分化等许多重要的细胞过程中发挥重要作用,细胞外信号调节激酶(Extracellular signal-regulated kinase,ERK)是MAPK信号通路的主要成员之一。研究表明,ERK-MAPK信号通路在间充质干细胞(Mesenchymal stem cells,MSCs)分化过程中影响成脂分化[18-19]。
1.5 cAMP/PKA信号通路
环磷酸腺苷(Cyclic 3′,5′-adenosine monophosphate,cAMP)是一个重要的胞内信号分子,其主要功能是激活cAMP依赖的蛋白激酶A(Protein kinase A,PKA),调控细胞的分化。cAMP/PKA信号可促进MSCs的成脂分化,抑制其成骨分化[20]。β3肾上腺素能受体(β3-adrenergic receptors,ADRB3)参与脂肪分解和产热[21],其对MSCs的成脂分化的影响是通过cAMP/PKA信号通路介导的[22]。
1.6 Hedgehog信号通路
Hedgehog(Hh)由Sonic hedgehog(Shh)、Indian hedgehog(Ihh)和Dersert Hedgehog(Dhh)3个成员组成,在细胞分化过程中发挥至关重要的作用。在脂肪细胞分化过程中Hh信号减弱,是成脂分化过程中一个必需的调节器,但并不足以触发成脂分化[23]。瘦素(Leptin)对发育、肥胖和肿瘤转移等瘦素调节过程具有重要作用,通过激活Hedgehog信号以改变控制PPARγ等细胞分化相关基因的表达[24]。
2 miRNAs在脂肪发育中的调控机制
R.C.Lee等[25]在Caenorhabditiselegans中发现了第一个小RNA,其长度只有22 nt,将其命名为lin-4。lin-4不编码蛋白,但可以通过反义RNA-RNA互作的方式调节lin-14基因的翻译。此后,越来越多的研究者开始关注小分子非编码RNA(sncRNAs)。MicroRNA(miRNAs)是一类内源性的小分子非编码RNA,其长度约为18~25 nt,主要通过与mRNA序列3′UTR完全或不完全的碱基互补配对,导致mRNA降解或抑制其表达,从而实现对基因表达的转录后调控。然而,也有少数miRNAs通过靶作用于启动子序列而诱导基因表达,如miR-373[26]。随着miRNAs研究方法的发展,已发现多个miRNAs在脂肪发育中发挥调控作用。一些miRNAs对脂肪细胞分化起正调控作用,而另一些则抑制脂肪细胞的分化。
2.1 促进脂肪细胞分化和脂肪沉积的miRNAs
表1列出了促进脂肪细胞分化相关的miRNAs及其靶基因。
表1 促进脂肪细胞分化相关的miRNAs及其靶基因
Table 1 The miRNAs promoting adipocyte differentiation and their target genes
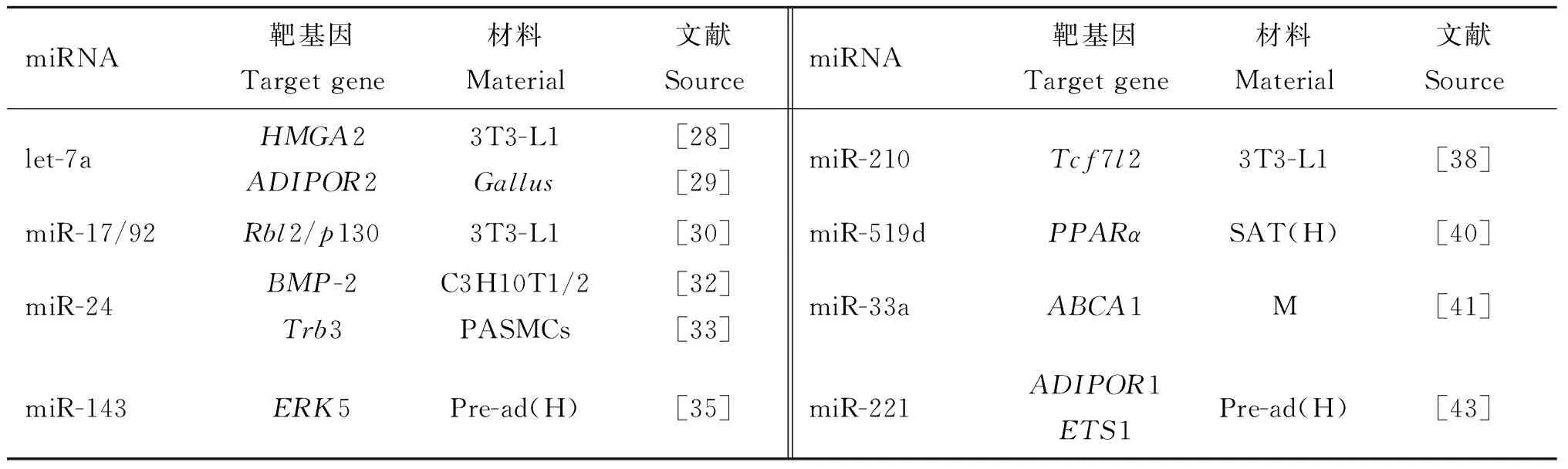
miRNA靶基因Targetgene材料Material文献SourcemiRNA靶基因Targetgene材料Material文献Sourcelet-7aHMGA2ADIPOR23T3-L1Gallus[28][29]miR-210Tcf7l23T3-L1[38]miR-17/92Rbl2/p1303T3-L1[30]miR-519dPPARαSAT(H)[40]miR-24BMP-2Trb3C3H10T1/2PASMCs[32][33]miR-33aABCA1M[41]miR-143ERK5Pre-ad(H)[35]miR-221ADIPOR1ETS1Pre-ad(H)[43]
3T3-L1.小鼠脂肪样成纤维细胞;C3H10T1/2.从C3H小鼠分离建立的间充质干细胞株;PASMCs.肺动脉平滑肌细胞;Pre-ad.前体脂肪细胞;H.人类;M.小鼠;SAT.皮下脂肪组织
3T3-L1.Mouse embryonic fibroblast adipose like cell line;C3H10T1/2.Mesenchymal stem cell lines separated from C3H mice;PASMCs.Pulmonary artery smooth-muscle cells;Pre-ad.Preadipocyte;H.Homosapiens;M.Musmusculus;SAT.Subcutaneous adipose tissue
Let-7家族是最早发现的具有物种间保守性的miRNA家族之一,在生物生长发育过程中具有广泛的作用,尤其对肿瘤细胞的生长发育发挥重要作用[27]。对3T3-L1前脂肪细胞分化过程中miRNA的表达谱研究发现,其家族成员let-7a具有促进脂肪细胞分化的能力,其靶基因为高迁移率族蛋白A2(High mobility group protein A2,HMGA2)基因[28]。let-7a与脂联素受体2(Adiponectin receptor 2,ADIPOR2)基因也存在潜在靶标关系[29]。这说明一个miRNA可能调节多个靶基因。
有些miRNAs在基因组上成簇存在。miR-17/92簇就是一个高度保守的基因簇。在小鼠3T3-L1脂肪细胞分化过程中,该基因簇促进细胞增殖,在脂肪细胞分化克隆扩增阶段显著上调。该基因簇与视网膜母细胞瘤(Retinoblastoma 2,Rbl2/p130)基因存在靶标关系[30]。其调控机理是miR-17/92簇通过靶作用于Rbl2/p130的3′UTR抑制其表达,进而使得转录因子E2F4和E2F5的编码基因上调。其中,这两个基因具有调控细胞周期和促进脂肪细胞分化的功能。
骨形态发生蛋白 2(Bone morphogenetic protein-2,BMP-2)可以诱导脂肪细胞分化[31]。F.Sun等[32]发现,miR-24可以上调BMP-2基因的表达,进而更有效地促进脂肪细胞分化;而miR-31是通过靶向作用于C/EBPα抑制脂肪细胞分化。该研究组单独过表达miR-24和miR-31,分别与阴性对照比较发现,它们对脂肪分化并没有明显的作用。说明这两个miRNAs是通过靶基因起作用的。此外,miR-24还靶向作用于毛球样蛋白-3(Tribbles-like protein-3,Trb3)基因Trb3[33],下调其表达。而Trb3蛋白在胰岛素抵抗和Ⅱ型糖尿病的发生中发挥作用[34]。
研究发现,miR-143在脂肪细胞分化过程中表达上调,而细胞外信号调节激酶5(ERK5)表达下降,但是在加入miR-143的抑制物后,ERK5的表达升高,说明ERK5是miR-143的靶基因[35]。R.Takanabe等[36]发现,miR-143的表达水平与脂肪细胞分化标志物PPARγ、ap2(Adipocyte-specific lipid-binding protein 2)以及leptin等的表达水平高度相关。之后,H.M.Xie等[37]对小鼠前脂肪细胞的研究也证实了miR-143的重要作用,并发现了与之具有相反作用的miR-103。
利用基因芯片技术和qRT-PCR的方法,发现miR-210靶向作用于Tcf7l2抑制Wnt信号通路,促进脂质形成[38]。同样,在成脂分化期间,如果阻滞内源性miR-210的表达则会显著抑制脂肪细胞的分化[39]。
R.Martinelli等[40]应用基因芯片技术,对未患糖尿病的严重肥胖者和非肥胖者的皮下脂肪组织中的1 458个miRNAs的表达谱进行分析,筛选出42个差异表达的miRNAs。进一步研究发现,miR-519d特异性和剂量依赖性地抑制PPARα蛋白的翻译,在脂肪细胞分化过程中促进脂质积累。
miR-33a是由SREBP-2的内含子转录得到的,可以抑制与脂质代谢和胆固醇逆转运相关的ATP结合盒式运载蛋白A1(ATP-binding cassette transporter A1,ABCA1)的表达,而造成脂质堆积进而引起心血管疾病[41]。核转录因子-κB(Nuclear factor-kappa B,NF-κB)的激活可导致SREBP-2和miR-33a表达增加[42]。
在人前体脂肪细胞中,leptin和TNF-α可下调miR-221的表达,而miR-221可以下调对脂肪沉积具有抑制作用的ADIPOR1和与血管生成相关的ETS1(V-ets erythroblastosis virus E26 oncogene homologue 1)的表达[43]。
2.2 抑制脂肪细胞分化的miRNAs
表2列出了抑制脂肪细胞分化相关的miRNAs及其靶基因。
骨髓间充质干细胞(Bone mesenchymal stem cells,BMSCs)具有多向分化为成骨细胞、软骨细胞、成脂细胞和成肌细胞等多种类型细胞的潜力[44]。Runx2是成骨分化早期的一个转录因子,抑制其内源性miRNA,即miR-31,调控成骨分化后期的另一重要转录因子Satb2[45]。Runx2在未分化的骨髓间充质干细胞中低表达,而高表达的miR-31可保持骨髓间充质干细胞处于未分化状态(图2)[46],从而抑制成脂分化[32]和成骨分化[47]。

图2 Runx2、Satb2和miR-31之间的调控图[46]Fig.2 Graphic representation of the regulatory feedback loop among Runx2,Satb2 and miR-31[46]
miR-103对胰岛素敏感具有负向调控作用,可能是糖尿病发病的病因[48]。PI3K-Akt-mTOR(Phosphatidylinositol-3-kinase-Akt-mammalian target of rapamycin)信号通路在细胞的增殖和生存中具有重要作用。miR-103下调可导致PI3K-Akt-mTOR下游通路过度激活而发生肥胖[49]。
利用荧光素酶报告载体的方法,在人类脂肪源性多能干细胞(Human multipotent adipose-derived stem cells,hMADS)中鉴定出PPARγ是miR-27b的靶基因,miR-27b直接靶向作用于PPARγ的3′UTR而阻遏成脂分化[50]。进一步的研究[51]证实,miR-27是冷应激时棕色脂肪调控网络中调控因子PRDM16(PRDI-BF1-RIZ1 domain containing 16)、PPARα、CREB(cAMP responsive element binding protein)、PPARγ和PGC1β(PPARγ coactivators 1β)的核心上游调节器(图3)。由于PRDM16与PPARα和PPARγ的功能绑定并共同起作用,因此,遭受寒冷环境后miR-27的下调机制与这些促进棕色脂肪细胞分化的关键基因的表达同步进行。miR-27通过作用于合成棕色脂肪的多个调控因子调节棕色脂肪细胞的分化。miR-130可靶向作用于PPARγ的编码区和3′UTR抑制其表达[52]。而miR-130受TNFα调控[53],上调了的miR-130通过靶标作用,使PPARγ下调,从而抑制脂肪细胞分化。
表2 抑制脂肪细胞分化相关的miRNAs及其靶基因
Table 2 The miRNAs associated with inhibiting adipocyte differentiation and their target genes
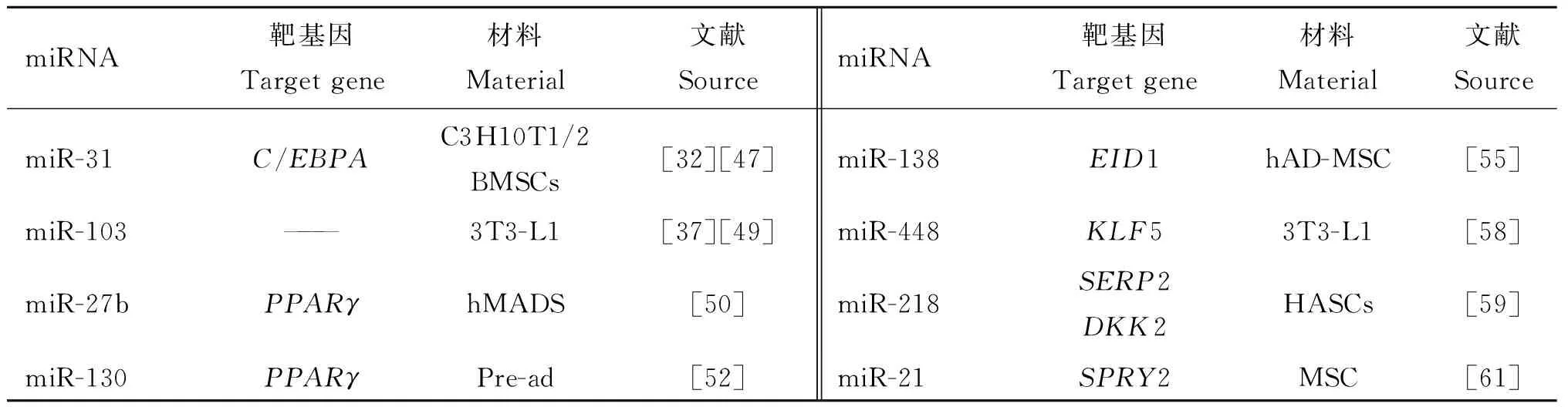
miRNA靶基因Targetgene材料Material文献SourcemiRNA靶基因Targetgene材料Material文献SourcemiR-31C/EBPAC3H10T1/2BMSCs[32][47]miR-138EID1hAD-MSC[55]miR-103———3T3-L1[37][49]miR-448KLF53T3-L1[58]miR-27bPPARγhMADS[50]miR-218SERP2DKK2HASCs[59]miR-130PPARγPre-ad[52]miR-21SPRY2MSC[61]
C3H10T1/2.从C3H小鼠分离建立的间充质干细胞株;BMSCs.骨髓间充质干细胞;3T3-L1.小鼠脂肪样成纤维细胞;hMADS.人类脂肪源性多能干细胞;Pre-ad.前体脂肪细胞;hAD-MSC.人脂肪组织源性骨髓间充质干细胞;HASCs.人类脂肪源性干细胞;MSC.间充质干细胞
C3H10T1/2.Mesenchymal stem cell lines separated from C3H mice;BMSCs.Bone mesenchymal stem cells;3T3-L1.Mouse embryonic fibroblast adipose like cell line;hMADS.Human multipotent adipose-derived stem cells;Pre-ad.Preadipocyte;hAD-MSC.Human adipose tissue derived mesenchymal stem cells;HASCs.Human adipose-derived stem cells;MSC.Mesenchymal stem cell
E1A样分化抑制剂1(Early region 1-A-like inhibitor of differentiation 1,EID1)是一种核受体共调因子,在白色脂肪分化的早期阶段抑制相关基因的活性,上调棕色脂肪组织中某些基因的表达[54]。利用RNAi沉默EID1后,脂肪细胞分化受到抑制[55]。miR-138可直接作用于EID1基因的3′UTR,抑制其发挥作用[55]。

图3 miR-27在皮下脂肪组织中米色/棕色成脂分化中的功能[51]Fig.3 The function of miR-27 in beige/brown adipogenesis of subcutaneous adipose tissue (SAT)[51]
锌指蛋白转录因子家族(Krüppel-like factors,KLFs)是一类保守的、重要的转录因子,在多种类型的细胞中均有表达,控制多种细胞进程[56]。在脂肪细胞分化的几个阶段中,KLF5是重要的调节因素。KLF5由C/EBPβ诱导,调控PPARγ的表达,具有促进脂肪细胞分化的功能[57]。M.Kinoshita等[58]利用荧光素酶报告载体鉴定出KLF5是miR-448的靶基因。进一步研究发现,过表达miR-448抑制了KLF5的表达和脂肪细胞的分化。
Dickkopf 2(DKK2)与SERP2(Stress-associated endoplasmic reticulum protein family member 2)是Wnt信号通路的阻遏物。miR-218可直接作用于SERP2和DKK2,增强Wnt信号活性,抑制人类脂肪源性干细胞(Human adipose-derived stem cells,hASCs)的成脂分化,并促进其成骨分化[59]。miR-21与miR-218有相似的作用,也可作用于DKK2,抑制其表达[60]。另有研究认为,在MSCs分化中,miR-21是通过抑制SPRY2(Sprouty2)的表达以维持ERK-MAPK信号通路的活性及作用时间[61]。
3 结 语
脂肪分化是一个复杂的过程,有多个信号通路和许多基因参与。miRNA是脂肪分化过程中基因调控和信号通路网络中不可或缺的调节器。了解脂肪分化相关的信号通路及其miRNAs的调节作用,可以在一定程度上揭示脂肪分化的调控机制。鉴于miRNAs、靶基因和miRNA抑制物的相互作用,研究与畜禽肉质性状和人类脂肪发育相关基因的miRNAs调控机制,对畜禽肉质性状的遗传改良和人类代谢性疾病的治疗具有重要的科学意义。
[1] LUKIC L,LALIC N M,RAJKOVIC N,et al.Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels:Potential targets for an efficient preventive intervention[J].IntJEnvironmentalResPublicHealth,2014,11(4):3586-3598.
[2] RAJKOVIC N,ZAMAKLAR M,LALIC K,et al.Relationship between obesity,adipocytokines and inflammatory markers in type 2 diabetes:Relevance for cardiovascular risk prevention[J].IntJEnvironmentalResPublicHealth,2014,11(4):4049-4065.
[3] ALONSO R,ANDRES E,MATA N,et al.Lipoprotein(a) Lipoprotein(a) levels in familial hypercholesterolemia:an important predictor of cardiovascular disease independent of the type of LDL receptor mutation[J].JAmCollCardiol,2014,63(19):1982-1989.[4] ZAHOOR M,CHA P H,CHOI K Y.Indirubin-3’-oxime,an activator of Wnt/β-catenin signaling,enhances osteogenic commitment of ST2 cells and restores bone loss in high-fat diet-induced obese male mice[J].Bone,2014,65:60-68.
[5] BARON R,KNEISSEL M.WNT signaling in bone homeostasis and disease:from human mutations to treatments[J].NatMed,2013,19(2):179-192.
[6] GONG M L,LIU C G,ZHANG L,et al.Loss of the TNFα function inhibits Wnt/β-catenin signaling,exacerbates obesity development in adolescent spontaneous obese mice[J].MolCellBiochem,2014,391(1-2):59-66.
[7] GUSTAFSON B,SMITH U.Cytokines promote wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes[J].JBiolChem,2006,281(14):9507-9516.
[8] CHOI H J,LEE D H,JEON H J,et al.Transcription factor 7-like 2 (TCF7L2) gene polymorphism rs7903146 is associated with stroke in type 2 diabetes patients with long disease duration[J].DiabetesResClinPrac,2014,103(3):e3-e6.
[9] SASAKI T,NAKATA R,INOUE H,et al.Role of AMPK and PPARγ1 in exercise-induced lipoprotein lipase in skeletal muscle[J].AmJPhysiol:EndocrinolMetab,2014,306(9):E1085-E1092.
[10] KISFALI P,POLGR N,SFRNY E,et al.Triglyceride level affecting shared susceptibility genes in metabolic syndrome and coronary artery disease[J].CurrMedChem,2010,17(30):3533-3541.
[11] FRAZIER-WOOD A C,ORDOVAS J M,STRAKA R J,et al.The PPAR alpha gene is associated with triglyceride,low-density cholesterol and inflammation marker response to fenofibrate intervention:the GOLDN study[J].PharmacogenomicsJ,2013,13(4):312-317.
[12] 林婄婄,高中元,乔利英,等.核受体因子PPARγ的研究进展[J].四川畜牧兽医,2011,(5):32-34. LIN P P,GAO Z Y,QIAO L Y,et al.Advances of nuclear hormone factor:peroxisome proliferator-actived recepter gamma[J].SichuanAnimal&VeterinarySciences,2011,(5):32-34.(in Chinese)
[13] 林婄婄,高中元,袁亚男,等.PPARα和PPARγ基因在不同脂尾型绵羊脂肪组织中的发育性表达研究[J].畜牧兽医学报,2012,43(9):1369-1376. LIN P P,GAO Z Y,YUAN Y N,et al.Developmental expression of PPARα and PPARγ mRNA in adipose tissues of different fat-tailed sheep[J].ActaVeterinariaetZootechnicaSinica,2012,43(9):1369-1376.(in Chinese)
[14] HORNEMANNA T,WORGALLB T S.Sphingolipids and atherosclerosis[J].Atherosclerosis,2013,226(1):16-28.
[15] ALVAREZ M S,ALVAREZ A F,CUCARELLA C,et al.Stable SREBP-1a knockdown decreases the cell proliferation rate in human preadipocyte cells without inducing senescence[J].BiochemBiophysicalResCommun,2014,447(1):51-56.
[16] KARASAWA T,TAKAHASHI A,SAITO R,et al.Sterol regulatory element-binding protein-1 determines plasma remnant lipoproteins and accelerates atherosclerosis in low-density lipoprotein receptor-deficient mice[J].ArteriosclerThrombVascBiol,2011,31:1788-1795.
[17] GOLDSTEIN J L,DEBOSE-BOYD R A,BROWN M S.Protein sensors for membrane sterols[J].Cell,2006,124(1):35-46.
[18] BOST F,CARON L,MARCHETTI I,et al.Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage[J].BiochemJ,2002,361(3):621-627.
[19] LIAO Q C,LI Y L,QIN Y F,et al.Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity[J].JCellBiochem,2008,104(5):1853-1864.
[20] YANG D C,TSAY H J,LIN S Y,et al.cAMP/PKA regulates osteogenesis,adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin[J].PLoSONE,2008,3(2):e1540-e1541.
[21] 武建亮,乔利英,刘建华,等.绵羊β3肾上腺素能受体基因在脂肪组织中的表达研究[J].畜牧兽医学报,2012,43(2):196-203. WU J L,QIAO L Y,LIU J H,et al.Expression of β3-adrenergic recept or gene in ovine adipose tissues[J].ActaVeterinariaetZootechnicaSinica,2012,43(2):196-203.(in Chinese)
[22] LI H F,FONG C C,CHEN Y,et al.Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway[J].MolCellEndocrinol,2010,323(2):201-207.
[23] FONTAINE C,COUSIN W,PLAISANT M,et al.Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells[J].StemCells,2008,26(4):1037-1046.
[24] CHOI S S,SYN W K,KARACA G F,et al.Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway[J].JBiolChem,2010,285(47):36551-36560.
[25] LEE R C,FEINBAUM R L,AMBROS V.TheC.elegansheterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14[J].Cell,1993,75(5):843-854.
[26] LI L C,POOKOT D,NOONAN E J,et al.MicroRNA-373 induces expression of genes with complementary promoter sequences[J].ProcNatlAcadSci,2008,105(5):1608-1613.
[27] PATEL K,KOLLORY A,TAKASHIMA A,et al.MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression[J].CancerLetters,2014,347(1):54-64.
[28] KAJIMOTO K,NARABA H,IWAI N.MicroRNA and 3T3-L1 pre-adipocyte differentiation[J].RNA,2006,12(9):1626-1632.
[29] 王乐乐.鸡let-7a的组织表达、功能预测及其靶基因ADIPOR2遗传效应分析[D].郑州:河南农业大学,2013. WANG L L.Expression profile and function prediction of chicken miRNA let-7a and genetic variation analysis of target genes ADIPOR2[D].Zhengzhou:Henan Agricultural University,2013.(in Chinese)
[30] WANG Q,LI Y C,WANG J,et al.miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130[J].ProcNatlAcadSciUSA,2008,105(8):2889-2894.
[31] HATA K,NISHIMURA R,IKEDA F,et al.Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor c during bone morphogenetic protein 2-induced adipogenesis[J].MolBiolCell,2003,14 (2):545-555.
[32] SUN F,WANG J,PAN Q,et al.Characterization of function and regulation of miR-24-1 and miR-31[J].BiochemBiophysResCommun,2009,380(3):660-665.[33] CHAN M C,HILYARD A C,WU C,et al.Molecular basis for antagonism between PDGF and the TGFβ family of signalling pathways by control of miR-24 expression[J].EMBOJ,2010,29(3):559-573.
[34] SATOH H,AUDREY NGUYEN M T,KUDOH A,et al.Yacon diet (Smallanthus sonchifolius,Asteraceae) improves hepatic insulin resistance via reducing Trb3 expression in Zucker fa/fa rats[J].NutrDiabetes,2013,3(5):e70.
[35] ESAU C,KANG X,PERALTA E,et al.MicroRNA-143 regulates adipocyte differentiation[J].JBiolChem,2004,279(50):52361-52365.
[36] TAKANABE R,ONO K,ABE Y,et al.Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet[J].BiochemBiophysResCommun,2008,376(4):728-732.[37] XIE H M,LIM B,LODISH H F.MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity[J].Diabetes,2009,58(5):1050-1057.
[38] QIN L M,CHEN Y S,NIU Y N,et al.A deep investigation into the adipogenesis mechanism:Profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/β-catenin signaling pathway[J].BMCGenomics,2010,11(320):1-10.
[39] LIANG W C,WANG Y,WAN D C,et al.Characterization of miR-210 in 3T3-L1 adipogenesis[J].JCellBiochem,2013,114(12):2699-2707.
[40] MARTINELLI R,NARDELLI C,PILONE V,et al.miR-519d overexpression is associated with human obesity[J].Obesity,2010,18(11):2170-2076.
[41] ROTLLAN N,RAMíREZ C M,ARYAL B,et al.Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr-/-Mice—Brief Report[J].ArteriosclerThrombVascBiol,2013,33(8):1973-1977.
[42] ZHAO G J,TANG S L,LV Y C,et al.NF-κB suppresses the expression of ATP-binding cassette transporter A1/G1 by regulating SREBP-2 and miR-33a in mice[J].IntJCardiol,2014,171(3):e93-e95.
[43] MEERSON A,TRAURIG M,OSSOWSKI V,et al .Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α[J].Diabetologia,2013,56(9):1971-1979.[44] BROWN P T,SQUIRE M W,LI W J.Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow[J].CellTissueRes,2014,358(1):149-164.
[45] ZHANG Y,XIE R L,STEIN J L,et al.A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2[J].PNAS,2011,108(24):9863-9868.
[46] DENG Y,WU S,ZHOU H F,et al.Effects of a miR-31,Runx2,and Satb2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells[J].StemCellDev,2013,22(16):2278-2286.
[48] TRAJKOVSKI M,HAUSSER J,SOUTSCHEK J,et al.MicroRNAs 103 and 107 regulate insulin sensitivity[J].Nature,2011,474:649-653.
[49] VINNIKOV I A,HAJDUKIEWICZ K,REYMANN J,et al.Hypothalamic miR-103 protects from hyperphagic obesity in mice[J].JNeurosci,2014,34(32):10659-10674.
[50] KARBIENER M,FISCHER C,NOWITSCH S,et al.microRNA miR-27b impairs human adipocyte differentiation and targets PPAR[J].BiochemBiophysResCommun,2009,390(2):247-251.
[51] SUN L,TRAJKOVSKIC M.MiR-27 orchestrates the transcriptional regulation of brown adipogenesis[J].Metabolism,2014,63(2):272-282.
[52] LEE E K,LEE M J,ABDELMOHSEN K,et al.miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor γ expression[J].MolCellBiol,2011,31(4):626-638.
[53] KIM C,LEE H,CHO Y M,et al.TNFα-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation[J].FEBSLetters,2013,587(23):3853-3858.
[54] LIZCANO F,VARGAS D.EID1-induces brown-like adipocyte traits in white 3T3-L1 pre-adipocytes[J].BiochemBiophysResCommun,2010,398(2):160-165.[55] YANG Z,BIAN C J,ZHOU H,et al.Differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1[J].StemCellDev,2011,20(2):259-267.
[56] HASHMI S,ZHANG J,SIDDIQUI S S,et al.Partner in fat metabolism:role of KLFs in fat burning and reproductive behavior[J].3Biotech,2011,1(2):59-72.
[57] MATSUBARA Y,AOKI M,ENDO T,et al.Characterization of the expression profiles of adipogenesis-related factors,ZNF423,KLFs and FGF10,during preadipocyte differentiation and abdominal adipose tissue development in chickens[J].ComparativeBiochemistryandPhysiology,PartB,2013,165:189-195.[58] KINOSHITA M,ONO K,HORIE T,et al.Regulation of adipocyte differentiation by activation of serotonin (5-ht) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5[J].MolEndocrinol,2010,24(10):1978-1987.
[59] ZHANG W B,ZHONG W J,WANG L.A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation[J].Bone,2014,58:59-66.
[60] KAWAKITA A,YANAMOTO S,YAMADA S I,et al.MicroRNA-21 promotes oral cancer invasion via the Wnt/β-Catenin pathway by targeting DKK2[J].PatholOncolRes,2013,20(2):253-261.
[61] MEI Y,BIAN C J,LI J,et al.miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation[J].JCellBiochem,2013,114(6):1374-1384.
(编辑 郭云雁)
Research Progress in Signaling Pathways and microRNA Regulation of Adipocyte Differentiation
JIA Xia-li#,PAN Yang-yang#,QIAO Li-ying,GUO Yun-yan,HU Zi-qiao,ZHANG Fang,LI Bao-jun,LIU Wen-zhong*
(CollegeofAnimalScienceandVeterinaryMedicine,ShanxiAgriculturalUniversity,Taigu030801,China)
Content of body fat in domestic livestocks and poultry determines the meat quality.Adipocyte differentiation is a complex biological process regulated by many factors.Understanding the adipose differentiation-related signaling pathways and their miRNA regulations can reveal the molecular mechanisms of adipocyte differentiation.Based on introduction to the 6 important signaling pathways regulating adipocyte differentiation,biological functions and mechanisms of 8 miRNAs for promoting and another 8 miRNAs for inhibiting adipocyte differentiation were discussed.The function of target genes of these miRNAs in adipogenesis was summarized.Studies on the related miRNAs and their target genes provide theoretical bases for genetic improvement of meat quality traits in domestic livestocks and poultry.
adipocyte;adipogenic differentiation;signaling pathways;microRNA;target gene
10.11843/j.issn.0366-6964.2015.04.002
2014-10-10
国家自然科学基金项目(31372292);山西省研究生优秀创新项目(20143059)
贾夏丽(1988-),女,山西洪洞人,硕士生,主要从事肉用绵羊的分子遗传育种研究,E-mail:jiaxialimm@163.com;潘洋洋(1989-),男,山东潍坊人,硕士生,主要从事肉用绵羊的分子遗传育种研究,E-mail:pyybiology@163.com。贾夏丽和潘洋洋并列为第一作者
*通信作者:刘文忠,教授,E-mail:tglwzyc@163.com
S813.3
A
0366-6964(2015)04-0518-08
