Pt/TiO2催化剂的载体结构对甲醇催化氧化性能的影响
2015-03-20金梅梅王新德贾建洪董华青
金梅梅,王新德,贾建洪,董华青
(浙江工业大学化学工程与材料学院,杭州310014)
1 Introduction
Among the various noble metal catalysts used in direct methanol fuels cells (DMFCs),Pt catalysts have attracted much attention due to their superior properties[1,2]. In spite of the intense research in the last decade,the slow methanol electro-oxidation reaction kinetics,high costs of Pt catalysts still exist.To overcome these problems,much efforts have been devoted,for example the development of PtM (M =Fe,Ni,Ru,etc)alloys and supported Pt catalysts.
Pt nanoparticles dispersed on diverse supports,such as carbon and graphene[3,4],in comparison to those supports,TiO2has attracted considerable attention in recent years,thanks to its unique properties such as environment friendly,low cost and excellent mechanical resistance[5,6]. The properties of TiO2have strong effects on the performance of supported Pt catalysts. For instance,the anatase TiO2supported Pt catalysts,may provide catalytic advantages for oxidation of methanol compared to the rutile and bookie TiO2. The morphology of TiO2has a strong influence on the properties of supported Pt catalysts[7]. Although several elegant researches had been focused on supported Pt catalysts on TiO2nanotube arrays (TNTAs),few studied on the aspect of TiO2nanowire arrays (TNWAs). Up to now,comparison of the performance for methanol oxidation of Pt supported on TNTAs and TNWAs has not been studied yet. It is well known that catalytic activity may be significantly altered when reaction is confined within a nanoscale environment[8].
Based on theoretical studies on TNTAs[9,10],in this work,we studied the performances of the supported Pt catalysts on TNTAs and TNWAs for methanol oxidation. We used electrochemical anodization and hydrothermal methods to prepare TNTAs and TNWAs on Ti foils directly. Pt/TNTAs and Pt/TNWAs were prepared by microwave heating method. The results demonstrate that Pt/TNTAs show a superior performance than supported on TNWAs for methanol oxidation. We speculate that the excellent activity of Pt/TNTAs might be due to the confinement effects. The study reveals the beneficial role of the support architecture in the performances of Pt catalysts for methanol oxidation.
2 Experimental
2.1 Synthesis of TiO2 nanotube arrays (TNTAs)and TiO2 nanowire arrays (TNWAs)
TNTAs were formed on the Ti foils (0.25 mm)by electrochemical anodization in ethylene glycol solution containing 0. 3 wt% NH4F and 2 vol% H2O.Prior to anodization,Ti foils were degreased by sonicating for 10 min in acetone and deionized water,respectively. In the first-step andoization,Ti foil was anodized at 60 V for 60 min,and then the as-grown nanotubular layer was ultrasonically removed in deionized water. Subsequently,a second anodization was performed at 60 V for 30 min. The as-anodized TNTAs were annealed in N2 at 450 ℃ for 2 h with a heating rate of 5 ℃/ min. TNWAs were synthesized on the Ti foils (0. 25 mm)by hydrothermal method[11].
Prior to hydrothermal,Ti foils were degreased by successively sonicating for 10 min in acetone,ethanol,and deionized water. Ti foils were placed in PPL-lined stainless steel autoclave,containing NaOH aqueous solution (1 M,20 mL). The steel autoclave was heated at 200 ℃for 24 h,and then air -cooled to room temperature. Ti foils were taken out,rinsed extensity with deionized water and immersed in HCl solutions (1 M,20 mL)for 10 min to replace Na+with H+,forming H2Ti2O4- (OH)2nanowire arrays. After that,H2Ti2O4(OH)2nanowire arrays were washed with deionized water again then dried at ambient temperature. TNWAs were obtained after a heat treatment in an oven at 500℃for 3 h.
2.2 Preparation of the supported Pt catalysts on TNTAs and TNWAs
Pt/TNTAs were easily synthesized by a microwave heating method as follows[12]:a growth solution was prepared by mixing 4ml H2PtCl6(3 mM),5ml ethylene glycol,and 0.125 ml KOH (0.4 mM)and 20mg TNTAs at room temperature using ultrasonication. Then,the growth solution was microwave -heated for 10 minutes at 180 ℃. The products were harvested after centrifugation and dried in vacuum at 80 ℃for 8 h. For comparison,Pt/TNWAs were also synthesized. The Pt loading was controlled at nearly 10 wt%.
2.3 Catalysts characterization
The X - ray diffraction (XRD)patterns of the samples were characterized by X'Pert Pro diffracto meter using Cu Kα radiation (λ = 1.54056). The surface morphologies of the samples were observed by scanning electron microscopy (SEM,Hitachi S4700)and their microstructures were investigated by transmission electron microscopy (TEM, Tecnai G2 F30). The exact Pt loading in the catalysts was confirmed by inductive coupled plasma atomic emission spectrometry (ICP - AES,Jarrel - Asm - Atom -Scan-2000).
2.4 Electrochemical measurements
All the electrochemical measurements were performed using a three - electrode cell with a CHI660 electrochemical workstation at 25 ℃in the solution of 0.5 M H2SO4with and without 1 M CH3OH. An ink was prepared by ultrasonically dispersing 3mg catalysts with 0.8 mg of Carbon Vulcan XC72 in 60 μl isopropyl alcohol and 40 μl 5wt% nafion solution(from Aldrich). The addition of Carbon Vulcan XC 72 was to enhance electronic conductivity of the catalytic layer. A drop of 4.0 μl catalyst ink was deposited to the glassy carbon (GC)electrode (3 -mm diameter)surface,which was used as the working electrode. A platinum wire and an Ag/AgCl reference electrode were used as the counter and reference electrode,respectively.
3 Results and discussion
3.1 SEM and TEM characterizations
Fig.1 shows the SEM images of the as - prepared TNTAs and TNWAs. Fig. 1 (a)and (c)show the top view and cross -sectional of TNTAs in which is highly ordered,compact,one - dimensional architechture is clearly evident. In addition,the nanotube arrays are smooth,and the average inner diameter is about 80 nm. Fig. 1 (b)and (d)are the typical SEM images of TNWAs. These images clearly indicate that the nanowires grown on the Ti foils with uniform morphology and the mean diameter of the TiO2nanowires is also nearly 80 nm. Moreover,the as -prepared TNWAs are less dense than the traditional ones[13],which is a benefit for loading.

Fig.1 SEM images of TNTAs (a,c)and TNWAs (b,d)
Fig. 2 shows the TEM images of the as-synthesized Pt/TNTAs and Pt/TNWAs. As evidenced in Fig. 2 (a)(b),after the growth solution was microwave-heated for 10 minutes at 180 ℃,Pt nanoparticles were uniformly dispersed on the nanotubes and nanowires. The growth directions of the TiO2nanotubes and nanowires are determined to be (101)plane. This is supported by the HRTEM images in Fig. 2 (b)(d),where the lattice fringes perpendicular to the growth direction have the spacing of 0.347 nm and 0.354 nm,which are nearly equal to the lattice parameter in the TiO2(101)plane[14]. From the HRTEM images in Fig.2 (c)(d),the lattice fringes of Pt nanoparticles can be clearly observed,suggesting the well-defined crystal structure. As shown in Fig.2 (c)(d),the lattice spacing of 0.233 nm and 0.227 nm originates from the (111)plane of Pt[15].
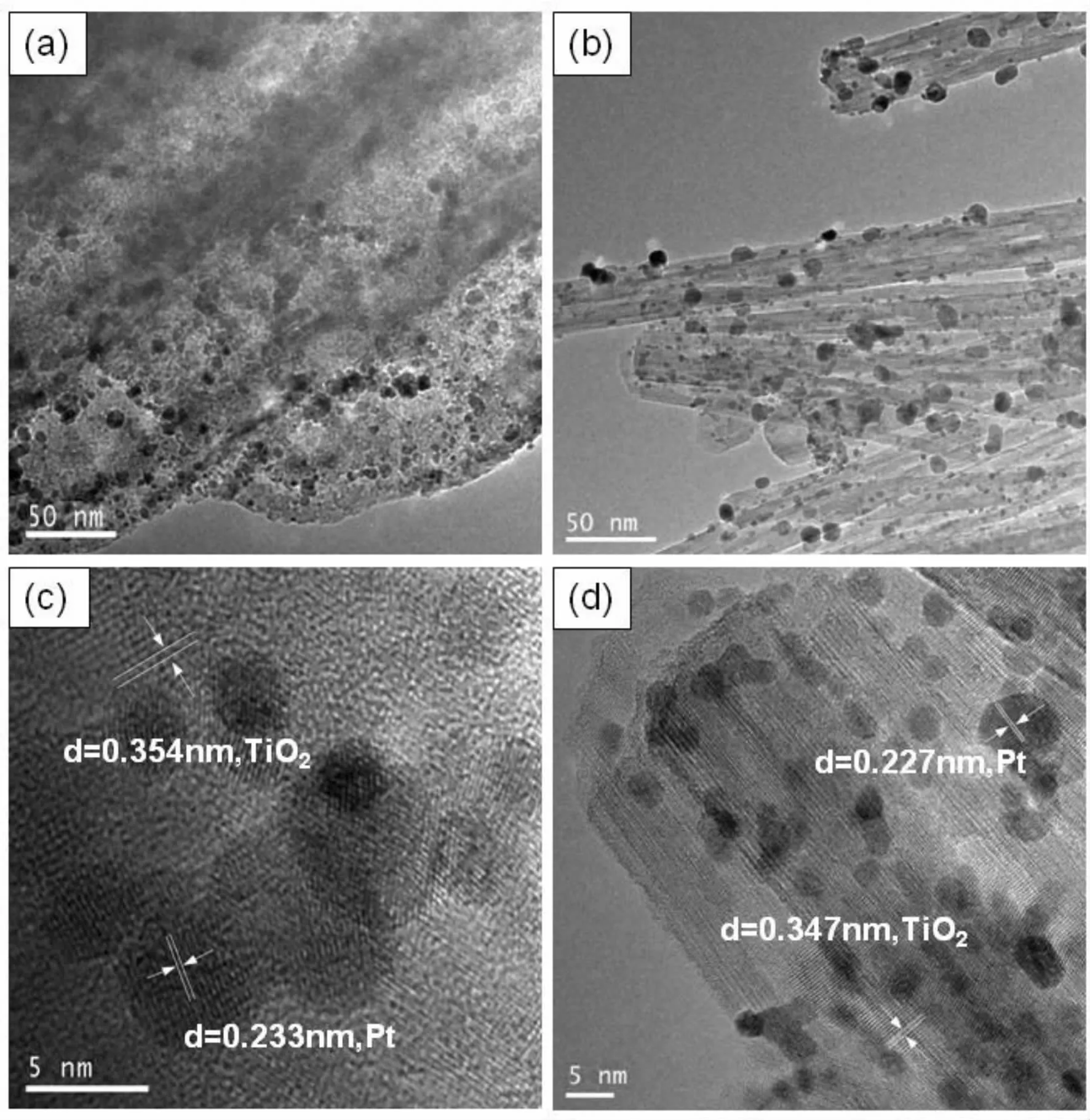
Fig.2 TEM and HRTEM images of Pt/TNTAs (a,c)and Pt/TNWAs (b,d)
3.2 XRD and ICP-AES measurements
Fig. 3 shows the XRD patterns of the as -synthesized Pt/ TNTAs and Pt/TNWAs. As shown in Fig. 3 (a),the diffraction peaks at about 25. 31°and 48.04° can be indexed to the (101)and (200)crystal planes of anatase TiO2[16]. This can be concluded that the amorphous TiO2were transformed into anatase phase after calcined at 450℃in N2for 2 h.The Pt nanoparticles exhibit diffraction peaks at 39.75°,46.23°and 67.45°,corresponding to the characteristic (111),(200),(220)reflections of a Pt face-centered cubic structure. The diffraction peaks of Pt/TNWAs (Fig. 3 (b))are not well defined as TNTAs,however,the diffraction peaks at about 25.16° and 47.78° are always obvious. The characteristics of the face - centered cubic Pt crystal structure are evident as indicated by the orientations along the Pt (111),Pt (200),and Pt (220)directions,at 40.04°,46.53° and 67.86°[17],respectively.

Fig.3 XRD patterns of the Pt/TNTAs (a)and Pt/TNWAs (b)
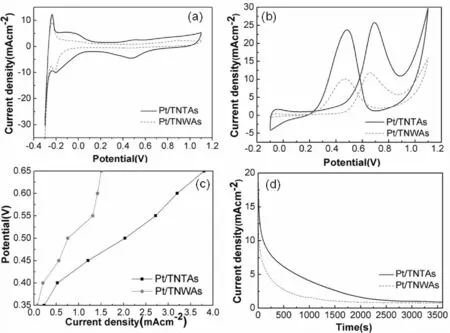
Fig.4 Cyclic voltammogram curves of Pt/TNTAs and Pt/TNWAs in 0. 5M H2SO4(a)and 0. 5M H2SO4 +1M CH3OH (b),Potentiostatic polarization curves in 0. 5M H2SO4 +1M CH3OH(c),Current–Time plots in 0.5 M H2SO4 +1.0 M CH3OH at 0.65 V (d)
The exact amount of Pt loadings on the two TiO2supports was determined by ICP -AES analysis. The nominal Pt loadings in Pt/TNTAs and Pt/TNWAs are 9.5 wt% and 10.95 wt%,respectively. Therefore,the comparison of supported Pt catalysts on TNTAs and TNWAs is reasonable since the close loading amount on two catalysts.
3.3 Electrochemical analysis
Fig. 4 (a)shows the CVs of Pt/TNTAs and Pt/TNWAs recorded in 0.5 M H2SO4at the scan rate of 50 mV·s-1,which was used to be background test.Hydrogen adsorption/desorption peaks are seen between - 0. 2 and 0 V. The electrochemical active surface area (EASA)of a catalyst can reflect the intrinsic electrocatalytic activity of the catalyst,which can be measured by using the integral of the charge under the Pt - H redox peaks in the lower potential region (-0.2V ~0V)[18]. EASAs of the Pt/TNTAs and Pt/TNWAs are estimated to be 79. 3 and 45. 9 m2g-1respectively,indicating that Pt/ TNTAs have the higher active surface area.
Fig. 4 (b)shows the typical CVs of Pt/TNTAs and Pt/TNWAs in 1M CH3OH + 0.5M H2SO4. The significant differences in onset potential and peak current density are observed illustrating the beneficial role of the TiO2structure in the performances of Pt catalysts for methanol oxidation. The current density of the methanol oxidation for Pt/TNTAs reaches a peak value of 25.77 mAcm-2,which is higher than that of Pt/TNWAs (11.76 mAcm-2). Moreover,the onset potential for Pt/TNTAs is found at even lower potential (0. 21 V)than that of Pt/TNWAs (0. 36 V). These results imply that the superior catalytic activity of the supported Pt catalysts on TNTAs than those on TNWAs for the methanol oxidation. We speculate that the excellent activity of Pt nanoparticles supported on TNTAs might be due to the confinement effects which may lead to a smaller barrier[19]. This can be further proved by potentiostatic polarization curves of methanol oxidation which was recorded for 30 min at each potential Point. As shown in Fig. 4(c),the overpotential for Pt/TNTAs is lower than that of Pt/TNWAs;in addition,it is higher current density of methanol oxidation on Pt/TNTAs at the same potential.
Fig. 4(d)shows the current -time plots recorded at 0.65 V in 1 M CH3OH + 0.5 M H2SO4. As shown in Fig. 4(d),the two catalysts display an initial current decay,which is due to the poisoning of the catalysts by intermediate products,such as COlike intermediates. A slower current decay and higher catalytic stability are detected of the Pt/TNTAs. In this study,Pt/TNTAs are able to maintain the higher current density in 3600 s,giving the better electrocatalytic performance. The results show that the architecture of the supporting materials not only influences the catalytic activity for methanol oxidation,but also the stability of the Pt catalysts.
4 Conclusion
In this work,we have prepared Pt catalysts supported on two different TiO2architectures (Pt/TNTAs,Pt/TNWAs)and discussed the influence of TiO2architecture on the performance of supported Pt catalysts for methanol oxidation. The results demonstrate that Pt/TNTAs show a superior performance(25.77 mAcm-2,0.21 V)than supported on TNWAs (11.76 mAcm-2,0.36 V)for methanol oxidation. We speculate that the excellent activity of Pt/TNTAs might be due to the confinement effects. This study is beneficial not only to understand the influence of TiO2architecture on the performance of supported Pt catalysts for methanol electro - oxidation,but also to design better supported Pt catalysts.
Reference:
[1] Leger J M. Mechanistic aspects of methanol oxidation on platinum - based electrocatalysts [J]. J. Appl.Electrochem.,2001,31:767.
[2] Wei Z D,Li L L,Luo Y H,et al. Electrooxidation of methanol on upd-Ru and upd -Sn modified Pt electrodes[J]. J. Phys. Chem. B,2006,110:26055.
[3] Wu J,Hu F P,Hu X D,et al. Improved kinetics of methanol oxidation on Pt/hollow carbon sphere catalysts[J]. Electrochim. Acta,2008,53:8341.
[4] Zhao Y Y,Zhou Y K,Xiong B,et al. Facile singlestep preparation of Pt/N -graphene catalysts with improved methanol electrooxidation activity[J]. J. Solid State Electrochem.,2013,17:1089.
[5] Yue Y X,Feng Q,Wang Y. Study on the optical and electronic properties of anatase TiO2co - doped with Mn-N,Mn-C and Mn-S[J]. J. At. Mol. Phys.(原子与分子物理学报),2013,30:479 (in Chinese)
[6] Chen P,Zhou X L,Lu L Y,et al. First -principles calculations for structural and thermodynamic properties of rutile TiO2under high pressure[J]. J. At.Mol. Phys.(原子与分子物理学报),2012,29(2):372 (in Chinese)
[7] Xing L,Jia J B,Wang Y Z,et al. Pt modified TiO2nanotubes electrode:Preparation and electrocatalytic application for methanol oxidation[J]. Int. J. Hydrogen Energy,2010,35:12169.
[8] Pan X L,BaoX H. The effects of confinement inside carbon nanotubes on catalysis[J]. Acc. Chem. Res.,2011,44:553.
[9] Xie Q,Meng Q Q,Zhuang G L,et al. Water oxidation on N -doped TiO2nanotube arrays[J]. Int. J.Quantum Chem.,2012,112:2585.
[10] Pan X,Cai Q X,Chen W L,et al. A DFT study of gas molecules adsorption on the anatase (0 0 1)nanotube arrays[J]. Comp. Mater. Sci.,2013,67:174.
[11] Lin Z H,Xie Y N,Yang Y,et al. Enhanced triboelectric nanogenerators and triboelectric nanosensor using chemically modified TiO2nanomaterials [J].ACS. Nano.,2013,7:4554.
[12] Liu B,Zheng L P,Liao S J,et al. Volume production of high loading Pt/C catalyst with high performance via a microwave - assisted organic colloid route [J]. J.Power Sources,2012,210:54.
[13] Feng X J,Shankar K,Varghese O K,et al. Vertically aligned single crystal TiO2nanowire arrays grown directly on transparent conducting oxide coated glass:synthesis details and applications [J]. Nano Lett.,2008,8:3781.
[14] Mohapatra S K,Misra M,Mahajan V K,et al. Enhanced photoelectrochemical generation of hydrogen from water by 2,6 -dihydroxyantraquinone -functionalized titanium dioxide nanotubes [J]. J. Phys.Chem. C,2007,111:8677.
[15] Formo E,Peng Z M,Lee E,et al. Direct oxidation of methanol on pt nanostructures supported on electrospun nanofibers of anatase[J]. J. Phys. Chem. C,2008,112:9970.
[16] Zhou W J,Yin Z Y,Du Y P,et al. Synthesis of few-layer MoS2nanosheet-coated TiO2nanobelt heterostructures for enhanced photocatalytic activities [J].Small,2013,9:140.
[17] Luo F,Liao S J,Chen D. Platinum catalysts supported on Nafion functionalized carbon black for fuel cell application[J]. J. Energy. Chem.,2013,22:87.
[18] Li X,Chen W X,Zhao J,et al. Microwave polyol synthesis of Pt/CNTs catalysts:Effects of pH on particle size and electrocatalytic activity for methanol electrooxidization[J]. Carbon,2005,43:2168.
[19] Halls M D,Schlegel H B. Chemistry inside carbon nanotubes:The Menshutkin S(N)2 reaction[J]. J.Phys. Chem. B,2002,106:1921.
