苯并噻二唑共轭有机分子合成研究进展
2015-03-20黄旦翔彭雄伟黎太浩程晓红
黄旦翔,夏 萌,彭雄伟,黎太浩,程晓红
(云南大学化学科学与工程学院,云南昆明 650091)
·专论与综述·
苯并噻二唑共轭有机分子合成研究进展
黄旦翔,夏 萌,彭雄伟,黎太浩,程晓红
(云南大学化学科学与工程学院,云南昆明 650091)
2,1,3-苯并噻二唑(BTD)共轭有机分子具有优异的光电特性,广泛应用于有机发光二极管、太阳能电池、液晶、荧光探针、光电管等方面。综述了以金属催化的偶联反应为关键步骤的2,1,3-苯并噻二唑(BTD)共轭有机分子的合成方法,包括Suzuki偶联反应,Stille偶合反应,Heck偶联反应,Sonogashir偶联反应,Nigishi偶联反应,Ullmann偶联反应等。
2,1,3-苯并噻二唑;偶联反应;合成
2,1,3-苯并噻二唑(BTD)具有较强的电子亲和势、共平面性和对化合物能隙较好的调节性,被广泛用于构建共轭有机分子,在有机发光二极管、太阳能电池、液晶、荧光探针、离子识别材料等领域有着广泛的应用[1-3],对这类共轭有机分子的合成方法的研究将对基于BTD新型光电材料的研发意义重大。文献[1]已经介绍了这类化合物在液晶材料研究方面的最新进展,本文重点对2,1,3-苯并噻二唑(BTD)共轭有机分子的合成研究进展作综述性的介绍。
1 4,7-二溴-2,1,3-苯并噻二唑的合成
4,7-二溴-2,1,3-苯并噻二唑(2)是用来制备苯并噻二唑(BTD)共轭有机分子的关键中间体之一[4],这一中间体可以从邻苯二胺及二氯亚砜出发,在碱性条件下先生成苯并噻二唑(1),1再经溴代得到2(见图1)[4-6]。当然,5,6位,4,5位-二溴-2,1,3-苯并噻二唑及单溴代的-2,1,3-苯并噻二唑也是制备2,1,3-苯并噻二唑(BTD)共轭有机分子的中间体。
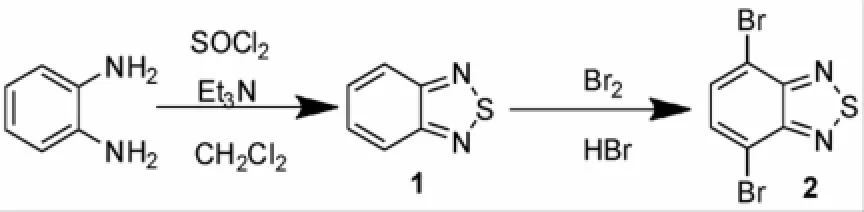
图1 4,7-二溴-2,1,3-苯并噻二唑的合成Fig.1 The synthesis of 4,7-dib romo-2,1,3-benzothiadiazole

图2 用于合成BTD共轭有机分子的偶合反应Fig.2 Coupling reactions used for the synthesis of BTD conjugated compounds
2 2,1,3-苯并噻二唑共轭有机分子的合成
通过过渡金属催化的偶合反应,如4,7-二溴-2,1,3-苯并噻二唑2与芳香硼酸间的Suzuki偶合反应,与三丁基锡化合物间的Stille偶合反应,与苯乙烯间的Heck反应,与芳香端炔间的Sonogashira反应,与芳香卤代物间的Nigishi反应,与氮杂环间的Ullmann反应等等,可以实现在2,1,3-苯并噻二唑(2)的4,7位以碳碳单键,碳碳双键,碳碳三键,杂碳键为连接单元的共轭延长,获取共轭有机分子,见图2。
2.1 Suzuki偶合反应
Suzuki偶联反应是构造2,1,3-苯并噻二唑共轭有机分子的最常见的方法,国内外多个小组都展开过这方面的研究。图3中列了通过该反应为关键步骤得到的一系列2,1,3-苯并噻二唑共轭有机分子3-11。合成这些化合物的Suzuki反应条件及其应用见表1。由表1可见,化合物3[7]、4、5[8]、6[9]、7[10]、8[11]、9[12]都是以Pd[PPh3]4做催化剂,Na2CO3为碱来制备;而Neto[13]报道的化合物10则采用了NCP-Pd做催化剂,CsF为碱性制备而得,CsF的碱性较Na2CO3强,所以说,碱性环境对于不同的Suzuki反应有较大的影响。Operamolla等采用Pd(OAc)2与S-Phos为催化剂,体积比为10∶1的四氢呋喃:水的异构介质为溶剂,通过Suzuki偶联反应得到聚合物11。因此Suzuki反应是构筑2,1,3-苯并噻二唑共轭有机分子最常见的途径。
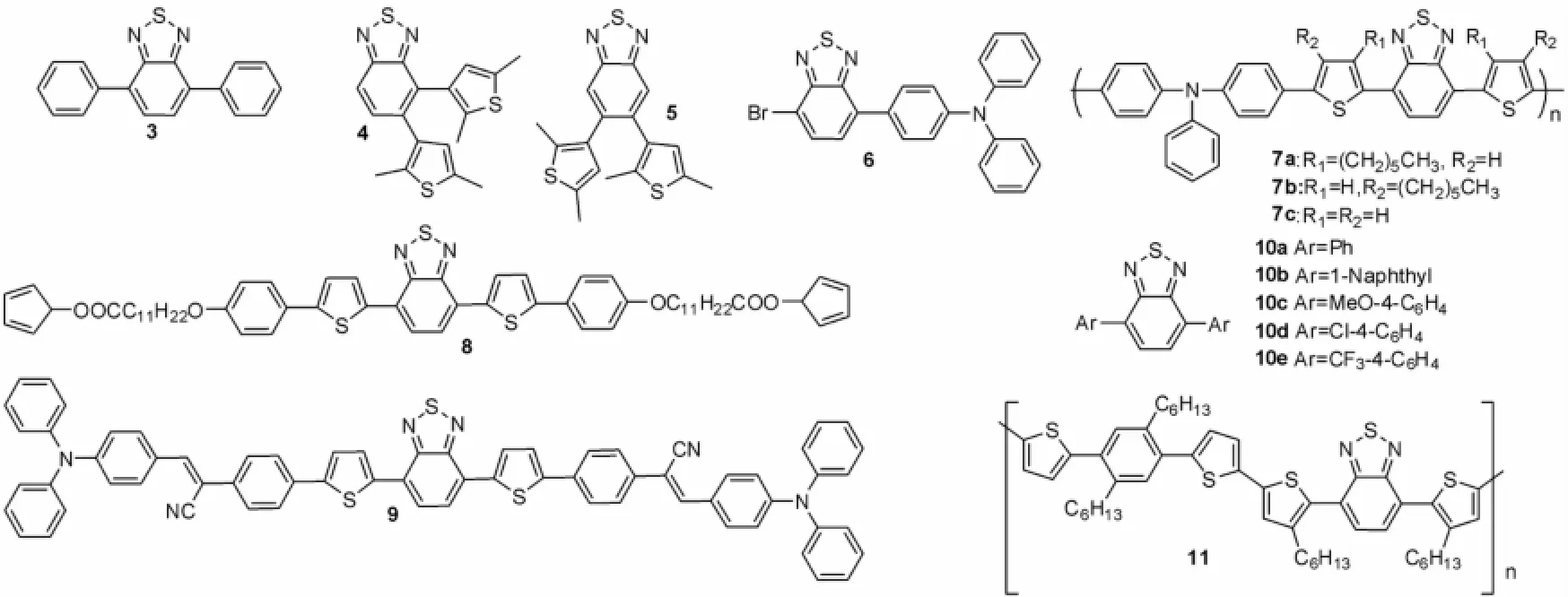
图3 化合物3~11Fig.3 Com pounds of 3-11

表1 制备化合物3-11的Suzuki反应条件及其应用Tab.1 Suzuki reaction conditions and the applications of compounds 3-11
2.2 Stille偶联反应
通过Stille偶联反应同样可以构筑2,1,3-苯并噻二唑共轭有机分子,图4中化合物12-22均是以Stille偶联反应为关键步骤合成而得。制备这些化合物的Stille反应条件及其应用列于表2中。13[15]、14[16]、15[17]、16[18]、17[19]的Stille偶联反应采用了甲苯为溶剂,Pd[PPh3]4为催化剂。Stille偶联反应除了采用甲苯为溶剂外,还可采用其他的溶剂,如化合物18[20]用了DMF,化合物20[12]用到了四氢呋喃,化合物21[21]用到了氯苯,化合物22[22]用到了邻二甲苯做溶剂;催化剂也可采用其他的,如化合物12[23],20用到了Pd[PPh3]2Cl2,化合物19[24]用到了原位Pd[As-Ph3]4,化合物22用到了Pd2(dba)3。反应条件可以不断完善,如化合物21,22还使用了微波来完成反应,大大缩短了反应时间。
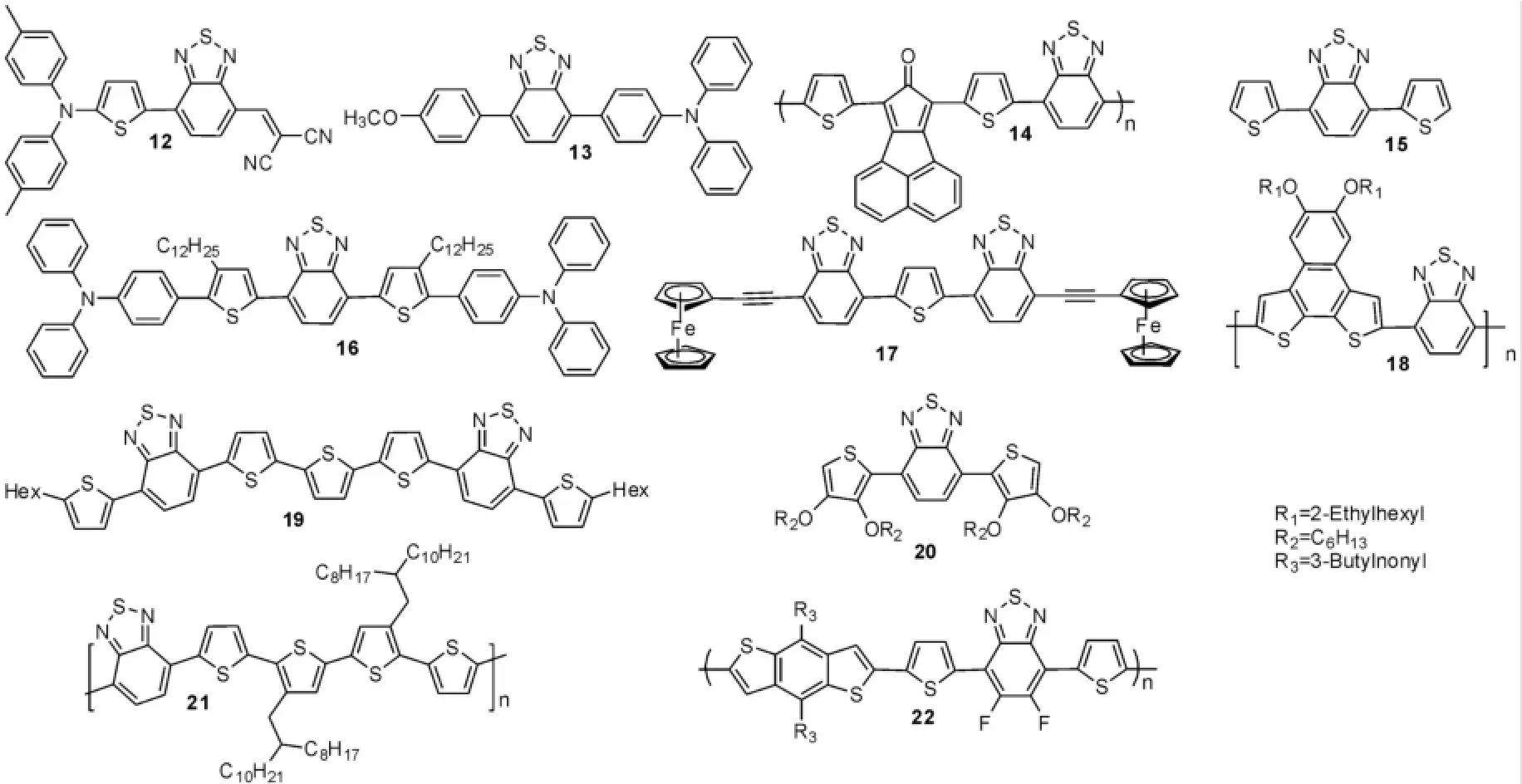
图4 化合物12~22Fig.4 The compounds of 12-22
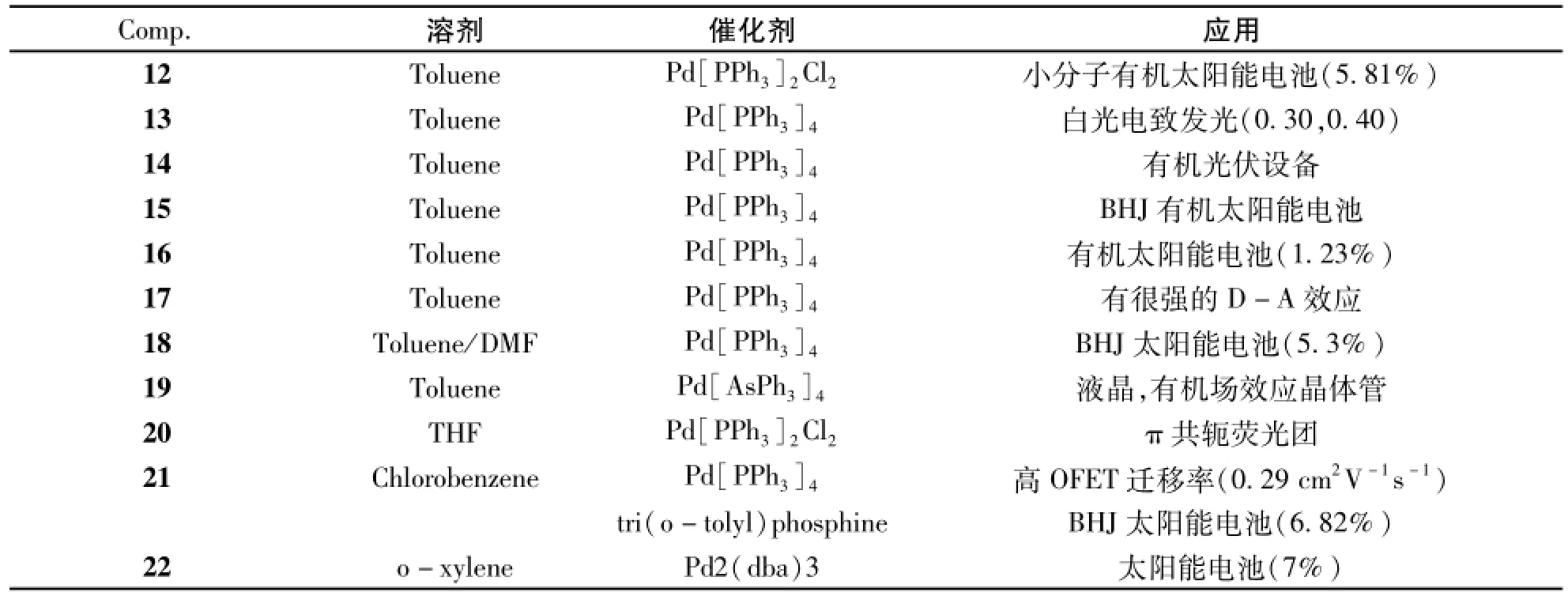
表2 化合物12-22的Stille偶联反应条件及其应用Tab.2 The Stille coupling reaction conditions and the applications of compounds12-22
2.3 Heck偶联反应
Heck偶联反应也能构造2,1,3-苯并噻二唑共轭有机分子,图5中,化合物23-26均是采用Heck偶联反应为关键步骤合成。Heck反应的反应条件及产物的应用总结于表3中。Heck偶联反应的溶剂都均采用DMF,催化剂为Pd[OAc]2,但是合成23[25]和26[26]的Heck偶联反应的钯配体采用P(o-tolyl)3,NEt3为碱;24[27]和25[28]的Heck偶联反应的钯配体采用NaOAc,碱性环境采用TBAB营造。在合成化合物25之前,李永舫等还用同样的条件[29]合成了有3条手臂的化合物。
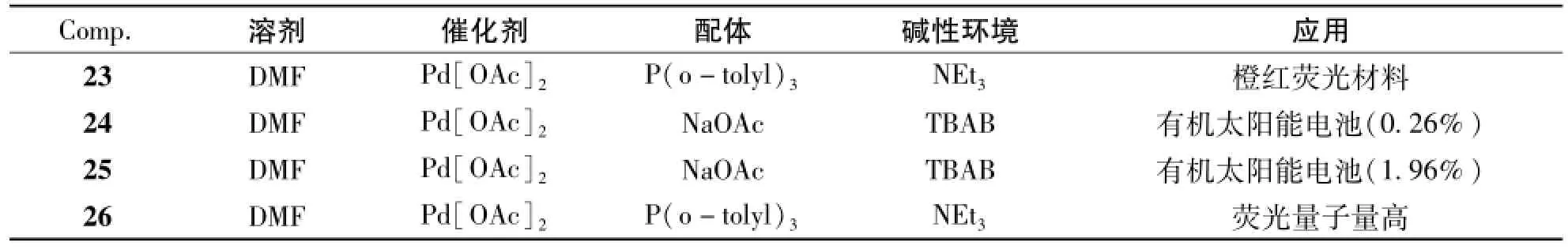
表3 化合物23-26的Heck反应条件及其应用Tab.3 The Heck coupling reaction conditions and the applications of compounds 23-26

图5 化合物23~26Fig.5 Compounds of 23-26
2.4 Sonogashira偶联反应
图5中列了Sonogashira偶联反应来构造2,1,3-苯并噻二唑共轭有机分子27~32。Sonogashira偶联反应的反应条件及产物的应用列于表4中。由表可见,Sonogashira反应的一般条件为:三乙胺为溶剂,Pd[PPh3]2Cl2和CuI为催化剂[30]。制备化合物27[31]时,催化剂变成CuBr;制备化合物28[32]时将溶剂换成三甲胺;制备化合物29[33]时将溶剂换成甲苯和i-Pr2NH的混合溶剂,催化剂Pd[PPh3]2Cl2换成Pd[PPh3]4;制备化合物31[34]时将催化剂Pd[PPh3]2Cl2换成Pd2(dba)3。制备化合物31时,还将溶剂换成i-Pr2NH后,发现产率略增但需要更多反应时间。此外,末端炔烃易快速分解,需要很快用于下一步的CuAAc点击反应形成化合物32。化合物32就是叠氮化糖与BTD-炔,用二价铜催化,在Na-抗坏血酸盐和TBAF的存在下,高产率得到的点击产物。
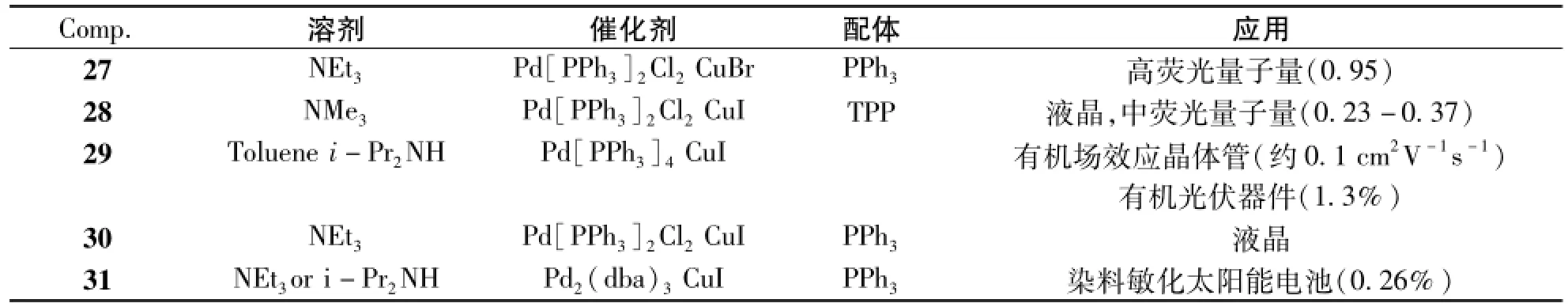
表4 化合物27~31的Sonogashira反应条件及其应用Tab.4 Sonogashira coupling reaction conditions and the applications of compounds27-31
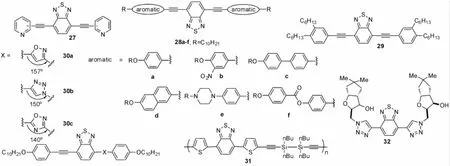
图6 化合物27~32Fig.6 Compounds of 27-32
2.5 Nigishi反应
Bijleveld等[35]用Nigishi反应合成了双苯并噻二唑衍生物35。化合物35的进一步反应得到的聚合物可以应用于制备太阳能电池,能量转换效率达到了2.5%。
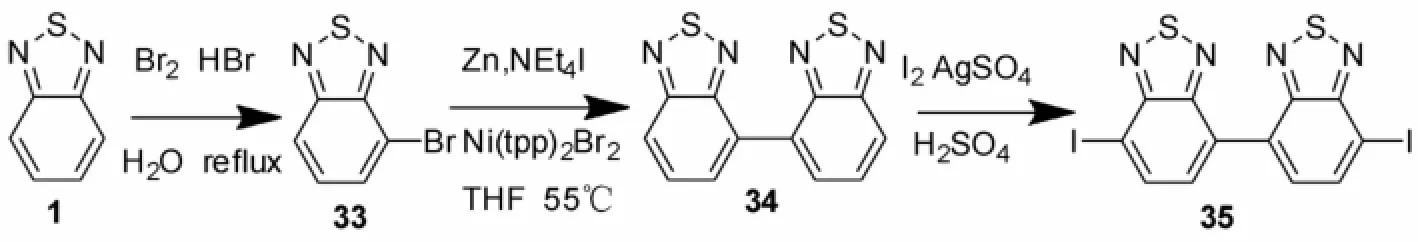
图7 化合物35的合成Fig.7 Synthesis of compound 35

图8 化合物36~43Fig.8 Compounds of 36-43
2.6 其他反应类型
还可以采用其他一些偶联反应来构建2,1,3-苯并噻二唑共轭有机分子,图8中列出了一些产物例子36~43。反应类型及反应条件及合成产物的的应用见表5。采用Ullmann反应合成化合物36[36]时,也有单边取代的副产物存在,改变2和咔唑单元的比例对于单,双取代产物的产率无明显影响。化合物38、39、40[37]都是同一个Mcmurry反应的产物,产率非常低。化合物4[38]和42[39]均采用honer-wittig反应制备,但是碱性环境不一样,化合物41用的碱是NaH,化合物42用的碱是t-BuOK。化合物37[40]是用亲核取代反应,而化合物43[41]使用亲核加成反应。
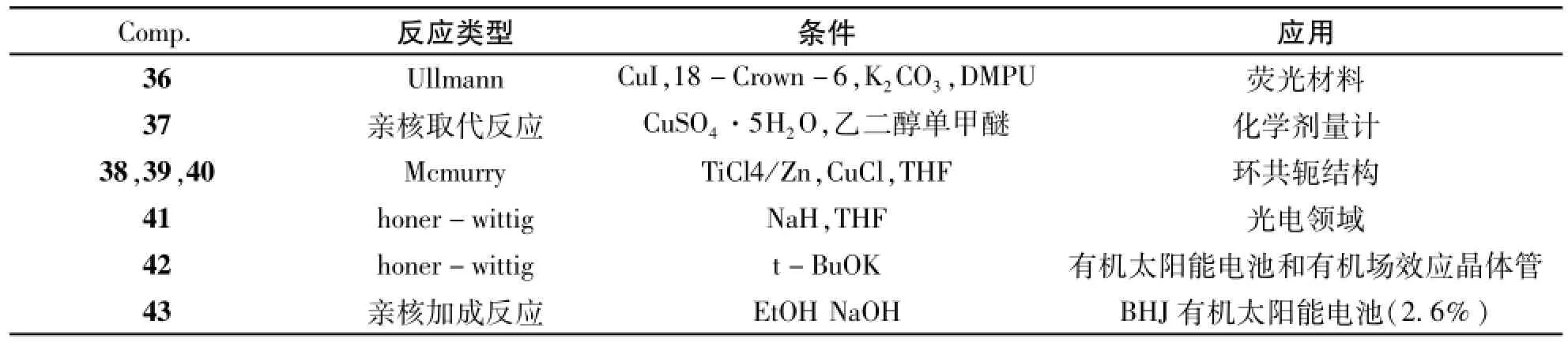
表5 化合物36-43的反应类型,条件及其应用Tab.5 Reaction types,conditions and the applications of compounds 36-43
3 其他方法
图9列出了一种新颖的合成含萘多氟代衍生物47的温和反应线路[42],芳基噻唑氨基化合物的分子内亲核环化提供了邻位取代2,1,3-苯并噻二唑共轭有机分子的新颖合成思路。
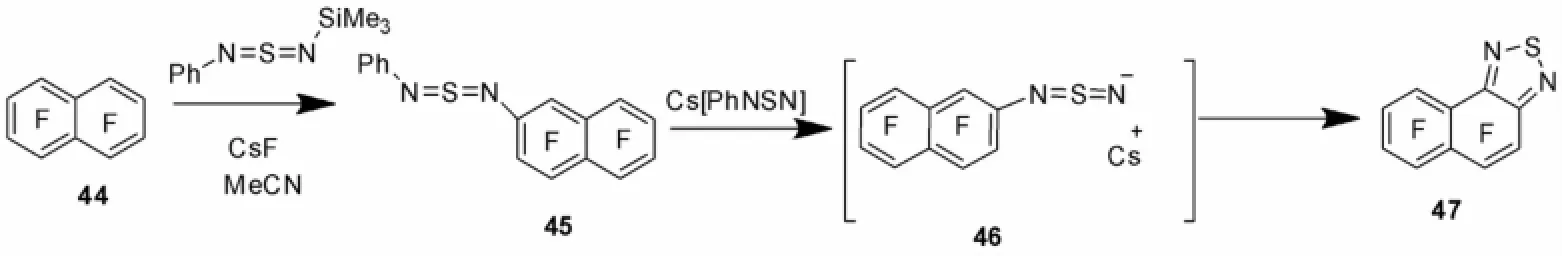
图9 BTD环的新合成Fig.9 New synthesis of BTD ring
4 结语
具有苯并噻二唑结构单元的化合物是一类非常重要的化合物,它拥有优良的光电性质和广泛的应用前景,因此,苯并噻二唑衍生物的合成已成为一个重要的研究课题。本文综述了最近发表的关于苯并噻二唑衍生物的合成方法。最有效的是从4,7-二溴-2,1,3-苯并噻二唑这个中间体开始,Pd催化的偶联反应,例如Suzuki反应,Stille反应,Heck反应及Sonogashira反应等。当然还有一些其他方法。以Pd为催化剂制备的方法仍然存在成本较高的缺憾,所以仍需要不断优化反应条件和探索更好的合成苯并噻二唑衍生物的高效途径,为这类功能材料的应用不断拓展新天地。
[1]高红飞,黄旦翔,程晓红,等.基于苯并噻二唑液晶化合物的研究[J].云南化工,2014,41(4):20-25.
[2]a)Lin P.Fluorescence of Organic Molecules in Chiral Recognition[J].Chem.Rev.,2004,104(3):1687-1716;b)Sun SS,Lees A J.Self-assembly triangular and square rhenium(I)tricarbonyl comp lexes:a comprehensive study of their preparation,electrochemistry,photophysics,photochemistry,and host-guest properties[J].J.Am.Chem.Soc.,2000,122(37):8956 -8967.
[3]Neto B A D,Lapis A A M,Dupont J,et al.2,1,3-Benzothiadiazole and derivatives:synthesis,properties,reactions,and applications in light technology of smallmolecules[J].Eur.J.Org.Chem.,2013,2013(2):228-255.
[4]a)Suzuki T.Regulation of crystal structure in organic redox systems by using intermolecular interactions induced by annelation of heterocycles[J].Asahi Garasu Zaidan JoseiKenkyu Seika Hokoku,1994,149-153;b)Tomura M,Akhtaruzzaman M,Suzuki K,et al.4,7-Diiodo-2,1,3-benzothiadiazole and 7,7'-diiodo-4,4'-bi(2,1,3-benzothiadiazole)[J].Acta. Cryst.,2002,58(7):373-375.
[5]Weinstock LM,Davis P,Handelsman B,et al.General synthetic system for 1,2,5-thiadiazoles[J].J. Org.Chem.,1967,32(7):2823-2829;b)Kom im A P,Street RW,Carmack M,Chemistry of 1,2,5-thiadiazoles.III.[1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazole[J].J.Org.Chem.,1975,40(19):2749-2752;c)Bryce M R,Use of piperidine-1-sulfenyl chloride as a sulfur-transfer reagent in reactions with diamines:the preparation of sulfur-nitrogen heterocycles[J].J.Chem.Soc.,1984,11:2591-2593.
[6]Pilgram K,Zupan M,Skiles R.Bromination of2,1,3-benzothiadiazoles[J].J.Heterocycl.Chem.,1970,7(3):629-633.
[7]Raimundo JM,Blanchard P,et al.Proquinoid acceptors as building blocks for the design of efficient pconjugated fluorophoreswith high electron affinity[J]. Chem.Commun.,2000,11:939-940.
[8]ZhuW H,Meng X L,Tian H,et al.Bisthienylethenes containing a benzothiadiazole unit as a bridge:photochromic performance dependence on substitution position[J].Chem.Eur.J.,2010,16(3):899-906.
[9]Hou Q,Xu X,Yang L T,et al.Synthesis and photovoltaic properties of fluorene-based copolymerswith narrow band-gap units on the side chain[J].Eur. Polym.J.,2010,46(12):2365-2371.
[10]Yasuda T,Shinohara Y,Ishi-i T,etal.Synthesis and photovoltaic properties of amorphous polymers based on dithienyl-benzothiadiazole-triphenyl-amine with hexyl side chains on different positions of thienyl groups[J].Ploym.Chem.,2013,51(12):2536 -2544.
[11]Aldred M P,Contoret A E A,Vlachos P,etal.Organic electroluminescence using polymer networks from smectic liquid crystals[J].Liquid Crystals.,2006,4(33):459-467.
[12]Zeng SH,Yin L X,Li K C,et al.D-A-D low band gap molecule containing triphenylamine and benzoxadiazole/benzothiadiazole units:synthesis and photophysical properties[J].Dyes and Pigments,2012,95(2):229-235.
[13]Neto B A D,Lopes A S A,Dupont J,et al.Photophysical and electrochemical properties of p-extended molecular 2,1,3-benzothiadiazoles[J].Tetrahedron,2005,61(46):10975-10982.
[14]Operamolla A,Colella S,Babudri F,et al.Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units:Synthesis,characterization and application in polymer solar cells[J].Sol.Energ. Mat.Sol.C.,2011,95(12):3490-3503.
[15]Liu J,Guo X,Jing X B,et al.White electroluminescence from a single-polymer system with simultaneous two-color emission:polyfluorene as blue host and 2,1,3-benzothiadiazole derivatives as orange dopants on the side chain[J].Adv.Funct.Mater.,2007,17(12):1917-1925.
[16]Ranjith K,Swathi SK,Kumar P,et al.Dithienylcyclopentadienone derivative-co-benzothiadiazole:an alternating copolymer for organic photovoltaics[J].Sol. Energ.Mat.Sol.C.,2012,98:448-454.
[17]Ranjith K,Swathi SK,Malavika A,et al.Random copolymers consisting of dithienylcyclopentadienone,thiophene and benzothiadiazole for bulk heterojunction solar cells[J].Sol.Energ.Mat.Sol.C.,2012,105:263-271.
[18]Shang H X,Li Y F,Zhan XW,et al.Solution processable D-A-D molecules based on triphenylamine for efficient organic solar cells[J].Sol.Energ.Mat. Sol.C.,2010,94(3):457-464.
[19]Misra R,Mobin SM,Thaksen J,et al.Donor acceptor ferrocenyl-substituted benzothiadiazoles:synthesis,structure,and properties[J].J.Org.Chem.,2013,78(10):4940-4948.
[20]Wang B,Tao Y,Wong M S,et al.Naphthodithiophene-2,1,3-benzothiadiazole copolymers for bulk heterojunction solar cells[J]Chem.Commun.,2011,47(33):9471-9473.
[21]Jheng JF,Lai Y Y,Hsu C S,et al.Influences of the non-covalent interaction strength on reaching high solid-state order and device performance of a low bandgap polymer with axisymmetrical structural units[J].Adv.Mater.,2013,25(17):2445-2451.
[22]Zhou H X,Yang L Q,You W,et al.Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7%efficiency[J].Angew. Chem.Int.Ed.,2011,50(13):2995-2998.
[23]Lin L Y,Chen Y H,Wong K T,et al.A low-energy-gap organic dye for high-performance smallmolecule organic solar cells[J].J.Am.Chem.Soc.,2011,133(40):15822-15825.
[24]Crivillers N,MelucciM,Zanelli A,et al.Self-assembly and electrical propertiesof a novel heptameric thiophene-benzothiadiazole based architectures[J]. Chem.Commun.,2012,48(100):12162-12164.
[25]Kato S I,Matsumoto T,Mataka S,et al.Novel 2,1,3-benzothiadiazole-based red-fluorescent dyes with enhanced two-photon absorption cross-sections[J].Chem.Eur.J.,2006,12(8):2303 -2317.
[26]Ishi-i T,Sakai M,Shinoda C.Benzothiadiazolebased dyes that emit red light in solution,solid and liquid state[J].Tetrahedron,2013,69(45):9475-9480.
[27]He Q G,He C,Li Y F,et al.Amorphous molecular material containing bisthiophenyl-benzothiadiazole and triphenylamine with bipolar and low-bandgap characteristics for solar cells[J].Thin Solid Films,2008,516(18):5935-5940.
[28]Yang Y,Zhang J,Li Y F,et al.Solution-processable organic molecule with triphenylamine core and two benzothiadiazole-thiophene arms for photovoltaic application[J].J.Phys.Chem.C.,2010,114(8):3701 -3706.
[29]Zhang J,Yang Y,Li Y F,etal.Solution-processable star-shaped photovoltaic organic molecule with triphenylamine core and benzothiadiazole-thiophene arms[J].Macromolecules,2009,42(20):7619 -7622.
[30]Gallardo H,Conte G,Molin F,et al.New luminescent liquid crystals based on 2,1,3-benzothiadiazole and bent five-membered N-heterocyclic cores[J]. Liquid Crystals.,2012,39(9):1099-1111.
[31]Akhtaruzzaman M,Tomura M,Zaman M B.Synthesis and characterization of new linearπ-conjugatedmolecules containing bis(ethynylpyridine)units with a benzothiadiazole spacer[J].J.Org.Chem.,2002,67(22):7813-7818.
[32]Behramand B,Molin F,Gallardo H.2,1,3-Benzoxadiazole and 2,1,3-benzothiadiazole-based fluorescent compounds:synthesis,characterization and photophysical/electrochemical properties[J].Dyes and Pigments,2012,95(3):600-605.
[33]Silvestri F,Marrocchi A,Taticchi A,et al.Solutionprocessable low-molecular weight extended arylacetylenes:versatile p-type semiconductors for fieldeffect transistors and bulk heterojunction solar cells[J].J.Am.Chem.Soc.,2010,132(17):6108 -6123.
[34]Tanaka D,Ohshita J,Harima Y,et al.Synthesis of disilanylene polymers with donor-acceptor-type p-conjugated units and applications to dye-sensitized solar cells[J].Journal of Organometallic Chemistry,2012,719:30-35.
[35]Bijleveld JC,Shahid M,Janssen R A J,et al.Copolymers of cyclopentadithiophene and electron-deficient aromatic units designed for photovoltaic applications[J].Adv.Funct.Mater.,2009,19(20):3262 -3270.
[36]TaoY M,LiH Y,You Z X,et al.Synthesis and characterization of efficient luminescent materials based on 2,1,3-benzothiadiazole with carbazole moieties[J].Synthetic Metals.,2011,161(9-10):718 -723.
[37]Chu CW,Horie M.Synthesis and characterization of cyclic conjugated architectures composed of thiophene and benzothiadiazole units[J].Asian J.Org.Chem.,2013,2(10):838-842.
[38]Liu Y M,Lai H,Fang Q,et al.New low bandgap molecules based on ethylene-separated benzothiadiazoles:synthesis and bandgap comparison[J].Tetrahedron Lett.,2010,51:4462-4465
[39]Wang J L,Xiao Q,Pei J.Benzothiadiazole-based D-π-A-π-D organic dyeswith tunable band gap:synthesis and photophysical properties[J].Org. Lett.,2010,12(18):4164-4167.
[40]Zou Q,Tian H.Chemodosimeters formercury(II)and methylmercury(I)based on 2,1,3-benzothiadiazole[J].Sensors and Actuators B.,2010,149(1):20 -27.
[41]Suresh P,Mikroyannidis JA,Stylianakis M M,et al. Effect of the incorporation of a low-band-gap small molecule in a conjugated vinylene copolymer:PCBM blend for organic photovoltaic devices[J].Appl.Mater.Interfaces.,2009,1(7):1370-1374.
[42]Lork E,Mews R,Zibarev A V,et al.Reactions of arylthiazylamides with internal and external fluoro electrophiles-formation of products with unusual structures[J].Eur.J.Inorg.Chem.,2001,8:2123-2134.
Advance in Syntheses of 2,1,3-Benzothiadiazole conjugated compounds
HUANG Dan-xiang,XIA M eng,PENG Xiong-wei,LI Tai-hao,CHENG X iao-hong
(School of Chemical Science and Technology,Yunnan University,Kunming 650091,China)
2,1,3-Benzothiadiazole conjugated compounds are one of themost active research area in the optoelectronicmaterials due to their outstanding optical and electrical properties and have great potentials as organic light-emitting diodes,solar cells,liquid crystals,fluorescent sensors and photovoltaic cells ect. This review covers the various synthetic methods reported for 2,1,3-Benzothiadiazole(BTD)conjugated compounds in recent years.Including Suzuki coupling reaction,Stille coupling reaction,Heck coupling reaction,Sonogashira coupling reaction,Nigishi coupling reaction,Ullmann coupling reaction ect.
synthesis;2,1,3-Benzothiadiazole;coupling reaction
TN104.3
A
1004-275X(2015)06-0028-08
10.3969/j.issn.1004-275X.2015.06.007
收稿:2015-09-14
国家自然科学基金(No.21274119,NO.21364017);云南省自然科学基金(2013FA007),云南省教育厅科学基金(ZD2015001)。
黄旦翔(1990-),男,硕士研究生;研究方向:超分子液晶化学。
