浙江丽水中华大蟾蜍和黑眶蟾蜍蝌蚪对水位变化的表型响应
2015-03-10唐小芬樊晓丽林植华姚婷婷周存通
唐小芬, 樊晓丽, 林植华, 姚婷婷, 李 香, 金 晶, 周存通
丽水学院生态学院, 丽水 323000
浙江丽水中华大蟾蜍和黑眶蟾蜍蝌蚪对水位变化的表型响应
唐小芬, 樊晓丽, 林植华*, 姚婷婷, 李 香, 金 晶, 周存通
丽水学院生态学院, 丽水 323000
全球气候变暖引发栖息地干涸将对生活在水中的无尾类幼体提出了挑战。通过浙江丽水中华大蟾蜍(Bufogargarizans)和黑眶蟾蜍(Duttaphrynusmelanosticus)蝌蚪在实验条件下对不同水位变化的表型响应,检测表型可塑性的遗传性和环境近因性影响。结果表明,水位变化对中华大蟾蜍蝌蚪早期发育历期、头宽和体重影响不显著,对体长影响显著,其中逐减水位最大、恒低水位最小,慢波、恒高与快波、逐增水位依次减少;水位变化对黑眶蟾蜍蝌蚪早期发育历期、体长、头宽和体重影响均显著;发育历期以恒高水位最大,恒低水位最小;体长以逐减水位最大,恒低、快波和慢波水位显著偏小,逐增和快波水位居中;头宽以恒低水位最小,逐增水位居中,其余较大;体重以恒低水位最小、恒高水位最大,其余居中。水位变化对中华大蟾蜍蝌蚪的变态时间、体长、头宽和体重影响均不显著;水位变化对黑眶蟾蜍蝌蚪的变态时间、体长和体重影响均显著,对头宽影响不显著;恒低水位的变态时间最长,恒高水位的变态时间最短,其他水位变化之间差异不显著;恒高水位的体长最大,恒低和快波水位最小,其他居中;逐增和快波水位的体重最大,恒低水位最小。研究结果表明,繁殖季节不同的中华大蟾蜍和黑眶蟾蜍蝌蚪响应水位变化的表型可塑性差异显著,长期在容易发生干旱和水位变化的冬季繁殖的中华大蟾蜍蝌蚪的表型可塑性低,在雨水充沛的春季繁殖的黑眶蟾蜍蝌蚪的表型可塑性高,表现出表型可塑性的种间差异和遗传性;在早期发育过程中,两种蝌蚪体长的共同的表型变异与缺乏遗传基础的环境近因性影响有关;黑眶蟾蜍蝌蚪对低水位或水位下降作出减速分化的消极响应,响应程度与环境信号的强弱直接相关。
中华大蟾蜍; 黑眶蟾蜍; 蝌蚪; 水位变化; 表型可塑性
全球气候变暖引起栖息地水温的升高,许多地区的降雨量减少[1- 2],湿地多样性和可利用性不断下降,临时性水体可能变干或者消失,持久性池塘也可能变得短暂[3- 5],增加了水域繁殖无尾类幼体的生存压力[6]。如果水体干涸速度超过无尾类幼体加快生长发育的能力,那么水体干涸将是致命的[7],水环境可利用性是影响无尾类幼体生长发育的最重要生态因子之一[8]。生活史复杂的无尾类幼体以最适的变态时间和大小完成从水生生境到陆地生境的迁移,通过在生长发育过程中产生适应性表型可塑性来提高其适合度,对于个体生存和种群动态变化至关重要[9- 11]。
不同无尾类幼体对栖息地水位的敏感性不同[6],存在长期进化形成的遗传特异性和特定的环境适应性。例如,二光肿肋蟾(Pleurodemadiplolister)[12]、哈蒙掘足蟾(Scaphiopushammondi)[13]、库氏掘足蟾(Scaphiopuscouchii)[14]、斑点合跗蟾(Pelodytespunctatus)[15]、Rhinellaspinulosa[16]、短头蛙(Sphaerothecabreviceps)[17]、强刃锄足蟾(Pelobatescultripes)[18]蝌蚪在响应水位下降时提前变态形成较小的变态个体;欧洲大蟾蜍(Bufobufo)和黄条背蟾蜍(Bufocalamita)蝌蚪在响应自然水体干涸时形成较小的变态个体,但是变态时间却没有改变[19];叙利亚锄足蟾(Pelobatessyriacus)蝌蚪通过加快发育提早完成变态来响应栖息地的干涸,但其变态时大小与恒定水位条件下所形成的变态个体差异不显著[20]。生活在沙漠中的库氏掘足蟾蝌蚪在低密度的池塘中会迅速完成变态发育,而在处于干旱条件下的高密度池塘中则很少能完成幼体的变态[21]。Wilbur和Collins 认为,利用临时性池塘进行繁殖的无尾类会比那些利用持久性水体繁殖的无尾类在蝌蚪期和变态大小这两个方面表现出更强的可塑性[22]。欧洲林蛙(Ranatemporaria)的数据表明,北方种群相对南方种群变态时间短,但缺乏对干涸风险的适应性响应[23]。
浙江丽水中华大蟾蜍(Bufogargarizans)和黑眶蟾蜍(Duttaphrynusmelanostictus)同域分布,在持久性池塘(终年有水)繁殖,前者在冬季12—3月繁殖,后者在春季3—5月繁殖[24],季节差异造成降雨量的差异,冬天多干旱缺水,春季多雨。本研究通过中华大蟾蜍和黑眶蟾蜍蝌蚪在实验条件下响应不同水位变化表型可塑性的种间差异,检测如下两个假设:(1)若水位变化诱导无尾类幼体的表型变异与个体的适合度密切相关,则不同季节物种的表型应表现出与其所在季节相对应的响应;(2)若遗传因素仅能部分地解释幼体表型的变异,则水位变化实验应能检测到不同季节物种共同的环境近因性影响形成的表型变异。
1 材料与方法
1.1 卵带的采集和孵化
2012年2月23日和2012年4月20日在浙江丽水学院校园(28°27′ N,119°53′ E)内的同一持久性池塘中分别采集当天产中华大蟾蜍和黑眶蟾蜍的部分卵带(各约300枚卵),带回两栖爬行动物实验室,分别置于塑料箱(700mm×500mm×400mm,200 mm水深)中孵化(室温(23±0.2) ℃),待蝌蚪长至能自由游泳的26—27期[25]用于实验。
1.2 实验设计与管理

图1 六种水位处理下,中华大蟾蜍和黑眶蟾蜍两种蝌蚪的变态成活率Fig.1 Survival rate of metamorphosis of B. gargarizans and D. melanostictus tadpoles under six different water level treatments
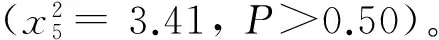
1.3 形态测定与发育历期鉴定
中华大蟾蜍和黑眶蟾蜍蝌蚪的水位实验分别在2月28日—3月25日和4月24日—5月22日完成。测定第1天(N= 20)、第14天和出现前肢(42期)时蝌蚪的湿重(BM: body mass)、体长(吻端到泄殖腔的距离,SVL: snout-vent length)、头宽(头部最宽处距离,HW: head width)和鉴定发育历期。用吸水纸吸干蝌蚪表面水分,用Sartorius电子天平称取湿重(± 0.001g);将蝌蚪和变态幼体放入底下有标尺的培养皿中,用Sony DSC-T100数码相机记录形态,用ImageJ 1.44p软件读出(± 0.01 mm);Nikon XTS30解剖显微镜鉴定蝌蚪的发育阶段[25]。
1.4 数据分析
用Statistica统计软件包完成所有数据的统计分析。统计分析前,检验数据正态性(Kolmogorov-Smirnov test)和方差同质性(F-max test)。经检验,数据无需转换符合参数统计的条件。用One-way ANOVA、One-way ANCOVA及后续的Tukey′s检验处理和比较相应的数据,非参数统计用x2-test。描述性统计值用Mean±SE表示,显著性水平设置为α = 0.05。
2 结果
2.1 实验蝌蚪初始发育历期与形态特征的种间比较
One-way ANOVA显示,实验用中华大蟾蜍蝌蚪和黑眶蟾蜍蝌蚪的初始发育历期差异不显著,中华大蟾蜍蝌蚪的体长显著大于黑眶蟾蜍。以体长为协变量的One-way ANCOVA显示,特定体长的中华大蟾蜍和黑眶蟾蜍蝌蚪的头宽差异不显著,中华大蟾蜍蝌蚪的体重显著大于黑眶蟾蜍蝌蚪(表1)。

表1 实验用中华大蟾蜍和黑眶蟾蜍蝌蚪的初始发育历期和形态特征Table 1 The initial Gosner stage and morphological characteristics of tadpoles in B. gargarizans and D. melanostictus
蝌蚪的发育历期和体长为One-way ANOVA,其体重和头宽均为以体长为协变量的One-way ANCOVA;BG:中华大蟾蜍(B.gargarizans),DM:黑眶蟾蜍(D.melanostictus)
2.2 水位变化对蝌蚪早期生长发育的影响
中华大蟾蜍蝌蚪和黑眶蟾蜍蝌蚪在6种水位条件下第14天的发育历期、个体的体长、头宽和体重的描述性统计见图2。种间One-way ANOVA显示,6种水位变化处理第14天时,中华大蟾蜍蝌蚪的发育历期显著小于黑眶蟾蜍蝌蚪,其体长显著大于黑眶蟾蜍蝌蚪(表2)。以体长为协变量的One-way ANCOVA显示,特定体长的中华大蟾蜍和黑眶蟾蜍两种蝌蚪的头宽和体重差异均不显著(表2)。

表2 水位变化对中华大蟾蜍和黑眶蟾蜍蝌蚪早期生长发育的影响Table 2 Effect of water levels on the early growth and development of tadpoles in B. gargarizans and D. melanostictus
发育历期和体长为One-way ANOVA,体重和头宽均以体长为协变量进行One-way ANCOVA;不同上标表示差异显著(Tukey′s test,α= 0.05, a > b > c); BG:中华大蟾蜍(B.gargarizans),DM:黑眶蟾蜍(D.melanostictus); 水位处理类型用不同数字表示,1:恒定低水位;2:恒定高水位;3:水位逐渐升高;4:水位逐渐降低;5:水位快速波动;6:水位慢速波动
以水位变化为因子One-way ANOVA显示,水位变化对中华大蟾蜍蝌蚪早期发育历期影响不显著,对体长影响显著,其中逐减水位最大、恒低水位最小,慢波、恒高与快波、逐增依次减少;以体长为协变量的One-way ANCOVA显示,水位变化对特定体长中华大蟾蜍蝌蚪的头宽和体重影响不显著(表2)。
以水位变化为因子One-way ANOVA显示,水位变化对黑眶蟾蜍蝌蚪早期发育历期和体长影响显著,发育历期以恒高水位最大、恒低水位最小,蝌蚪体长以逐减水位最大、恒低、快波和慢波水位显著偏小,逐增和快波居中。以体长为协变量的One-way ANCOVA显示,水位变化对特定体长蝌蚪的头宽和体重影响显著,头宽以恒低水位最小、逐增水位居中、其余较大,体重恒低水位最小、恒高水位最大、其余居中(表2)。

图2 6种水位处理下对第14天中华大蟾蜍和黑眶蟾蜍两种蝌蚪的发育历期和形态特征的描述性统计值Fig.2 Descriptive statistics of Gosner stage and morphological traits of B. gargarizans and D. melanostictus tadpoles under six different water level treatments. The bar graphs show mean Gosner stage, SVL, HW, and BM on the 14th day (n=4 tanks/treatment); error bars represent ± SE
2.3 水位变化对蝌蚪变态时间和大小的影响
中华大蟾蜍蝌蚪和黑眶蟾蜍蝌蚪在6种水位条件下的变态时间、变态个体的体长、头宽和体重的描述性统计见图3。种间One-way ANOVA显示,不同水位变化处理下中华大蟾蜍蝌蚪的变态时间和体长显著大于黑眶蟾蜍蝌蚪,以体长为协变量的One-way ANCOVA显示,特定体长的中华大蟾蜍和黑眶蟾蜍刚变态个体头宽差异不显著,中华大蟾蜍刚变态个体的体重显著大于黑眶蟾蜍(表3)。

表3 水位变化对中华大蟾蜍和黑眶蟾蜍蝌蚪变态时间和大小的影响Table 3 Effect of water levels on the time and size at metamorphosis of tadpoles in B. gargarizans and D. melanostictus
变态时间和体长为One-way ANOVA,体重与头宽均为以体长为协变量的One-way ANCOVA; 不同上标的平均值差异显著(Tukey′s test,α= 0.05, a > b > c); 水位处理类型用不同数字表示,1:恒定低水位;2:恒定高水位;3:水位逐渐升高;4:水位逐渐降低;5:水位快速波动;6:水位慢速波动
以水位变化为因子One-way ANOVA显示,水位变化对中华大蟾蜍蝌蚪的变态时间和体长影响不显著;水位变化对黑眶蟾蜍蝌蚪的变态时间和体长影响显著,恒低水位的变态时间最长,恒高水位的变态时间最短,其他水位变化之间的变态时间差异不显著;恒高水位下的体长最大,恒低和快波水位最小,其他居中(表3)。以体长为协变量的One-way ANCOVA显示,不同水位变化对特定体长的中华大蟾蜍、黑眶蟾蜍蝌蚪变态时头宽和中华大蟾蜍蝌蚪变态时体重影响不显著,对黑眶蟾蜍蝌蚪变态时体重影响显著,以逐增和快波水位的体重最大,恒低水位最小(表3)。
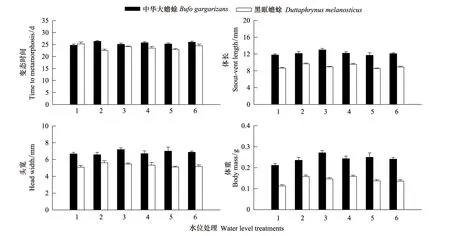
图3 6种水位处理下,中华大蟾蜍和黑眶蟾蜍两种蝌蚪的变态时间和形态特征的描述性统计值Fig.3 Descriptive statistics of time to metamorphosis and morphological traits of toadlets of B. gargarizans and D. melanostictus under six different water level treatments. The bar graphs show mean time to metamorphosis, SVL, HW, and BM at metamorphosis (n=4 tanks/treatment); error bars represent ± SE
3 讨论
3.1 中华大蟾蜍和黑眶蟾蜍蝌蚪对水位变化的早期表型响应
发育早期(第14天),中华大蟾蜍和黑眶蟾蜍蝌蚪的体长均表现出对六种水位处理的生长响应(即两种蝌蚪的体长在逐减水位体长最大,表2),即不同季节物种应对环境变化的共同反应[23]。
两种蝌蚪体长的这种变化与同域分布但主要在临时性水体繁殖的虎纹蛙(Hoplobatrachuschinensis)不同[26],反映了无尾类持久性池塘繁殖者幼体的早期生长发育比临时性水体繁殖者对水位的敏感性高。野外观察虎纹蛙蝌蚪喜栖息水体底部,常处于静止或作短距离游动,故早期栖息地的充足水体不会触发其对干涸信号的生理响应机制。而中华大蟾蜍和黑眶蟾蜍这两种蝌蚪喜好在持久性池塘中上层群聚游动,这也许有利于感知水位的下降,触发它们快速摄食而促进生长。与恒高水位相比,饲养在恒低水位下的中华大蟾蜍蝌蚪和黑眶蟾蜍蝌蚪的体长分别缩短了6.0%和5.6%(图2)。由于本研究设置的密度非常低(3只/箱),消除了密度制约效应[28],可能原因是恒低水位限制了这两种蝌蚪的游动范围,继而减少摄食,导致生长过程的抑制[13]。
与冬季繁殖的中华大蟾蜍蝌蚪相比,春季繁殖的黑眶蟾蜍蝌蚪的发育历期、体重和头宽还表现出不同水位处理间的显著差异(表2),表现出了表型可塑性的种间差异和物种特异性,即表型可塑性的遗传性[23]。黑眶蟾蜍蝌蚪在早期阶段表现出发育可塑性来响应水位变化,即恒高水位下发育最快,恒低水位下发育最慢,其他水位居中(表2),黑眶蟾蜍蝌蚪早期生长发育对恒低水位作出消极响应[13, 29]。
3.2 中华大蟾蜍和黑眶蟾蜍蝌蚪对水位变化的变态表型响应
水位变化对中华大蟾蜍蝌蚪的变态时间和大小均不产生显著影响(图2,表2),这表明该物种响应水位变化的可塑性程度低。虽然中华大蟾蜍蝌蚪在水位变化早期阶段能感知到水体的减少,但后期通过补偿性生长保持平衡[30]。在发育上,中华大蟾蜍蝌蚪之所以不对水位变化发生适应性的发育响应,这也可能与该物种的繁殖时间密切相关。中华大蟾蜍通常在冬季选择持久性池塘作为繁殖地[24],相对于黑眶蟾蜍的春季繁殖,该季节相对寒冷干燥、雨水较少,水位经常发生变化,但一般不发生干涸,长期的适应可能是中华大蟾蜍不敏感的原因。
研究表明,黑眶蟾蜍蝌蚪的变态时间受水位影响显著(图2,表2)。首先,恒低水位下发育最慢,变态时间最长,而恒高水位下发育最快,变态时间最短,前者比后者平均延迟了2.7 d左右,这与已有关于Hylapseudopuma[31]、二光肿肋蟾与Rhinellagranulose[16],以及Discoglossuspictus[26]的研究结果一致,但与另外一些关于哈蒙掘足蟾[13]、虎纹蛙[26]、Ranatemporaria[32]和Scaphiopuscouchii[33]的研究结果不同,这表明了黑眶蟾蜍蝌蚪对低水位很敏感。其次,黑眶蟾蜍蝌蚪在其他水位变化下的平均变态时间分别为慢波(约24.5 d)、逐减(约24.1 d)、逐增(约23.5 d)和快波(约22.9 d),与恒高水位相比而言,快波、逐增、逐减和慢波依次延迟0.3 d、0.9 d、1.5 d和1.9 d发生变态,这表明水位慢波变化中较长的恒低水位可能限制了该物种蝌蚪的发育,通过减慢发育延迟变态响应水位的逐减,逐增水位下黑眶蟾蜍蝌蚪的变态时间更接近于恒高水位,而非恒低水位,证实了黑眶蟾蜍蝌蚪适应于在雨后高水位水体中繁殖。
无尾类幼体的加快发育通常与变态时个体变小有关[22],而本研究发现,6种水位处理下对黑眶蟾蜍蝌蚪变态时体长影响显著,其中快波和恒低水位体长显著最短,恒高水位体长显著最长,若不考虑快波水位的情况,黑眶蟾蜍蝌蚪在恒低水位下的变态时间最长,但体长最短,而恒高水位下变态时间最短,但个体最大,这表明恒低水位同时降低黑眶蟾蜍蝌蚪生长发育速率。而恒低水位大大地限制了其游动空间影响其新陈代谢,这一结果与Székely等人[20]的研究结果一致。
因此,冬季繁殖的中华大蟾蜍和春季繁殖的黑眶蟾蜍蝌蚪面对干涸风险的表型可塑性差异显著,长期在容易发生干旱和水位变化的冬季繁殖的中华大蟾蜍的可塑性低,在雨水充沛的春季繁殖的黑眶蟾蜍的表型可塑性高,表现出表型可塑性的种间差异和遗传性;在早期发育过程中,蝌蚪个体大小的共同的表型变异,这种变异与缺乏遗传基础的环境近因性影响有关;黑眶蟾蜍蝌蚪对低水位或水位下降作出减速分化的消极响应,响应程度与环境信号的强弱直接相关。
[1] Le Treut H, Somerville R, Cubasch U, Ding Y, Mauritzen C, Mokssit A, Peterson T and Prather M. Historical Overview of Climate Change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL ed. Climate Change 2007: The Physical Science Basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Nork York: Cambridge University Press. 2007. 1- 36.
[2] Macrae M L, Brown L C, Duguay C R, Parrott J A, Petrone R M. Observed and projected climate change in the Churchill region of the Hudson Bay Lowlands and implications for pond sustainability. Arctic, Antarctic, and Alpine Research, 2014, 46(1): 272- 285.
[3] Araújo M B, Thuiller W, Pearson R G. Climate warming and the decline of amphibians and reptiles in Europe. Journal of Biogeography, 2006, 33(10): 1712- 1728.
[4] McMenamin S K, Hadly E A, Wright C K. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(44): 16988- 16993.
[5] Elsner M M, Cuo L, Voisin N, Deems J S, Hamlet A F, Vano J A, Lettenmaier D P. Implications of 21st century climate change for the hydrology of Washington State. Climatic Change, 2010, 102(1/2): 225- 260.
[6] Walther G R, Post E, Convey P, Menzel A, Parmesank C, Beebee T J C, Fromentin Jean-Marc, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature, 2002, 416(6879): 389- 395.
[7] Leips J, McManus M G, Travis J. Response of tree frog larvae to drying ponds: comparing temporary and permanent pond breeders. Ecology, 2000, 81(11): 2997- 3008.
[8] Kulkarni S S, Gomez-Mestre I, Moskalik C L, Storz B L, Buchholz D R. Evolutionary reduction of developmental plasticity in desert spadefoot toads. Journal of Evolutionary Biology, 2011, 24(11): 2445- 2455.
[9] Altwegg R, Reyer H. Patterns of natural selection on size at metamorphosis in water frogs. Evolution, 2003, 57(4): 872- 882.
[10] Johansson F, Hjelm J, Giles B E. Life history and morphology ofRanatemporariain response to pool permanence. Evolutionary Ecology Research, 2005, 7(7): 1025- 1038.
[11] Gervasi S S, Foufopoulos J. Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Functional Ecology, 2008, 22(1): 100- 108.
[12] Maciel T A, Juncá F A. Effects of temperature and volume of water on the growth and development of tadpoles ofPleurodemadiplolisterandRhinellagranulosa(Amphibia: Anura). Zoologia, 2009, 26(3): 413- 418.
[13] Denver R J, Mirhadi N, Phillips M. An experimental analysis of adaptive plasticity in amphibian metamorphosis: developmental response ofScaphiopushammondiitadpoles to habitat desiccation. Ecology, 1998, 79(6): 1859- 1872.
[14] Morey S R, Reznick D N. The relationship between habitat permanence and larval development in California spadefoot toads: field and laboratory comparisons of developmental plasticity. Oikos, 2004, 104(1): 172- 190.
[15] Richter-Boix A, Llorente G A, Montori A. Effects of phenotypic plasticity on post-metamorphic traits during pre-metamorphic stages in the anuranPelodytespunctatus. Evolutionary Ecology Research, 2006, 8(2): 309- 320.
[16] Márquez-García M, Correa-Solis M, Sallaberry M, Méndez M A. Effects of pond drying on morphological and life-history traits in the anuranRhinellaspinulosa(Anura: Bufonidae). Evolutionary Ecology Research, 2009, 11(5): 803- 815.
[17] Mogali S M, Saidapur S K, Shanbag B A. Receding water levels hasten metamorphosis in the frog,Sphaerothecabreviceps(Schneider, 1799): a laboratory study. Current Science, 2011, 101(9): 1219- 1222.
[18] Gomez-Mestre I, Kulkarni S, Buchholz D R. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PloS One, 2013, 8(12): e84266.
[19] Brady L D, Griffiths R A. Developmental responses to pond desiccation in tadpoles of the British anuran amphibians (Bufobufo,B.calamitaandRanatemporaria). Journal of Zoology, 2000, 252(1): 61- 69.
[20] Székely P, Tudor M, Cogălniceanu D. Effect of habitat drying on the development of the Eastern spadefoot toad (Pelobatessyriacus) tadpoles. Amphibia-Reptilia, 2010, 31(3): 425- 434.
[21] Newman R A. Effects of density and predation onScaphiopuscouchiitadpoles in desert ponds. Oecologia, 1987, 71(2): 301- 307.
[22] Wilbur H M, Collins J P. Ecological aspects of amphibian metamorphosis. Science, 1973, 182(4119): 1305- 1314.
[23] Laurila A, Karttunen S, Merilä J. Adaptive phenotypic plasticity and genetics of larval life histories in twoRanatemporariapopulations. Evolution, 2002, 56(3): 617- 627.
[24] 赵丽华. 浙江丽水中华大蟾蜍和镇海林蛙繁殖地选择及蝌蚪特征的比较研究 [D]. 杭州: 杭州师范大学, 2012.
[25] Gosner K L. A simplified table for staging anuran embryos and larvae with notes of identification. Herpetologica, 1960, 16(3): 183- 190.
[26] Fan X L, Lin Z H, We J. Effects of hydroperiod duration on developmental plasticity in tiger frog (Hoplobatrachuschinensis) tadpoles. Zoological Research, 2014, 35(2): 124- 13.
[27] Enriquez-Urzelai U, San Sebastián O, Garriga N, Llorente G A. Food availability determines the response to pond desiccation in anuran tadpoles. Oecologia, 2013, 173(1): 117- 127.
[28] Reques R, Tejedo M. Reaction norms for metamorphic traits in natterjack toads to larval density and pond duration. Journal of Evolutionary Biology, 1997, 10(6): 829- 851.
[29] Alford R A, Harris R N. Effects of larval growth history on anuran metamorphosis. The American Naturalist, 1988, 131(1): 91- 106.
[30] Richter-Boix A, Tejedo M, Rezende E L. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecology and Evolution, 2011, 1(1): 15- 25.
[31] Crump M L. Effect of habitat drying on developmental time and size at metamorphosis inHylapseudopuma. Copeia, 1989, 1989(3): 794- 797.
[32] Loman J. Early metamorphosis in common frogRanatemporariatadpoles at risk of drying: an experimental demonstration. Amphibia-Reptilia, 1999, 20(4): 421- 430.
[33] Newman R A. Developmental plasticity ofScaphiopuscouchiitadpoles in an unpredictable environment. Ecology, 1989, 70(6): 1775- 1787.
Phenotypic responses to water level change inBufogargarizansandDuttaphrynusmelanosticustadpoles at Lishui, Zhejiang
TANG Xiaofen, FAN Xiaoli, LIN Zhihua*, YAO Tingting, LI Xiang, JIN Jing, ZHOU Cuntong
CollegeofEcology,LishuiUniversity,Lishui323000,China
Habitat drying caused by global warming will raise a challenge for anuran larvae living in water. We investigated the phenotypic response to six different patterns of water level change inBufogargarizansandDuttaphrynusmelanosticustadpoles under laboratory conditions. The aim of this study was to examine the heritable basis and environmental proximate causes of phenotypic plasticity of these two species tadpoles. The results showed that all the six water level treatments had no significant effect on the early development Gosner stage (GS), head width (HW), or body mass (BM) ofB.gargarizanstadpoles on the 14th day, but there was a significant effect on their snout-vent length (SVL). The SVL ofB.gargarizanstadpoles raised in decreasing water levels were the longest, while the ones raised in constant low water levels had the shortest SVL than the remaining groups. Conversely, the six water level treatments had respectively significant effects on the GS, SVL, HW and BM ofD.melanosticustadpoles on the same day, respectively. Firstly, those tadpoles raised in constant high water level developed most fast, while the ones raised in constant low water level developed most slowly. Secondly, the tadpoles raised in decreasing water level had greater SVL, while the ones raised in constant low, fast fluctuation and slow fluctuation water levels had smaller SVL than the remaining groups. Thirdly, HW of the tadpoles raised in constant low water levels was the narrowest, followed by the ones raised in increasing water levels, the others raised in the remaining water level groups had the biggest HW. Lastly, BM of tadpoles in constant low water levels was the smallest, while the ones raised in constant high water level had heavier BM than the remaining groups. The water level treatments had no significant effect on the time of metamorphosis and body size at metamorphosis including SVL, HW, and BM inB.gargarizan. However, there were significant effects of the water level changes on the time of metamorphosis and body size at metamorphosis including SVL and BM, except for HW, inD.melanosticus. Tadpoles raised in the constant low water levels had protracted metamorphosis, whereas the tadpoles raised under the constant high water levels had shortened metamorphosis. SVL at metamorphosis ofD.melanosticusin the constant high water levels was the largest, while the ones raised in constant low and rapidly fluctuating water levels were the shortest. BM at metamorphosis ofD.melanosticusraised in the increasing and rapidly fluctuating water levels were the biggest, while the ones raised in the constant low water levels were the smallest. Our results suggest that there are significant interspecific differences in the phenotypic plasticity respond to desiccation risks betweenB.gargarizansandD.melanosticustadpoles: the former was weaker than the latter. Winter-breederB.gargarizanstadpoles experienced habitat drying more frequently, while spring-breederD.melanosticustadpoles experienced habitat drying rarely. This showed the interspecific differences and hereditary of the phenotypic plasticity. During the early development of the two toad tadpoles, the common phenotypic variations in their SVL were associated with lack of genetic basis of environmental proximate causes. The response to constant low or decreasing water level inD.melanosticustadpoles was negative (deceleration of differentiation), and the response degree was directly related to the strength of the environmental signals.
Bufogargarizans;Duttaphrynusmelanosticus; tadpoles; water level change; phenotypic plasticity
国家自然科学基金项目(31270443, 30970435); 浙江省大学生科技创新活动计划(2012R429022)
2014- 04- 28;
日期:2014- 07- 07
10.5846/stxb201404280845
*通讯作者Corresponding author.E-mail: zhlin1015@126.com
唐小芬, 樊晓丽, 林植华, 姚婷婷, 李香, 金晶, 周存通.浙江丽水中华大蟾蜍和黑眶蟾蜍蝌蚪对水位变化的表型响应.生态学报,2015,35(3):911- 918.
Tang X F, Fan X L, Lin Z H, Yao T T, Li X, Jin J, Zhou C T.Phenotypic responses to water level change inBufogargarizansandDuttaphrynusmelanosticustadpoles at Lishui, Zhejiang.Acta Ecologica Sinica,2015,35(3):911- 918.
