Dynamic Analysis of Nitric Oxide and Total Oxidant Capacity in Cow Uterine Secretion with Subclinical Endometritis
2015-03-01SongXueLiDejunFengGuofengLiBeiandLiuYunfeng
Song Xue,Li De-jun,Feng Guo-feng,Li Bei,and Liu Yun-feng
College of Veterinary Medicine,Northeast Agricultural University,Harbin 150030,China
Introduction
Subclinical endometritis (SCE),as one of the most important disorders in dairy cows during the post-partum period,impacts reproduction (Sheldon et al.,2006;Dubuc et al.,2010) and causes economic losses (Azawi,2008).Earlier diagnose and treatment of SCE play an important role in controlling clinical endometritis,yet the index of the early diagnosis are still not clear (Ahmadi et al.,2005;Gilbert,2005;Ribeiro et al.,2013;Mohammad et al.,2014).
Polymorphonuclear neutrophils (PMN) in uterine secretion is an indicator reflecting uterine health state (Kasimanickam,2004).Excessive amounts of neutrophilic granulocytes,macrophages,lymphocytes,eosinophilic granulocytes and various epithelial cells of the uterine tissue in uterine fluid are considered as a response to prevent exogenous pathogenic bacterium in the part of inflammation of the uterus (Singh,2008).In addition,the level of pro-inflammatory cytokines,such as tumor necrosis factor α (TNF-α),interleukin (IL) 1β,IL-6,IL-8 and some other molecules such as nitric oxide (NO) are increased during the period of infections (Sheldon et al.,2001;Li et al.,2010;Loyi et al.,2013).As an inflammatory mediator,NO which synthesized by macrophages causes smooth muscle relaxation and mediates cytoimmunity and inflammation toxicity.Excess NO is produced during inflammation as a primer defense system (Subandrio et al.,2000).Study showed that NO content is increased during inflammation diseases (Rawlingson,2003;Abdorrahman et al.,2005).In addition,substances derived from oxidation of NO,such as peroxynitrite,changed antioxidant balance of the bacteria (Onur et al.,2010).
In the process of inflammation,pro-inflammatory cytokines and cytotoxic radicals which are released from macrophages and granulocytes inhibit cellular metabolic pathways and lipid peroxidation (Ewa et al.,2012).Several studies showed that increase in lipid peroxidation during endometritis decreases the levels of some antioxidant molecules,such as vitamin E (VE) and vitamin C (VC),which lead to an increase in oxidative stress (Kankofer et al.,2005;Lorraine and Stacey,2009).Oxidative stress is a result of unbalance between oxidant and antioxidant levels (Lykkesfeldt and Svendsen,2007;Onur et al.,2010),occurs different pathological events,such as mastitis,metritis,and retained fetal membranes during the periparturient period in cows (Lykkesfeldt and Svendsen,2007;Lorraine and Stacey,2009).Due to membrane lipid peroxidation and oxidative stress,mammalian tissues such as cellular can be damaged by the accumulation of reactive oxygen species (Lorraine and Stacey,2009).Therefore,earlier diagnosis and treatment of the endometritis,especially SCE,are important to minimize economic losses.Antioxidant is used to treat mastitis in goats,bovines and mares,during the reproductive state (Abdorrahman et al.,2005;Eyassu et al.,2007;Lorraine and Stacey,2009);however,few reports show the change of the antioxidant in SCE.
Thus,with the aim to reveal the early diagnosis index of the subclinical endometritis,the levels of NO,VC and VE,total oxidant capacity (TOC) and PMN percentage from uterine secretion in cows were examined.
Materials and Methods
Experimental animals
Totally 80 Holstein cows from Dairy Tarm were enrolled in this study (Sarkar et al.,2006;Kasimanickam et al.,2004).All the Holstein cows were routinely examined once between 28 and 33 days after calving,included inspection of the vulva,tail,and perineum,vaginoscopy,and transrectal palpation of the cervix,uterus,and ovaries.Uterine discharge was classified as clear mucus,mucus with flecks of pus,mucopurulentand orpurulent according to the criteria described previously (Sarkar et al.,2006;Kasimanickam et al.,2004).Briefly,normal cows with no abnormal uterine discharge were selected according external inspection and vaginoscopy.SCE was diagnosed by endometrial cytology and histopathology.The cows were divided into two groups,which included 20 normal cows and 60 cows with SCE.Blood and uterine secretion were collected at the same time of inspection.None of cows had other diseases requiring systemic treatment.
Sample collection
Uterine secretion was collected as described previously(Sioutas et al.,2008).In brief,uterine washings were collected aseptically using two ways 18 gauges.Sterilized stainless steel catheters were fixed in the uterine horn with 10-15 mL air,then withdrew introducer,40-50 mL of washing fluid (sterile PBS pH 7.0) was infused into the uterine horn and mixed with the intrauterine contents by massaging the uterus per rectum.40 mL uterine washings were centrifuged at 1 000 g for 10 min,and the supernatant was frozen at –80℃ until all the samples were collected.
The percentage of PMN was counted with a minimum of 100 cells at 400 magnification.
Blood sample from vena cervicalis was collected in EDTA-vacutainer tubes,and centrifuged at 4℃ for 15 min at 3 000 g.Plasma was removed into Eppendorf tubes by pipette and stored at –20℃ until usage.
Biochemical analysis of sample
NO concentration in uterine secretion was measured by the Griess reaction according to Miranda et al (2001) (Nanjing Jiancheng Biology Engineering Research Institute,China).In order to measure TOC,VC and VE accurately in uterine secretion,uterine secretion samples were diluted 10 times with physiologic saline and filtered to obtain transparent secretion samples,which was slightly different from the method of Erel (2005).Then,secretion samples were centrifuged at 1 000 g for 10 min,and the supernatant was frozen until all the samples were collected.The concentrations of TOC,VC and VE were detected by a commercially available assay kit (Nanjing Jiancheng Biology Engineering Research Institute,China),following manufacturer recommendations (Li et al.,2010).
Statistical analysis
The data were analyzed by a one-way analysis of variance procedure followed by a Student's t-test (SPSS 17.0 software;SPSS Inc.,Chicago,IL,USA).Normal distribution of the data was analyzed by Anderson-Darling Normality test.Statistical significance was indicated in two levels: P<0.05 and P<0.01.P-values of <0.05 were considered to be statistically significant and P-values of <0.01 were considered markedly significant difference.All the data were presented as the mean±SD.
Results
Concentrations of NO (P<0.05) and TOC were signification greater (P<0.01) for cows with SCE compared with normal cows.In contrast,the levels of VC and VE were significantly lesser (P<0.01) in uterine secretion with SCE compared to those from normal cows.The percentage of PMN were significantly greater (P<0.01) in uterine secretion with SCE compared to those from normal cows (Table 1).Statistically significant positive correlation (r=0.235,P<0.01) was detected between PMN and NO concentrations in uterine secretion (Fig.1).
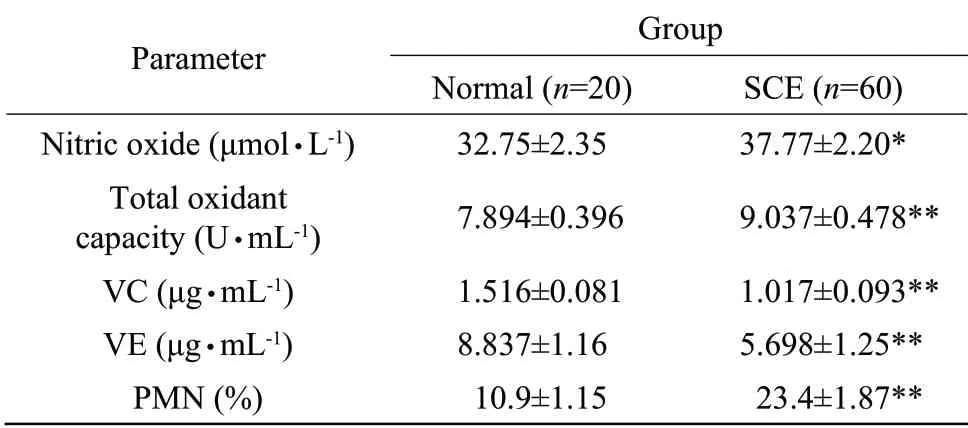
Table1 Levels of nitric oxide,total oxidant capacity,VC,VE and percentage of PMN in uterine secretion

Fig.1 Correlation between PMN and NO concentrations from uterine secretion in cows with SCE
Discussion
SCE is caused by the calving environment,diet,inadequate hygiene,stress,improper breeding and management conditions (Azawi,2008).NO is an important product during the developmental process of inflammation (Riku and Tuula,2001;Mukherjee,2008).Study showed that NO is an inflammatory mediator that causes inflammation toxicity and mediates cytoimmunity and smooth muscle relaxation (Li et al.,2010;Kamran et al.,2014).SCE leads to increase the level of NO in uterine sections from cows,therefore,the increased NO concentration in uterine sections and serum is speculated as an inflammatory response of uterine.In this regard,alteration in uterine section NO levels could be used as a diagnostic index to detect inflammation during SCE.The percentage of PMN indicated the level of inflammation with SCE (Anna et al.,2014).In the present study,there was a statistically significant positive correlation (P<0.05) between PMN percentage and NO concentration,which indicated the positive relationship between them.
During inflammation,increased NO reacted with superoxide anions leading to formation of peroxide nitrite radical (Savenkova et al.,2002;Cross and Wilson,2003),which led to the increase in lipid peroxidation and formation of the free radicals (Kumar et al.,2014).Studies showed that TOC was important components of the antioxidant defense system that could efficiently protect against oxidantive damage (Vessey,1993;Steenvoorden and Beijersbergen,1997).Therefore,in this study,increased TOC in uterine secretion from cows with SCE indicated that SCE induced oxidative damage by increasing NO concentration.
VC is the most effective antioxidant in plasma.VE,as a mundificant,cleans single oxygen and superoxide anion radical,more importantly,as a blocking agent,intercepts lipid peroxidation.Therefore,in this study,the decreased VC and VE levels in uterine secretion from cows with SCE indicated that SCE induced the imbalance of oxidant/antioxidant and the decrease of the antioxidant property.Studies showed that due to increased oxidant levels,oxidative stress changed metabolism and physiological functions (Zhong and Zhou,2013).Early studies suggested that dairy cows in oxidative stress,supplement certain antioxidant could ameliorate metabolism and guard against infectious diseases (Lorraine and Stacey,2009).Therefore,we deduced that SCE induced oxidative stress,which damaged the physiological function of the uterine.
In conclusion,SCE increased NO concentrations,leading to oxidative stress and oxidative damage.In addition,the positive correlation between PMN percentage and NO concentration indicated that the alteration of NO concentration in uterine sections could be used as an early diagnosis index of SCE.
Abdorrahman S A,Douglas N F,Cathy S C,et al.2005.Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis.American Journal of Reproductive Immunulogy,53: 230-237.
Ahmadi M R,Khodakaram T A,NazifiS,et al.2005.The comparative evaluation of uterine and cervical mucosa cytology with endometrial histopathology in cows.Comp Clin Path,14: 90-94.
Anna D,Janine M,Maike H,et al.2014.Peripheral blood leukocytes of cows with subclinical endometritis show an altered cellular composition and gene expression.Theriogenology,81: 906-917.
Azawi O I.2008.Postpartum uterine infection in cattle.Animal Reproduction Science,105(3/4): 187-208.
Cross R K,Wilson K T.2003.Nitric oxide in inflammatory bowel disease.Inflamm Bowel Dis,9: 179-189.
Dubuc J,Duffield T F,Leslie K E,et al.2010.Randomized clinical trial of antibiotic and prostaglandin treatments for uterine health and reproductive performance in dairy cows.J Dairy Sci,94: 1325-1338
Erel O.2005.A new automated colorimetric method for measuring total oxidant status.Clinical Biochemistry,38: 1103-1111.
Ewa G C,Katarzyna H,Sigurd L.2012.Is there a role for neuronal nitric oxide synthase (nNOS) in cytokine toxicity to pancreatic beta cells? Nitric Oxide,27(4): 235-241.
Eyassu S,Donkin E F,Buys E M.2007.Potential of lactoperoxidase to diagnose subclinical mastitis in goats.Small Ruminant Research,69: 154-158.
Gilbert R O,Shin S T,Guard C L,et al.2005.Prevalence of endometritis and its effects on reproductive performance of dairy cows.Theriogenology,64: 1879-1888.
Kankofer M,Lipko J,Zdunczyk S.2005.Total antioxidant capacity of bovine spontaneously released and retained placenta.Pathophysiology,11(4): 215-219.
Kasimanickam R,Duffield T F,Foster R A,et al.2004.Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows.Theriogenology,62: 9-23.
Kumar A,Chen S H,Kadiiska M B,et al.2014.Inducible nitric oxide synthase is key to peroxynitrite-mediated,LPS-induced protein radical formation in murine microglial BV2 cells.Free Radical Biologyand Medicine,73: 51-59.
Li D J,Liu Y F,Liu Y,et al.2010.Significance of nitric oxide concentration in plasma and uterine secretes with puerperal endometritis in dairy cows.Vet Res Commun,34: 315-321.
Lorraine M S,Stacey L A.2009.Immpact of oxidatice stress on the health and immune function of dairy cattle.Veterinary Immunology and Immunopathology,128(1/2/3): 104-109.
Loyi T,Kumar H,Nandi S,et al.2013.Differential expression of proinflammatory cytokines in endometrial tissue of buffaloes with clinical and sub-clinical endometritis.Research in Veterinary Science,4(94): 336-340
Lykkesfeldt J,Svendsen O.2007.Oxidants and antioxidants in disease: oxidative stress in farm animals.Veterinary Journal,173: 502-511.
Miranda K M,Espey M G,Wink D A.2001.A rapid,simple spectrophotometric method for simultaneous detection of nitrate and nitrite.Nitric Oxide: Biology and Chemistry,5: 62-71.
Mohammad R A,Arsalan H,Hamid R G,et al.2014.Preliminary trial in treatment of postpartum endometritis with intrauterine application of hyperimmune serum in dairy cows.Asian Pacific Journal of Tropical Disease,4(Supplement 1): S360-S365.
Mukherjee R.2008.Selenium and vitamin E increases polymorphonuclear cell phagocytosis and antioxidant levels during acute mastitis in riverine buffaloes.Veterinary Research Communications,32: 305-313.
Onur A,Hasan O,Emine A,et al.2010.Subclinical mastitis causes alterations in nitric oxide,total oxidant and antioxidant capacity in cow milk.Research in Veterinary Science,89(1): 10-13.
Rawlingson A.2003.Nitric oxide,inflammation and acute burn injury.Burns,Nov 29: 631-40.
Ribeiro E S,Lima F S,Greco L F,et al.2013.Prevalence of periparturient disease and effects on fertility of seasonally calving grazing dairy cows supplemented with concentrates.Journal of Dairy Science,193(1): 5682-5697.
Riku A,Tuula M.2001.Nitrous oxide use in paediatric surgery.Best Practice &Research Clinical Anaesthesiology,15(3): 467-475.
Sarkar P,Kumar H,Rawat M.2006.Effect of administration of garlic extract and PGF2α on hormonal changes and recovery in endometritis cows.Asian-Australasian Journal of Animal Science,19(7): 964-969.
Savenkova L,Gercberga Z,Muter O,et al.2002.PHB-based films as matrices for pesticides.Process Biochemistry,37(7): 109-114.
Singh J,Murray R D,Mshelia G,et al.2008.The immune status of the bovine uterus during the peripartum period.The Veterinary Journal,175: 301-309.
Sioutas A,Ehren I,Lundberg J O,et al.2008.Intrauterine nitric oxide in pelvic inflammatory disease.Fertility and Sterility,89: 948-952.
Sheldon I M,Noakes D E,Rycroft A,et al.2001.Acute phase protein response to postpartum uterine bacterial contamination in cattle.Vet Rec,148: 172-175.
Sheldon I M,Lewis G S,LeBlanc S,et al.2006.Defining postpartum uterine disease in cattle.Theriogenology,65: 1516-1530
Steenvoorden D P T,Beijersbergen van Henegouwen G M J.1997,The use of endogenous antioxidants to improve photoprotection.Journal of Photochemistry and Photobiology Part B: Biology,41: 1-10.
Subandrio A L,Sheldon I M,Noakes D E.2000.Peripheral and intrauterine neutrophil function in the cow: the influence of endogenous and exogenous sex steroid hormones.Theriogenology,53(8): 1591-1608.
Kamran A,Suna D M,Lain C.2014.Uterine physiology.Anaesthesia &Intensive Care Medicine,15(3): 133-135.
Vessey D A.1993.The cutaneous antioxidant system.Clinical Dermatology,8: 81-103.
Zhong R Z,Zhou D W.2013.Oxidative stress and role of natural plant derived antioxidants in animal reproduction.Journal of Integrative Agriculture,12(10): 1826-1838.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Transgenic DREB Toybean Dongnong50 on Diversity of Soil Nitrogen-fixing Bacteria
- SWOT Analysis and Countermeasures of Ecological Agricultural Development of Jianshan Farm
- Evolution Analysis About Soybean MIR166 Family
- Influence of FSH Treatment on Expression of CDC25A,TSSK3 and P53 in Vitro Cultured Sertoli Cells of Calf
- Antioxidant Activities of Nine Selected Culinary Spices from China
- Fault Line Selection Method Considering Grounding Fault Angle for Distribution Network
