THE FIRST RECORDS OF TRICHODINID ECTOPARASITES (CILIOPHORA, PERITRICHIA) FROM WILD MARINE FISHES IN THE SOUTH CHINA SEA
2015-02-27WANGWenQiangTANGFaHuiandZHAOYuanJun
WANG Wen-Qiang, TANG Fa-Hui and ZHAO Yuan-Jun
(Chongqing Key Laboratory of Animal Biology, Chongqing Normal University, Chongqing 401331, China)
THE FIRST RECORDS OF TRICHODINID ECTOPARASITES (CILIOPHORA, PERITRICHIA) FROM WILD MARINE FISHES IN THE SOUTH CHINA SEA
WANG Wen-Qiang, TANG Fa-Hui and ZHAO Yuan-Jun
(Chongqing Key Laboratory of Animal Biology, Chongqing Normal University, Chongqing 401331, China)
Several marine fishes were surveyed in the South China Sea, from which, four trichodind species (Ciliophora, Peritrichia) belonging to the genus Trichodina were isolated and studied. They are Trichodina puytoraci Lom, 1962, Trichodina japonica Imai, et al., 1991, Trichodina rectuncinata Raabe, 1958 and Trichodina fugu Imai, et al., 1997. This survey has revealed that Trichodina fugu Imai, et al., 1997 was the pathogen for the host Takifugu vermicularis and could cause mortality in wild condition. Taxonomic and morphometric data for these trichodinids based on dry silver nitrate-impregnated specimens are presented in the paper. To our best knowledge, this study is the first formal report on these trichodinids from the South China Sea.
Trichodina; First record; Marine fishes; South China Sea
As part research work of investigations of fish parasitology in China, the main aquatic parasites reported are mainly myxosporea and trichodinid groups and usually caused serious diseases in the recent findings[1—8]. Among them, trichodinid ciliates, as wellknown ectoparasites of fishes or mollusks, often parasitize on maricultured and freshwater animals. Up to date, more than 300 nominal trichodinid species have been reported from different environments around the world[6,9—15].
In China, a series of research works on trichodinids have been carried out in the recent fifteen years from all kinds of freshwater and marine environments[16—24]. Although some trichodinids have been found from the Bohai Bay and the Yellow Sea in China[20—24], in marine and brackish-water of China, trichodinid ciliophorans still remain a poorly studied group, and these ciliophorans from the South China Sea have been never reported and need to be further studied systematically.
In the present research work, we report four trichodinid species collected from wild marine fishes from the South China Sea, and compared them with previously reported population from other host fishes from other sea areas and discussed the possible reasons for their flourish, hoping to provide some valuable information of the pathogen Trichodina fugu Imai, et al., 1997 to the industry of mariculture and marine ecology.
1 Materials and methods
The wild host fishes (Gerres filamentosus Cuvier, 1829; Mugil cephalus Forskal, 1775; Leiognathus bindus Valenciennes, 1835; Takifugu vermicularis Temminck and Schlegel 1850) were caught in the South China Sea during March 2011 to June 2012 in Sanya City, China. The fishes were adults and no apparent symptom of disease, deformity or malnutrition to be found by eye inspect except some puffer fish (Takifugu vermicularis) in Dadonghai sea showed dermohemia, damaged gills, more mucus over the body and emptiness of the digestive track. Gills or tissue smears were prepared as air dried specimens from freshly collected fishes. These specimens were washed with distilled water to get rid of chloride ion, impregnated with 1% silver nitrate solution for 15min, exposed to incandescent light for 5min, examined under the LEICA DM750 microscope and microphotographed using LEICA DM6000B. The macronucleus morphology, the micronucleus position relative to the macronucleus and all measurements were performedfollowing the uniform specific characteristic system proposed by Lom (1958)[25]. Denticle characteristics were described following the method given by Lom (1958) and Van As and Basson (1989)[14,25]. Measurements were presented in mircometres (μm).
2 Results
Subclass Peritrichia Stein, 1859
Order Mobilida Kahl, 1933
Family Trichodinidae Claus, 1874
Genus Trichodina Ehrenberg, 1838
Trichodina japonica Imai, et al., 1991
Host and site: Gerres filamentosus (Cuvier, 1829), gills.
Locality: Yalong Bay (109.7°E, 18.2°N), Sanya City, China.
Body: Small-sized marine Tichodina species with diameter of 24.0—28.5 (26.0±1.75)
Adhesive disc: 21.0—26.0 (23.2±1.64) in diameter.
Denticle ring: 12.0—4.0 (13.1±0.85) in diameter.
Border membrane: Whitish, 1.0—1.7 (1.47±0.31) wide.
Number of denticles: 18—19.
Number of radial pins per denticle: 6—7.
Dimensions of denticle: Length: 2.5—3.0 (2.64± 0.15), blade length: 1.5—2.0 (1.84±0.17), central part length: 0.5—1.5 (0.99±0.15), ray: 2.5—3.0 (2.64± 0.15), span: 5.5—6.5 (5.95±0.14).
Nuclear apparatus: Macronucleus horseshoeshaped, micronucleus not observed.
Adoral spiral: About 380°.
Denticle morphology (PlateⅠ-1): Broad blade, fitting most space between Y+1 axis; round distal blade surface with curve to anterior blade surface and lower than tangent point; round and smooth blade tangent point; anterior and posterior surfaces convergent almost straightly and heavily to the centre; anterior surface closed to Y + 1 axis, invisible blade apophysis and posterior projection; slender central part with round point fitting loosely into preceding denticle and extending more than halfway to Y–1 axis; robust ray connection, tapering to the sharp point; absent ray apophysis.
Remarks. T. japonica was originally described by Imai, et al. (1991) from the gills of cultured Japanese eel, Anguilla japonica and redescribed by Xu, et al. (1999) from the gills of cultured percoids, Lateolabrax japonicus and Chrysophrys major in Qingdao, China[20,26]. Later, it was reported by Mitra and Bandyopadhyay from the gills of Lates calcarifer in India[27]. The population presented in our study is identical in morphometry and denticle shape to T. japonica originally described by Imai, et al.[26].
The present host Gerres filamentosus, which is a coastal inhabitant and collected from Yalong Bay of China, is a new host record for T. japonica. According to our study, T. japonica seems more likely to infect coastal marine fishes in Asia, as currently it is only seen in Asia and present in nearly the smallest population compared to other reported ones. Coincidently, it is also geographically distributed at the lowest latitude, which contributes to expanding its host range.
Trichodina puytoraci Lom, 1962
Host and site: Mugil cephalus (Forskal, 1775), gills.
Locality: Shore of Dadonghai (109.5°E,18.2°N), Sanya City, China.
Body: Medium-sized marine Tichodina speices, with diameter of 27.0—38.5 (33.76±3.18).
Adhesive disc: 23.7—34.4 (30.08±3.06) in diameter.
Denticle ring: 14.4—22.4 (18.78±2.45) in diameter.
Border membrane: Finely striated and 1.0—3.0 (1.67±0.56) wide.
Number of denticles: 20—25.
Number of radial pins per denticle: 7—9.
Dimensions of denticle: Length: 3.2—4.8 (3.89± 0.47), blade length: 2.3—4.2 (3.20±0.46), central part length: 0.7—1.9 (1.44±0.33), ray: 2.5—5.3 (3.65±0.83), span: 6.7—10.0 (8.67±0.95).
Nuclear apparatus: Macronucleus U-shaped; micronucleus oval, usually situated in +y position.
Adoral spiral: About 380°.
Denticle morphology (PlateⅠ-2): Broad blade with slightly falcate, fitting most space between Y+1 axis; truncated or flat distal blade surface parallel to border membrane when situated close to it and almost at the same level with tangent point; blunt and smooth tangent point; smoothly down-curved anterior surface almost touching Y+1 axis, forming a shallow apex; absent anterior and posterior blade apophysis; delicate blade connection; slender central part with blunt point fitting tightly with preceding denticle; similar sections above and below X-axis; straight ray with throughout same thickness, but thicker end and invisible round tip; visible ray apophsis. Posterior margin forms shallow, semilunar curve with deepest point lying lower than apex.
Remarks. T. puytoraci was originally described by Lom from the gills of Mugil auratus, Mugil salieus and Mugil cephalus from the Black Sea coast in Rumania (PlateⅠ-3). Based on its morphology, the present species was identified as T. puytoraci. However, it is comparatively smaller in size than the previously reported ones from Mugil auratus, Mugil saliens, Mugilplatanus and Mugil cephalus[9,28,29](Tab. 1). This species can be easily distinguished from other seawater trichodinids by the presence of several thickly dotted granules in the centre of adhesive disc, and by the truncated distal blade margin, widened and swollen tip of the ray and distinct ray apophysis. Moreover, T. puytoraci seems to have a narrower host range, because it was mainly found from the genus of Mugil Linnaeus, 1758. Thus, the identification is beyond doubt. This is the first report of T. puytoraci in the South China Sea, while the difference in body size from other populations from different sea areas in the world such as the Black Sea and Samborombón Bay, Argentina, is considerable. Our population has the smallest body size and denticle numbers compared with those found from areas with the lowest latitude. The different population variation within one trichodinid species from different regions or hosts can differ a lot, as the case that T. rectuncinata and T. puytoraci reported here from Mugil cephalus in the South China Sea apparently have denticles with smaller size than other populations described by Lom (1962)[9](Tab. 1).
Besides, another trichodinid species named as Trichodina chittagongesis was found to be similar to the present species (PlateⅠ-4). T. chittagongesis was described from the gills of Labeo bate from Karnaphuli River, India by Asmat, et al.[30]. It is remarked by Asmat, et al. that T. chittagongesis is distinguished from T. puytoraci in the aspects such as the distal margin, the tip of the ray, the ray apophysis etc., and shows high morphologic similarity with the original one described by Lom[9]. The relationship between T. chittagonesis and T. Puytoraci maybe needs to be clarified with more data (PlateⅠ-3) (Tab. 1).
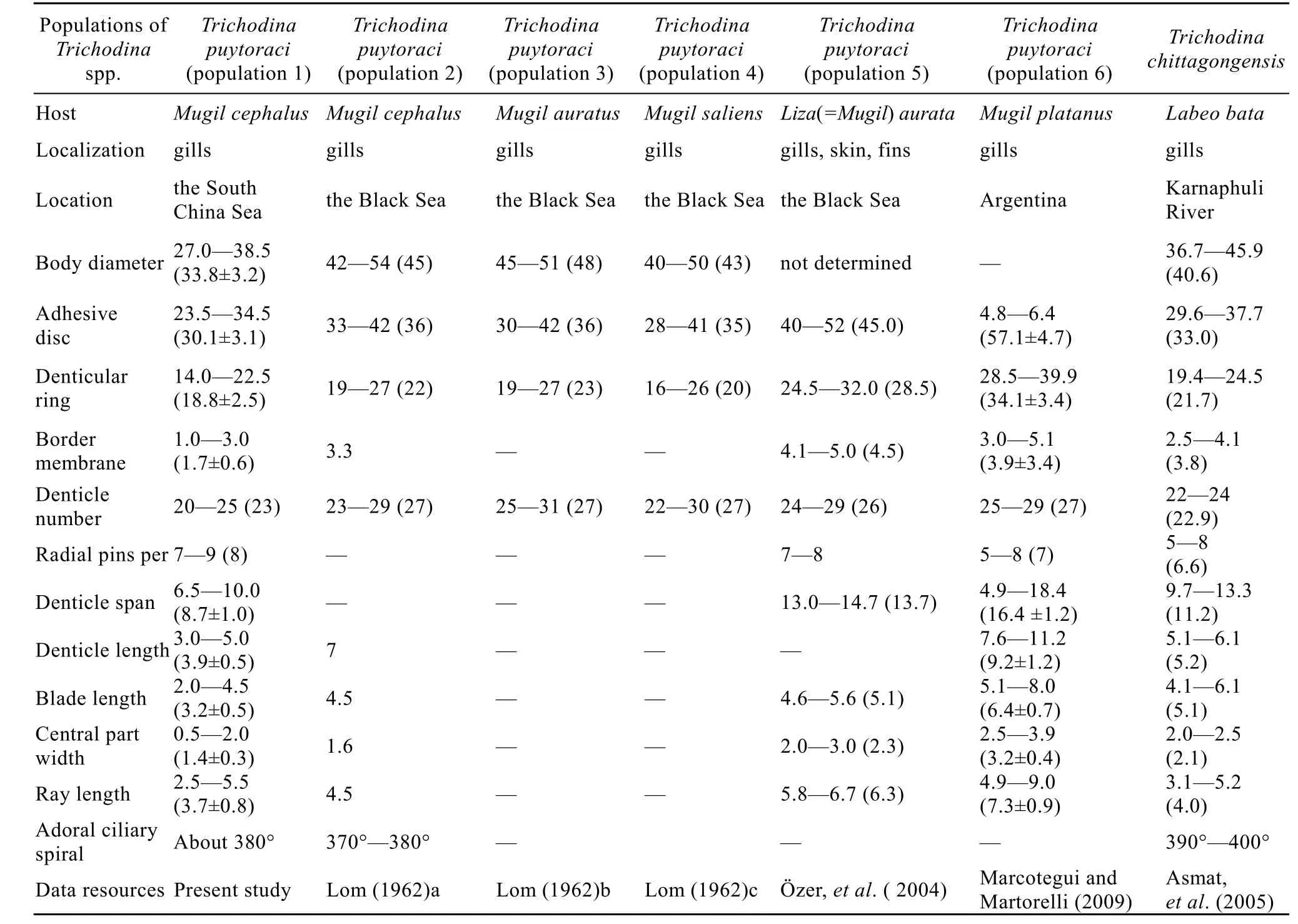
Tab. 1 Morphometric comparison of different populations for Trichodina puytoraci Lom, 1962 and Trichodina chittagongensis Asmat, et al., 2005
Trichodina rectuncinata Raabe, 1958
Host and site: Gerres filamentosus (Cuvier, 1829), gills. Leiognathus bindus (Valenciennes, 1835), gills.
Locality: G. filamentosus from Yalong Bay (109.7°E, 18.2°N), and L. bindus from reefs of Wuzhizhou Island (109.8°E,18.3°N), Sanya City, China.
The following descriptions are based on the specimens from L. bindus.
Body: Small-sized marine Tichodina species, with diameter of 18.9—24.9 (22.50±1.54).
Adhesive disc: 16.5—21.9 (19.79±1.48) in diameter.
Denticle ring: 8.5—12.80 (10.82±1.35) in diameter.
Border membrane: 0.9—1.7 (1.25±0.20) wide.
Number of denticles: 19—22.
Number of radial pins per denticle: 5—7.
Dimensions of denticle: Length: 1.6—3.1 (2.37± 0.44), blade: 2.0—4.0 (3.17±0.56), central part: 0.7—1.9 (1.04±0.28), ray: 0.4—0.9 (0.76±0.15), span: 4.0—6.6 (5.45±0.73).
Nuclear apparatus: Macronucleus C-shaped; micronucleus oval, usually situated in +y position.
Adoral spiral: About 400°.
Denticle morphology (Plate Ⅱ). Straight, triangular-shaped blade with round outline, fitting a small proportion between Y+1 axis; smooth, round distal blade surface higher than tangent point; round, bulbous and indistinct tangent point; almost straight anterior and posterior surfaces; posterior surface nearly on the Y axis; hardly determined blade apex and deepest curve point; absent anterior and posterior blade apophysis; wide blade connection; robust central part with blunt point fitting tightly with preceding denticle; flat and short ray, tapering directly backwards at the ends.
Remarks. T. rectuncinata is a worldwide marine trichodinid. It was originally described by Raabe from four species of Adriatic fishes and later reported by Lom on Gaidropsis mediterranaeus and Crenilabrus griseus from the Romanian coast off Black Sea and Hippocampus guttulatus and Blennius pholis from Dinard and Brittany coast of France[31]. Grupcheva, et al. studied T. rectuncinata from the gills of C. ocellatus, B. sanguinolentus, B. tentacularis, B. sphinx, B. gattorugine, Gobius guadrimaculatus, Syngnathus typhle argentatus, S. nigrolineatus, H. hippocampus microcoronatus from the Bulgarian Sea coast of the Black Sea and the Banyuls-sur-Mer coast of the Mediterranean Sea[32]. Later, Loubser, et al. described two populations from the Bay of Dakar, Senegal[33]. In China, only two different populations of this species were reported by Xu, et al. (2001) from the gills of Lateolabrax japonicus and Agrammus agrammus in the Yellow Sea and the Bohai Sea[22]. In our present research, another two populations of T. rectuncinata were isolated from the gills of Gerres filamentosus and Leiognathus bindus, respectively, and their morphological and morphometric data fit well within the range of original population of T. rectuncinata (Raabe, 1958)[31].
T. rectuncinata showed a wide variation range in denticle morphology. However, it can be easily recognized by the triangular blade with cavity in the adhesive disc centre although the blade cavity was not clearly visible in all specimens and the ray shape and width of the centre part differ a lot from other populations from other hosts or regions in the world. The hosts, G. filamentosus and L. bindus, are new records for T. rectuncinata, which expands the host range of this parasite (PlateⅡ).
Trichodina fugu Imai, et al., 1977
Based on the collection way, host fishes can be divided into two groups: Group A, fishes fishing in the sea, and Group B, dead or dying fishes on the shore, which were heavily infected with T. fugu. The following descriptions are based on the specimens from Group B, as there were more valid specimens in the group.
Host and site: Takifugu vermicularis (Temminck and Schlegel 1850), gills, body surface, fins, urogenital sinus.
Locality: Haitang Bay (109.5°E, 18.2°N) and Dadonghai Bay (109.8°E, 18.4°N), Sanya, China.
Body: Medium-sized, hat-shaped trichodinid with diameter of 33.0—53.7 (45.64±4.66).
Adhesive disc: 29.1—49.0 (41.30±5.08) in diameter.
Denticle ring: 20.2—34.1 (28.82±3.50) in diameter.
Border membrane: Finely striated and, 0.9—2.8 (1.59±0.45) wide.
Number of denticles: 24—30.
Number of radial pins per denticle: 7—9.
Dimensions of denticle: Length: 2.7—5.8 (4.51± 0.75), blade length: 3.6—5.5 (4.78± 0.45), central part length: 1.4—2.9 (2.21±0.42), ray: 3.3—6.6 (5.35±0.88), span: 9.6—14.8 (13.09±1.37).
Nuclear apparatus: Macronucleus horseshoe-shaped and micronucleus not observed.
Adoral spiral: about 400°.
Denticle morphology (Plate Ⅲ). Bar-shaped, narrow blades, filling a small portion between Y axes; smooth, round distal blade surface slightly higher than tangent point; round, bulbous and indistinct tangent point; straight anterior and posterior surfaces, nearly parallel to each other, making blade apex and deepest curve point difficult to determine; anterior surface far away from Y+1 axis; absent blade apophysis and prosterior projection; comparably robust, cylindershaped,central part fitting tightly into preceding denticle and extending to Y-1 axis; broad and short ray connection; not clearly visible ray apophysis; straight ray slanted more or less distinctively forward and with equal thickness to round points; ratio of denticle above axis to denticle below axis is about one (PlateⅢ- 9, 10).
Division. Several development stages of T. fugu were observed in silver-impregnated specimens. During binary fission, the cell split into two daughter-cellscontaining half the number of denticles compared with the mature individuals (Plate Ⅲ-11, 12). On the periphery, the new denticles are generated, gradually forming the blade, the central part, and the ray. The specimens also display the adhesive disc with a dark centre and no visible radical pins, and has a body diameter approximately half of that of the mature ones (Plate Ⅲ-12)
Remarks. The present described population of T. fugu found from Takifugu vermicularis in Sanya (the South China Sea, China) has coincident morphometric data of that originally from Takifugu rubripes from Japan described by Imai, et al.[34]. T. fugu was also discovered from Takifugu rubripes from Qingdao (the Yellow sea, China) by Xu, et al. (2007) and showed very high similarity with our population[24]. However, the denticle dimensions and number of our population are comparatively smaller than those of the populations from Nagasaki and Shizuoka by Imai, et al. (1997). Although Imai, et al. (1997) used formalin-fixed cells for silver impregnation[34], which made the adhesive disc of the species was not well impregnated, the number of the denticles was clear. The denticle number was 24—30 (27) (Sanya) and 23—30 (27) (Qingdao), smaller than those of 26—33 (29) (Nagasaki) and 29—35 (31) (Shizuoka) (Tab. 2).
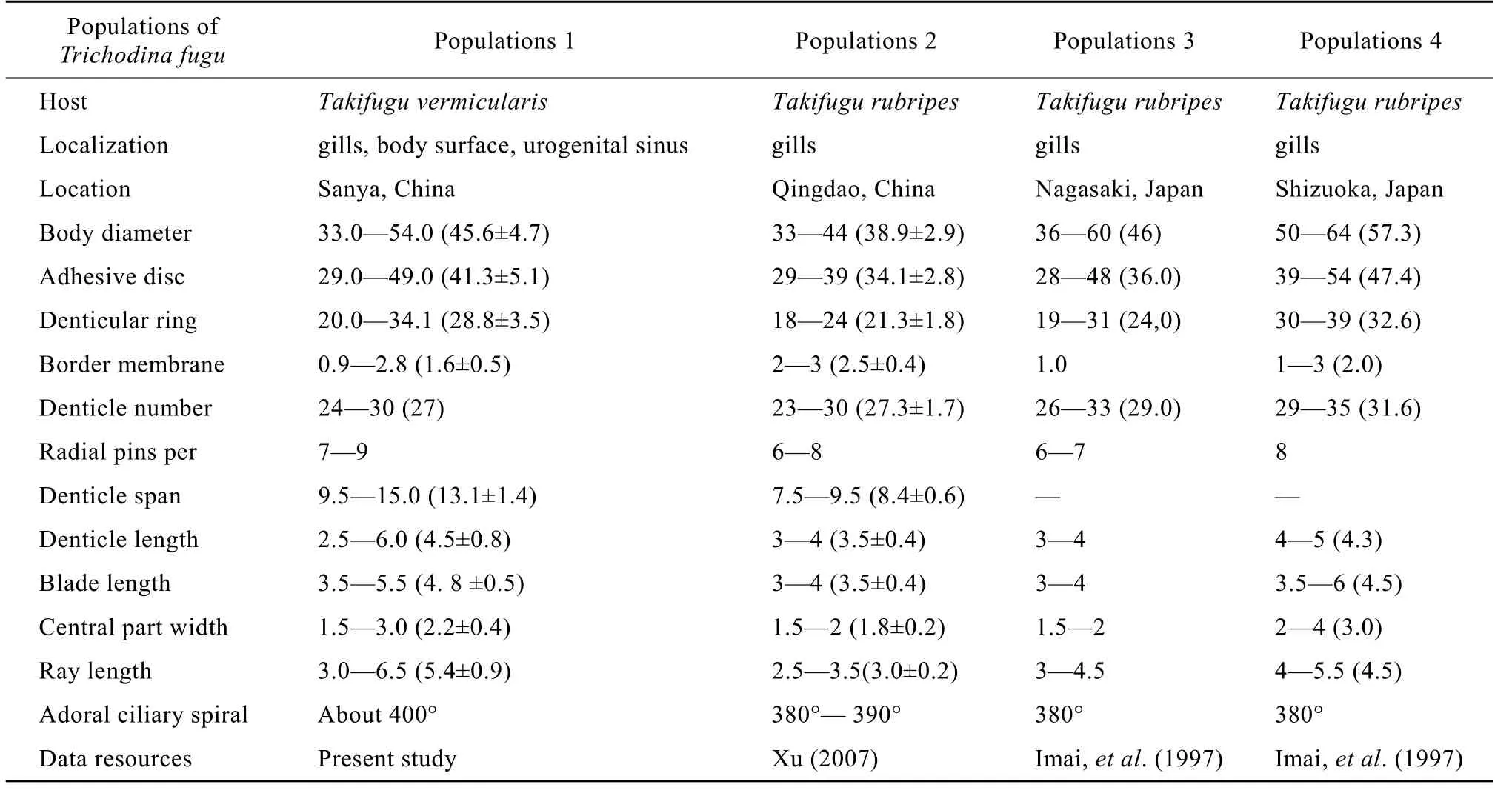
Tab. 2 Morphometric comparison (in micrometers) of different populations on Trichodina fugu Imai et al., 1997
T. fugu is similar to Trichodina urinaria Dogiel, 1940, Trichodina oviduct Poljansky, 1955 and Trichodina nephritica Lom, 1958, all of which are members of endozoic trichodinids. As noted by Kostenko and Karaev, the narrow denticle shape and higher denticle number are typical for endocommensal members of the genus, such as T. oviduct and T. nephritica. As expected, we found the species was parasitic on urogenital sinus. However, endohabitat is not the only choice for the species. Besides, the denticle number is apparently smaller than that of T. oviduct and T. nephritica. Last, it is not a commensal species but a pathogen for purple puffer.
In general, the outbreak of trichodinids in aquaculture is due to high host population density, eutrophication or poor water quality. We speculate that both high host population density and water condition are responsible for the death of puffer fish. As mentioned by Xu (2007), T. fugu seems to have rather narrow host range and has only been found from the tiger puffer Takifugu rubripes. In Takifugu rubripes (by Xu), the intensity of T. fugu is lower than other Trichodina species[24]. However, in our study, T. fugu is the only parasite and has extremely high intensity (more than 20 individual trichodinids could be found per slide) in diseased fish Takifugu vermicularis, which proved that Takifugu vermicularis is more vulnerable to T. fugu. Furthermore, T. vermicularis was heavily infected with T. fugu, leading to the host mortality in wild environment. Mortality of other fishes caused by trichodinds have also been observed under controlled experiments in some study[35,36], in which most of them are ectocommensal on fish while feed on suspended bacteria.
Parasitic specificity could control the population of a species not being too large. It is well-known that the dynamic of infectious diseases are related to the density of host populations. In Sanya, however, the population of Takifugu vermicularis can not be that high, because many fisheries are fully or over-exploited and the mariculture is not corelated in the region. Thus, the number of both the puffer fish and its prey are reduced recently. In spite of this, it is still possible that there are other cultured fishes that share the parasites of T. fugu, but are not as vulnerable as the puffer fish, Tiger puffer, such as Takifugu rubripes, a breeding object in some Asia fiery. On the other hand, unlike other microparasites such as viruses and bacteria, which do not have free-living stage in their life cycle, the trichodinids can spread more than by hostto-host contact, as previously proved by other authors that the trichodinids only can temporarily leave the host to find new individual host. Moreover, aquatic micro-parasites may also be transported by water movement[37,38]. In other parts of the coastal South China Sea, such as Wanning City, which is 120 kilometers away from Sanya, the number of farmed marine fish increases rapidly with aquaculture growth. Marine aquaculture facilities are typically open to the surrounding ecosystem and, therefore, wild and farmed populations are connected by their shared parasite. Furthermore, protististic parasites have already caused serious problems in marine aquaculture in Wanning. If this is true, the influence of aquaculture on the marine ecosystems may be more serious.
Because trichodinids are not fed on their hosts, so the flourish of the parasite may not always in accordance with the population density of the hosts. In eutrophic water, the high concentration of bacteria and suspended particles in the water flourished the parasites, and the parasites infected hosts. Incidentally, the dead puffer fish (with seriously infected with trichodinids) was found in Dadonghai where the on-boardrestaurants dumped food scrap directly to the sea in the present research, therefore, the water condition should be taken into consideration.
3 Discussion
Based on the morphometric data and geographical distribution of each population for T. japonica, T. puytoraci and T. fugu, we found an interesting phenomenon that the body size and denticle number seem to be enlarged with the increase of latitude. The relationship between size and latitude is harder to show by the data of T. rectuncinata, as previous studies revealed considerable variations in morphometric data and in denticle morphology of the species. Grupcheva, et al. (1989) and Loubser, et al. (1995) suggested that T. rectuncinata requires comprehensive revision[32,33].
As is vividly depicted in the chart (Fig. 1, 2), the overall trend shows that the size of trichodinid and the denticle number increase with the latitude. However, regularity is not clear possibly due to the following reasons. On the one hand, the latitude is only one of several factors influencing the body size of trichodinid, as marine environment is very complex and the change of the environment and faunas are not strictly followed by the increase of latitude. On the other hand, some populations reported previously are from cultured fishes imported from other places, and the trichodinid can translocate via the introduction of their fish hosts. Last, trichodinid in the present study are more related to the cultured fish or experimental condition. Thus, more research and data are needed to reveal a clearer trend for supporting our hypothesis.
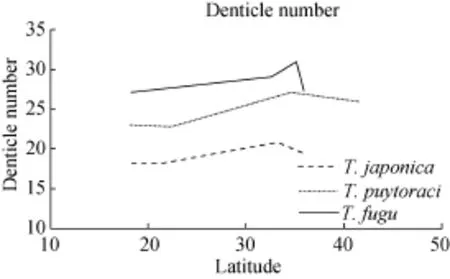
Fig. 1 The relationship between denticle number and geographical distribution (with the increase of latitude)
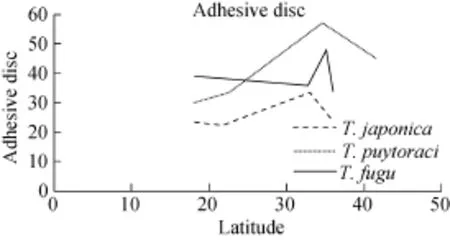
Fig. 2 The relationship between adhesive disc and geographical distribution (with the increase of latitude)
In brief, each trichodinid population in our work is almost the smallest one compared to other high latitude populations. Their hosts are wild fishes in the South China Sea, therefore, the study could reveal the relationship among geographical distribution and intra-specific variation to some extent.
[1] Liu Y, Whipps C M, Gu Z M, et al. Myxobolus honghuensis n. sp. (Myxosporea: Bivalvulida) parasitizing the pharynx of allogynogenetic gibel carp Carassius auratus gibelio (Bloch) from Honghu Lake, China [J]. Parasitology Research, 2012, 110(4): 1331—1336
[2] Tang F H, Zhao Y J. Taxonomic study on three species of Trichodina Ehrenberg, 1838 with pathologic research on gill tissue of Carassius auratus caused by Trichodina heterodentata Duncan, 1977. A study on trichodinids from freshwater fishes in Chongqing II [J]. Journal of Chongqing Normal University, 2007, 24(3): 8—12 [唐发辉, 莙赵元 .三种鲫鱼外寄生车轮虫车轮虫分类学及异齿车轮虫致鳃组织病理学研究——重庆地区淡水车轮虫研究Ⅱ. 重庆师范大学学报(自然科学版).2007, 24(3): 8—12]
[3] Tang F H, Zhao Y J. Study of trichodinids (Protozoa, Ciliophora) parasitic on gills of freshwater fishes from Chongqing, China, and identification of a new species, Trichodina cyprinocola sp. nov [J]. African Journal of Microbiology Research, 2011, 5(26): 5523—5527
[4] Tang F H, Zhao Y J, Liu C N. Two trichodinids of Paratrichodina Lom, 1963 (Ciliophora, Peritrichida, Trichodinidae) infecting gills of Ietalurus punetaus from Chongqing, China [J]. African Journal of Microbiology Research, 2012, 6(9): 2145—2149
[5] Tang F H, Zhao Y J, Warren A. Phylogenetic analyses of trichodinids (Ciliophora, Oligohymenophora) inferred from 18S rRNA gene sequence data [J]. Current Microbiology, 2013, 66(3): 306—313
[6] Song W B. Pathogenic Protozoa in Mariculture [M]. Beijing: Science Press. 2003, 429—483 [宋微波. 海水养殖中的危害性原生动物. 北京: 科学出版社. 2003, 429—483]
[7] Zhao Y J, Tang F H. Trichodinid ectoparasites (Ciliophora: peritricha) from Misgurnus anguillicaudatus (Cantor) and Anodonta woodiana (lea) in China, with descriptions of two new species of Trichodina Ehrenberg, 1838 [J]. Systematic Parasitology, 2007, 67: 65—72
[8] Zhao Y J, Li N N, Tang F H, et al. Remarks on the validity of Myxobolus ampullicapsulatus and Myxobolus honghuensis (Myxozoa: Myxosporea) based on SSU rDNA sequences [J]. Parasitology Research, 2013, 112(11): 3817—3823
[9] Lom J. Trichodinid ciliates from fishes of the Rumanian Black Sea Coast [J]. Parasitology, 1962, 52(1-2): 49—61
[10] Lom J. Trichodinid ciliates (Peritrichida: Urceolariidae) from some marine fishes [J]. Folia Parasitologica, 1970, 17: 113—125
[11] Lom J, Haldar D P. Ciliates of the genera Trichodinella, Tripartiella and Paratrichodina (Peritricha, Mobilina) invading fish gills [J]. Folia Parasitologica, 1977, 24: 193—210
[12] Lom J, Hoffman J L. Geographical distribution of some species of Trichodinids (Ciliata: Peritricha) parasitic on fishes [J]. Parasitology, 1964, 50(1): 30—35
[13] Lom J, Laird M. Parasitic protozoa from marine and euryhaline fish of Newfoundland and New Brunswick. I. Peritrichous ciliates [J]. Canadian Journal of Zoology, 1969, 47(6): 1367—1380
[14] Van As J G, Basson L. A further contribution to the taxonomy of Trichodinidae (Ciliophora: peritricha) and a review of the taxonomic status of some fish ectoparasitic trichodinids [J]. Systematic Parasitology, 1989, 14(3): 157—179
[15] Van As J G, Basson L. Trichodinid ectoparasites (Ciliophora: Pertrichida) of freshwater fishes of the Zambesi River System, with a reappraisal of host specificity [J]. Systematic Parasitology, 1992, 22(2): 81—109
[16] Tang F H, Zhao Y J, Chen H. Trichodinid ectoparasites from golden carp, with a description of Trichodina paranigra sp. nov [J]. Acta Hydrobiologica Sinica, 2005, 29(1): 75—80 [唐发辉, 莙赵元 , 陈辉. 鲫寄生车轮虫一新种的描述. 水生生物学报, 2005, 29(1): 75—80]
[17] Tang F H, Zhao Y J, Tang A K. Presence of ectoparasitic trichodinids (Ciliophora, Oligohymenophorea, Peritrichida) on the gills of cultured freshwater fish, Carassius auratus in Chongqing, China, with the the description of a new species of the genus Trichodina [J]. Acta Zootaxonomica Sinica, 2005, 30(1): 35—40
[18] Tang F H, Zhao Y J, Tao Y F. Trichodinids (Ciliophora: Peritrichida) parasitic on gills of freshwater fishes, Carassius auratus and Aristichthys nobilis from China, with the description of Trichodina subtilihamata sp. nov [J]. Zootaxa, 2007, 1582: 39—48
[19] Tang F H, Zhao Y J, Liu C N. First records of three Tripartiella species (Ciliophora, Oligohymenophora, Peritrichida) from freshwater fishes along Yangtze River in China [J]. Zootaxa, 2013, 3681: 169—174
[20] Xu K D, Song W B, Warren A. Trichodinid etoparasites (Ciliophora: Peritrichida) from the gills of cultured marine fishes in China, with the description of Trichodinella lomi n. sp [J]. Systematic Parasitology, 1999, 42(3): 219—227
[21] Xu K D, Song W B, Warren A. Observations on trichodinid ectoparasites (Ciliophora: Peritricha) from the gills of maricultured molluscs in China, with descriptions of three new species of Trichodina Ehrenberg, 1838 [J]. Systematic Parasitology, 2000, 45(1): 17—24
[22] Xu K D, Song W B, Warren A, et al. Trichodinid ectoparasites of some marine fishes from coastal regions of the Yellow and Bohai Sea [J]. Systematic Parasitology, 2001, 50: 69—79
[23] Xu K D, Song W B, Warren A. Taxonomy of trichodinids from the gills of marine fishes in coastal regions of the Yellow Sea, with descriptions of two new species of Trichodina Ehrenberg, 1830 (Protozoa: Ciliophora: Peritrichia) [J]. Systematic Parasitology, 2002, 51: 107—120
[24] Xu K D. Trichodinid ectoparasites (Ciliophora, Peritrichia) from the tiger puffer Takifugu rubripes in the Yellow Sea, with revision of Trichodina jadranica Raabe, 1958 [J]. Acta Protozoology, 2007, 46(4): 311—324
[25] Lom J. A contribution to the systematics and morphology of endoparasitic trichodinids from amphibians, with a proposal of uniform specific characters [J]. Journal of Protozoology, 1958, 5(4): 251—263
[26] Imai S, Miyazaki H, Nomura K. Trichodinid species from the gills of cultured Japanese eel, Anguilla japonica, with the description of a new species based on light and scanning electron microscopy [J]. European Journal of Protistology, 1991, 27(1): 79—84
[27] Mitra A K, Bandyopadhyay P K. First records of Trichodina japonica Imai, Miyazaki et Nomura 1991 and Trichodina mutabilis Kazubski et Migala 1968 (Ciliophora, Trichodinidae) from Indian fishes [J]. Protistology, 2005, 4(2): 121—127
[28] Özer A, Öztürk T. Trichodina puytoraci Lom, 1962 and Trichodina lepsii Lom, 1962 (Peritrichia: Ciliophora) infestations on mugilids caught at the Black Sea coast of Sinop Turkey [J]. Turkish Journal of Zoology, 2004, 28(2): 179—182
[29] Marcotegui P S, Martorelli S R. Trichodinids (Ciliophora: Peritrichida) of Mugil platanus (Mugiliformes: Mugilidae) and Micropogonias furnieri (Perciformes: Sciaenidae) from Samborombon Bay, Argentina, with the description of a new species [J]. Folia Parasitologica, 2009, 56(3): 167—172
[30] Asmat G S M, Afroz F, Mohammad N. Four new species of Trichodina Ehrenberg, 1830 (Ciliophora: Trichodinidae) from Bangladeshi fishes [J]. Research Journal of Agriculture and Biological Sciences, 2005, 1(1): 23—29
[31] Raabe Z. On some species of Trichodina (Cialiata–Peritricha) of gills of Adriatic fishes [J]. Acta Parasitologica Polonica, 1958, 6: 355—362
[32] Grupcheva G, Lom J, Dykova I. Trichodinids (Ciliate: Urceolaridae) from gills of some marine fishes with the description of Trichodina zaikai sp. n [J]. Folia Parasitologica, 1989, 36(3): 193—207
[33] Loubser G J. Trichodinid ectoparasites (Ciliophora: Peritrichida) of some fishes from the Bay of Dakar, Senegal (West Africa) [J]. Acta Protozoology, 1995, 34(3): 211—216
[34] Imai S, Inouye K, Kotani T, et al. Two trichodinid species from the gills of cultured tiger puffer, Takifugu rubripes, in Japan, with the description of new species [J]. Fish Pathology, 1997, 32(1): 1—6
[35] Subasinghe R P. Effects of controlled infections of Trichodina sp. on transmission of epizootic ulcerative syndrome (EUS) to naive snakehead, Ophicephalus straitus [J]. Bloch Journal Fish Disease, 1997, 16(2): 161—164
[36] Obiekezie A I, Ekanem D A. Experimental infection of Heterobranchus longifilis with Trichodina maritinkae (Ciliophora: Peritrichida) [J]. Aquatic Living Resources, 1995, 8(4): 439—443
[37] Gustafson L L, et al. Hydrographics and the timing of infectious salmon anemia outbreaks among Atlantic salmon (Salmo salar L.) farms in the Quoddy region of Maine, USA and New Brunswick, Canada [J]. Preventive Veterinary Medicine, 2007, 78(1): 35—56
[38] Viljugrein H, et al. Integration of hydrodynamics into a statistical model on the spread of pancreas disease (PD) in salmon farming [J]. Diseases of Aquatic Organisms, 2009, 88(1): 35—44
Q959.117
A
1000-3207(2015)03-0564-10
10.7541/2015.74
Received date: 2014-08-27; Accepted date: 2014-11-09
Foundation item: The National Natural Science Foundation of China (No. 31101637, No. 31172068); the Project of Chongqing Science & Technology Commission (No. CSTC, 2010CA1010; No. cstc2014jcyjA80008); the Science Research Foundation of the Education Committee of Chongqing (No. KJ1400530)
Brief introduction of author: Wang Wen-Qiang (1986—), male, Chengdu, China; Master’s degree graduates; mainly engaged in Fish Parasitology. E-mail: 181827190@qq.com
Zhao Yuan-Jun, E-mail: zhaoyuanjuncqnu@126.com
