Effi cacy and safety of glucocorticoids in the treatment of community-acquired pneumonia: A meta-analysis of randomized controlled trials
2015-02-08LipingChenJunhuiChen2YingChenChaoWuXiaohongYang
Li-ping Chen, Jun-hui Chen2, Ying Chen, Chao Wu, Xiao-hong Yang
1Department of Respiratory and Critical Care Medicine, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi 830001, China
2Department of Pharmacy, Sixth Affi liated Hospital of Xinjiang Medical University, Urumqi 830002, China
Corresponding Author:Xiao-hong Yang, Email: clp651@126.com
Meta-analysis
Effi cacy and safety of glucocorticoids in the treatment of community-acquired pneumonia: A meta-analysis of randomized controlled trials
Li-ping Chen1, Jun-hui Chen2, Ying Chen1, Chao Wu1, Xiao-hong Yang1
1Department of Respiratory and Critical Care Medicine, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi 830001, China
2Department of Pharmacy, Sixth Affi liated Hospital of Xinjiang Medical University, Urumqi 830002, China
Corresponding Author:Xiao-hong Yang, Email: clp651@126.com
BACKGROUND:Community-acquired pneumonia (CAP) is pneumonia acquired infectiously from normal social contact as opposed to being acquired during hospitalization. CAP is a leading cause of illness and death. This review aims to determine the effi cacy and safety of glucocorticoids in the treatment of community-acquired pneumonia (CAP).
DATA SOURCES:We searched randomized controlled trials (RCTs) from Pubmed, EMBASE, Cochrane Library, Chinese Journal Full-text Database, and Chinese Biomedical Literature Database to obtain the information by using steroids, glucocorticoids, cortisol, corticosteroids, community-acquired pneumonia and CAP as key words. The quality of RCTs was evaluated. A Meta-analysis was made using RevMan 5.0 provided by the Cochrance Collaboration.
RESULTS: Seven RCTs involving 944 patients were included in the meta-analysis. The mean length of hospital stay in glucocorticoids treatment group was significantly shorter than that in standard treatment group (WMD=–1.70, 95%CI 2.01–1.39, Z=10.81, P<0.00001). No statistically significant differences were found in the mortality rate (RR=0.77,95%CI 0.46–1.27, Z=1.03, P=0.30), the mean length of hospital stay in ICU (WMD=1.17, 95%CI 1.68–4.02, Z=0.81, P=0.42), the incidence of super infection (RR=1.32, 95%CI 0.66–2.63, Z=0.79, P=0.43), the incidence of hyperglycemia (RR=1.84, 95%CI 0.76–4.41, Z=1.36, P=0.17), the incidence of upper gastrointestinal bleeding (RR=1.98, 95%CI 0.37–10.59, Z=0.80, P=0.42) between the standard treatment group and the glucocorticoids treatment group.
CONCLUSIONS:The use of glucocorticoids in patients with community-acquired pneumonia can signifi cantly shorten the duration of illness and have a favorable safety profi le. However, it could not reduce the overall mortality.
Glucocorticoids; Community-acquired pneumonia; Meta-analysis
INTRODUCTION
Community-acquired pneumonia (CAP) is pneumonia acquired infectiously from normal social contact as opposed to being acquired during hospitalization. Causes of CAP include bacteria, viruses, fungi, and parasites. CAP can be diagnosed simply by symptoms and physical examination or by X-rays, sputum test and others. CAP is a leading cause of illness and death. Its morbidity in the general population is 2‰–12‰, whereas its mortality is up to 20%–50%.[1]CAP is primarily treated with antibiotics. In recent years, treatments including administration of high-efficient broad spectrum antibiotics and mechanical ventilation have been widely used clinically, but there was no marked decrease ofthe CAP mortality.[2–4]Therefore, researches of CAP treatment are turning to the subsidiary measure of nonantibiotics. Experiments have shown that the key factor for poor prognosis of CAP patient is the excessive immune-inflammatory reactions of the lungs and the whole body. Moreover, adrenal insufficiency has been diagnosed in gravis CAP patients, which is related to severity of the disease and its poor prognosis.[5,6]Glucocorticoids (GCS) which have pronounced effects on inflammation and adrenal insufficiency remain to be the key adjuvant medicine. However, the use of GCS for the treatment of CAP remains debatable as it may disguise disease process to complicated diagnosis and delayed treatment, resulting in untoward reaction of GCS. Hence, this study aims to evaluate the relevant results from randomized controlled trials (RCTs) that have been published in recent years.
METHODS
Inclusion criteria
The included were patients aged more than 18 years old who had been diagnosed with CAP in accordance with China CAP Guidelines and the guidelines of the American Thoracic Society (ATS). The patients were treated at the emergency room or hospitalized.
Exclusion criteria
Clinical studies involved CAP patients with HIV and those with hospital acquired pneumonia were excluded. Studies with limited information, inaccurate number of cases, literature reviews, case reports, etc were also excluded.
Interventions
GCS combined with conventional antibiotic treatment or conventional antibiotic treatment only was given to the patients.
Measurement index
The mortality, lengh of hospital stay, lengh of ICU stay, occurrence of adverse reaction, including super infection, upper gastrointestinal bleeding (UGB), hyperglycemia were measured.
Retrieval method
A literature search was conducted with the following key words such as steroids, glucocorticoids, cortisol, corticosteroids, community-acquired pneumonia, CAP from Pubmed (January 1966 to December 2012), Embase (January 1974 to September 2012), and Cochrane Library (2009, 3rd). These words were also searched from Wanfang Database (January 1982 to September 2012), Chinese Journal Full-text Database (January 1979 to September 2012) and Chinese Biomedical Study Database, and CBM (January 1978 to September 2012). Pubmed and Embase retrieval is limited to clinical studies. Searching language is limited to Chinese and English.
Study screening and data extraction
Two researchers separately screened the studies, collected and cross-checked data, and distinguished differences through discussion with another researcher. Missing materials were supplemented by contacting the authors through telephone or mails. Collected information mainly consisted of name of the authors, publication date, study resource, size of participating centers, type of the study design, sample size, description of intervention, location of diagnosis and treatment as well as observational indices.
Quality evaluation
Quality evaluation was done according to the quality evaluation standard recommended by Cochrane handbook 5.0: (1) whether the randomized method is correct or not; (2) whether allocation concealment method is used or not; (3) whether blinding or not is adopted; (4) whether there is bias caused by data deficient or not; (5) whether there is bias caused by selective report or not; (6) whether there are other types of biases or not. Each quality standard will be divided into "yes", "no" and "not clear".
Statistical treatment
Meta-analysis was made using RevMan 5.0 provided by the Cochrance Collaboration Center. Enumeration data evaluation adopted relative incidence (RR). When each document used the same measurement and unit to one index, measurement data counted weighted mean difference (WMD). Each effect size was expressed in 95% confidence interval (CI). The heterogeneity of each study was evaluated using the chi-square test. If there was no heterogeneity (P<0.1, I2>50%) between two studies, a fixed effect model was used for analysis; if there was heterogeneity (P<0.1, I2>50%), the data source and associated factors were analyzed. If there is statistical heterogeneity without clinical heterogeneityand statistical significance between studies, a random effect model was used for analysis. If the heterogeneity between the two groups was too large to search for the data source, descriptive analysis was used.
RESULTS
Search results
According to the designated searching method, 2 471 relevant articles were retrieved in initial stage. Irrelevant articles which were eliminated after a review included essays, reviews, mails, meeting reports and repeated study reports. Thirteen articles were selected. After full-text review and in-depth information evaluation, the unstudied-researches, nonclinical study, medical interventions unrelated to GCS and studies with incomplete data were excluded. Final analysis included 7 articles that met the inclusion criteria.
Quality evaluation and general characteristics of the included studies
The quality evaluation of 7 articles is shown in Table 1 and their general characteristics are shown in Table 2. All articles reported the results from randomized studies, and random concealment was inadequate. In three studies[8,10,11]a single-blind method was used and a total of 944 patients were included.
Meta-analysis
Improvement of mortality
Six of the seven articles described the mortality of community-acquired pneumonia in both GCS treatment group and conventional treatment group, and they were subjected to meta-analysis. The heterogeneity (P=0.47, I2=0%) was not estimated using a fixedeffect mode. Compared to the conventional treatment group, the mortality of the GCS treatment group was reduced (RR=0.77, 95%CI 0.46–1.27), but the difference was not statistically significant (Z=1.03, P=0.30) (Figure 1).
Mean length of hospital stay
The mean length of hospital stay was described in six of the seven studies of CAP patients from both treatment groups. The heterogeneity (P=0.04, I2=56%) was estimated using a fixed-effect model. The mean length of hospital stay of CAP patients in the GCS treatment group was shorter (WMD=–1.70, 95%CI –2.01 to –1.39) than that in the conventional treatment group (Z=10.81, P<0.01) (Figure 2).
Because of the heterogeneity in the six studies, a random effect model was used to re-analyze the study results for further verification of the stability of metaanalysis. The results showed that the mean length of hospital stay of CAP patients from the GCS treatmentgroup was shorter than that from the conventional treatment group (WMD=–1.66, 95%CI –2.37 to –0.94) (Z=10.81, P<0.01).

Table 2. The general characteristics of the studies included
Mean length of hospital stay in ICU
Three of the seven studies described the mean length of hospital stay in ICU for both groups. The heterogeneity (P=0.07, I2=62%) was estimated using a fixed-effect model. The mean length of hospital stay in ICU for the GCS treatment group was longer than that for the conventional treatment group (WMD=1.17, 95%CI –1.68 to 4.02), but there was no significant difference (Z=0.81, P=0.42) (Figure 3).
Because of the heterogeneity in the three studies, a random effect model was adopted to re-analyze for further verification of the stability of the result from meta-analysis. The mean length of hospital stay of CAP patients from the GCS treatment group was shorter than that from the conventional treatment group (WMD =1.17, 95%CI –4.39 to 6.73), but there was no significant difference (Z=0.41, P=0.68).Super infection
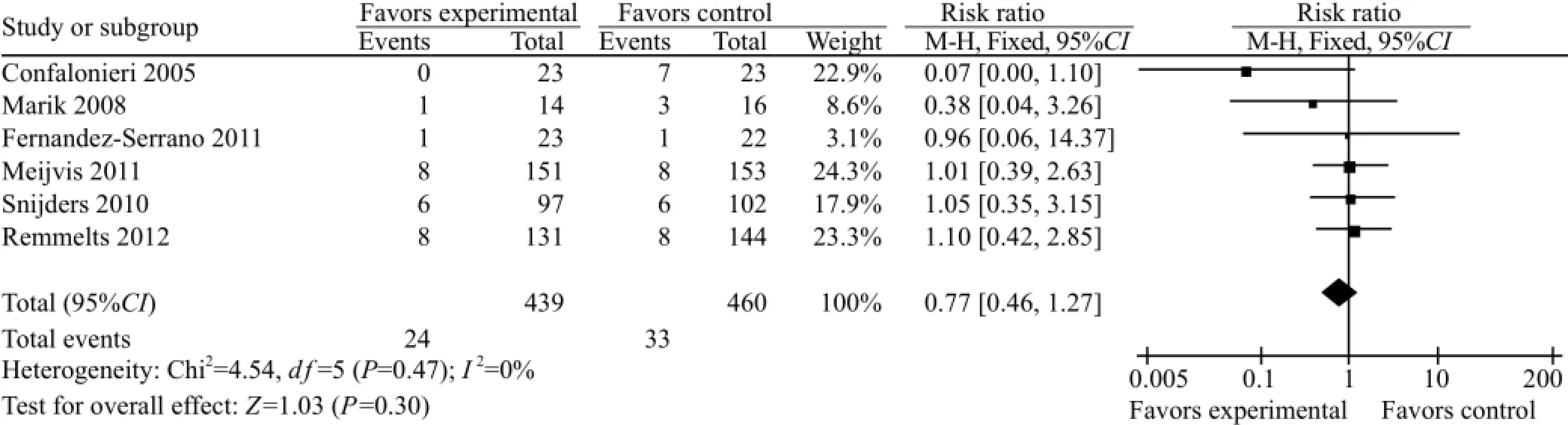
Figure 1. Comparison of mortality between the GCS treatment group and standard treatment group.
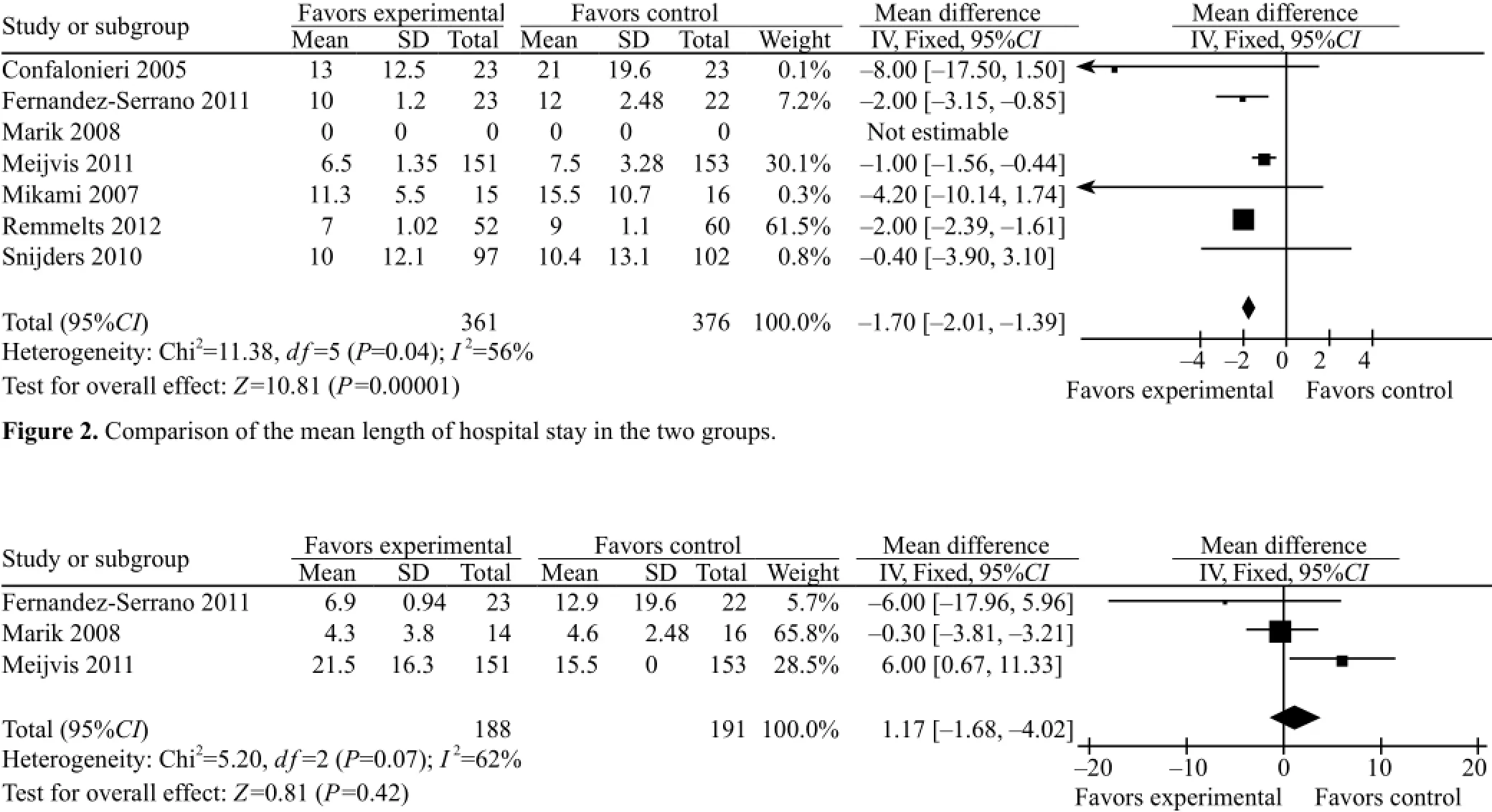
Figure 3. Comparison of the mean length of hospital stay at ICU in the two groups.
Three of the seven studies described the incidence of super infection in both groups. The meta-analysis included these three studies. The heterogeneity (P=0.11,I2=55%) was estimated using a fixed-effect model. The incidence of super infection in the GCS treatment group was higher than that in the conventional treatment group (RR=1.32, 95%CI 0.66 to 2.63), but there was no signifi cant difference (Z=0.79, P=0.43) (Figure 4).
Owing to the heterogeneity in the 3 studies, a random effect model was adopted to re-analyze for further verifi cation of the stability of the result from this meta-analysis. The incidence of super infection of CAP patients from the GCS treatment group was higher than that from the conventional treatment group (RR=1.25, 95%CI 0.35 to 4.45), but there was no significant difference (Z=0.34, P=0.73).
Hyperglycemia
Three of the seven studies described the incidence of hyperglycemia (HG) in the two groups. The metaanalysis analyzed the data from the 3 studies. The heterogeneity (P=0.79, I2=0) was not estimated using a fixed-effect model. The incidence of HG in the GCS treatment group was higher than that in the conventional treatment group (RR=1.84, 95%CI 0.76 to 4.41), but the difference was not statistically significant (Z=1.36, P=0.17) (Figure 5).
Upper gastrointestinal bleeding
Three of the 7 studies described the incidence of upper gastrointestinal bleeding in both treatment groups. The meta-analysis included the 3 studies. And the heterogeneity (P=0.83, I2=0%) was not estimated using a fixed-effect model. The incidence of upper gastrointestinal bleeding in the GCS treatment group was higher than that in the conventional treatment group (RR=1.98, 95%CI 0.37 to 10.59), but there wasno statistically significant difference (Z=1.36, P=0.17) (Figure 6).
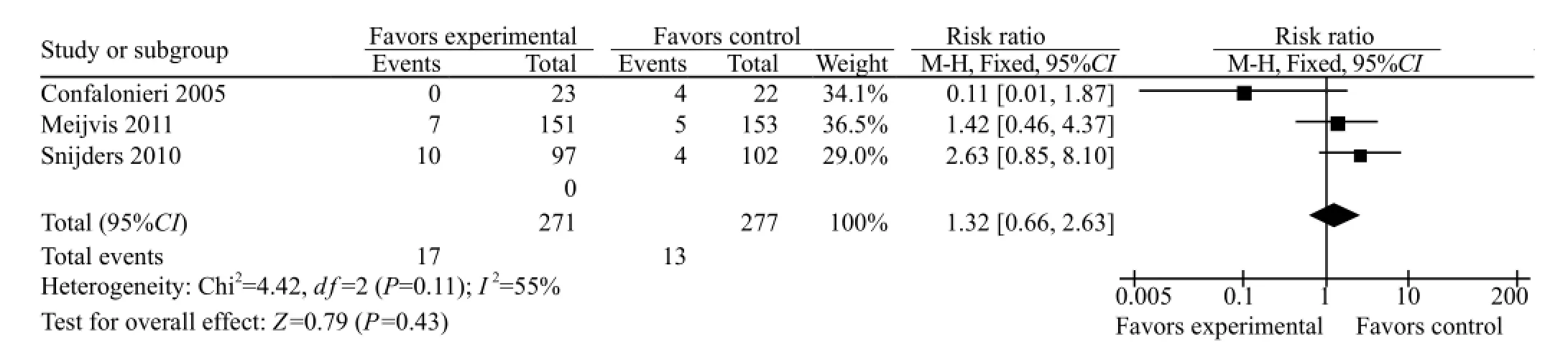
Figure 4. Comparison of the incidence of super infection in the two groups.

Figure 5. Comparison of the incidence of hyperglycemia in the two groups.

Figure 6. Comparison of the incidence of upper gastrointestinal bleeding in the two groups.
DISCUSSION
The infl ammatory response of the host is a key factor determining the prognosis in CAP, which aims to destroy microorganism and control infection, but excessive inflammatory response is harmful to the host, resulting in early in-hospital mortality in patients with CAP. GCS as the most effective anti-inflammatory agents may be effective for severe CAP patients with adrenal insuffi ciency. Systemic GCS treatment is recommended in consensus guidelines of the Infectious Diseases Society of America/American Thoracic Society on the management of severe CAP in patients, but the evidence was from small sample sizes in RCTs.
A systematic review showed that administration of GCS in patients with severe CAP was associated with a lower mortality rate than those treated with placebo.[14]The current meta-analysis demonstrated that GCS treatment decreased the mortality of CAP, but no significant difference was detected. GCS treatment could shorten the length of hospital stay (LOS), but didn’t shorten the length of ICU stay of patients with severe CAP. This fi nding might be due to the insuffi cient information from primary publications and the accuracy of the contents. Therapeutic benefi t and safety could be expected during the treatment of severe CAP with GCS; however, severe side effects such as super infection, hyperglycemia, gastroduodenal bleeding, and muscle weakness after prolonged GCS treatment may occur in patients with severe CAP. There was no statistically significant difference in adverse effect in patients treated with GCS compared with patients treated with standard methods, indicating that the safety of GCS treatment as an adjunctive therapy for CAP.
In this meta-analysis, 944 patients were hospitalized for CAP, including those with mild to severe CAP. Adrenal function was not assessed in most RCTs. In addition, the doses and duration of GCS treatment were different among the studies, which contributed to a significant clinical heterogeneity in systematic evaluation if no subgroup analysis was performed in the RCTs. Heterogeneity was found in pooled analysis on the length of hospital stay, the length of ICU stay and super infection with GCS treatment in RCTs. Therefore, sensitivity and subgroup analyses were performed again in this meta-analysis to assess the length of hospital stay, the length of ICU stay and super infection comparing GCS with routine treatment of CAP patients. The results were consistent with those from the original studies, showing the stability of meta-analysis.
This meta-analysis has certain limitations. The scientific integrity of results and conclusions from meta-analyses could be influenced by the type of GCS, the dosage, and the treatment duration in each RCT. Further, the seven RCTs included in this meta-analysis have methodological differences: (1) four RCTs did not depict the concealment of randomization allocation; (2) only four RCTs were performed using doubleblinding methods, the remaining three were performed using a single-blind method; (3) the articles included in the analysis were limited to publications in English or Chinese only.
Hence, GCS as an adjunctive therapy is valuable for patients with CAP. When it is cautiously used in clinical setting, complications should be determined if necessary. Further RCTs with a large sample size, higher quality, and a long-term follow-up period are warranted to defi ne the indication of GCS for patients with CAP and to evaluate the appropriate type of GCS, dosage, and the duration of treatment.
Funding:None.
Ethical approval:Not needed.
Confl icts of interest:The authors declare that there is no confl ict of interest relevant to the content of the article.
Contributors:Chen LP proposed the study, analyzed the data and wrote the fi rst draft. All authors contributed to the design and interpretation of the study and to further drafts.
1 Sibila O, Agusti C, Torres A. Corticosteroids in severe pneumonia. Eur Respir J 2008; 32: 259–264.
2 Anderson RN, Smith BL. Deaths: leading causes for 2002. Natl Vital State Rep 2005; 53: 1–89.
3 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/ American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: S27–S72.
4 Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest 2007; 131: 1205–1215.
5 Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P. Diagnosis of adrenal insuffi ciency in severe sepsis and septic shock. Am J Respir Crit Care Med 2006; 174: 1319–1326.
6 de Jong MF, Beishuizen A, Spijkstra JJ, Groeneveld AB. Relative adrenal insuffi ciency as a predictor of disease severity, mortality, and benefi cial effects of corticosteroid treatment in septic shock. Crit Care Med 2007 ; 35: 1896–1903.
7 Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Effi cacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010; 181: 975–982.
8 Remmelts HH, Meijvis SC, Heijligenberg R, Rijkers GT, Oosterheert JJ, Bos WJ, et al. Biomarkers define the clinical response to dexamethasone in community-acquired pneumonia. J Infect 2012; 65: 25–31.
9 Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe communityacquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005; 171: 242–248.
10 Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Effi cacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007; 185: 249–255.
11 Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36: 1937–1949.
12 Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377: 2023–2030.
13 Fernández-Serrano S, Dorca J, Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Fernández-Agüera A, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Critical Care 2011; 15: R96.
14 Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother 2008; 62: 661–668.
Received January 21, 2015
Accepted after revision May 19, 2015
10.5847/wjem.j.1920–8642.2015.03.002
World J Emerg Med 2015;6(3):172–178
杂志排行
World journal of emergency medicine的其它文章
- Albuterol in the treatment of acute respiratory distress syndrome: A meta-analysis of randomized controlled trials
- Performance of cardiopulmonary resuscitation during prolonged basic life support in military medical university students: A manikin study
- Peer-assisted learning to train high-school students to perform basic life-support
- Feasibility study of fi rst-year medical students identifying cardiac anatomy using ultrasound in rural Panama
- Sequential invasive-noninvasive mechanical ventilation weaning strategy for patients after tracheostomy
- Paraoxonase-1 gene in patients with chronic obstructive pulmonary disease investigation Q192R and L55M polymorphisms
