Effects of low nutrition on photosynthetic capacity and accumulation of total N and P in three climber plant species
2015-02-07•
•
Effects of low nutrition on photosynthetic capacity and accumulation of total N and P in three climber plant species
Deke Xing•Yanyou Wu
To study the effects of low nutrition on photosynthetic capacity and accumulation of total nitrogen (N)and phosphorus(P)in three climber plant species,Pharbitis nil(Linn.)Choisy,Lonicera japonicaThunb.andParthenocissus tricuspidata(Sieb.et Zucc.)Planch,all climber plants were exposed to low nutrition at 6 levels (Hoagland solution as control,1/2,1/4,1/8,1/16 and 1/32-strength Hoagland solution)for 30 days.Photosynthetic capacity was determined by measuring leaf chlorophyll fuorescence,chlorophyll content,carbonic anhydrases activity and growth.Accumulation of total N and P was studied by measuring N and P content in plant tissues.Low nutrition decreased the photosynthetic capacity ofP.nil, whileL.japonicamaintained high photosynthetic capacity under low nutrition.Photosynthetic apparatus ofP.tricuspidatasuffered no damage when exposed to low nutrition.L.japonicaandP.tricuspidatahad better adaptability to low nutrition thanP.nil.With a faster growth rate,P.nilconsumed more nutrition(N and P),and its growth was mainly affected by P defciency under low nutrition. AlthoughL.japonicasuffered damage from N and P defciency simultaneously,but the nutrient defciency was not serious except for 1/32-strength Hoagland solution.P.tricuspidatagrew slowly,so its requirement of N and P were the least,even if it was mainly affected by the P defciency,it could still grow well under low nutrition. With the consideration of fertilizing N and P fertilizers in karst areas which were with lower N and P contents,plant species,N/P ratio threshold and low nutrition level should be taken into account synchronously.This study could provide a general consideration for the planning and developing low nutrition resistant plants and fertilizing the three climber plant species in the low nutrition environment.
Carbonic anhydrase·Chlorophyll fuorescence·N,P accumulation·Karst environment· Plant growth
Abbreviations
CA Carbonic anhydrase
ChlF Chlorophyll fuorescence
N Nitrogen
P Phosphorus
P.nilPharbitis nil(Linn.)Choisy
L.japonicaLonicera japonicaThunb
P.tricuspidataParthenocissus tricuspidata
(Sieb.et Zucc.)Planch
PSII Photosystem II
WA Wilbur and Anderson
Fv/Fm Maximum quantum yield of PSII
1 Introduction
Karst terrain covers a vast 0.34 million km2land in southwestern China(Yuan 2001),which gives rise to a fragile environment and low productive economy.In fact,the lack of mineral elements is an inherent factor leading to the fragile agro-ecological environment in the karst areas. Available element contents in cultivated soil delivered from carbonate rocks are merely 31.1%of those from noncarbonate rocks(Chen and Bi 2011).Nutrient(nitrogen and phosphorus)limitation has strongly affected competition between plant species,as species vary in their ability to cope with low nutrient resources(Koerselman and Meuleman 1996).Nitrogen(N)and phosphorus(P)are essential macronutrients for plant growth and development.However,inorganic P is one of the least available nutrients in the soils of several terrestrial ecosystems(Vance et al. 2003;Qiu and Lian 2012),causing problems that could lead P defciency,especially in the karst areas where dissolved P concentration was no more than 0.33 mmol/L (Alloush et al.2003).According to those researchers,P defciency signifcantly infuenced leaf photosynthesis and carbon metabolisms in plants(Rao 1996;Foyer and Spencer 1986;Fredeen et al.1989).Besides,the karst vegetations were also generally at N-and P-co-limited stresses,N also played an important role in the formation of chloroplast structure and hence to the photosynthetic intensity and productivity of plants(Du et al.2011;Doncheva et al.2001;Liu et al.2013).But the question was how to select appropriate kinds of plants for the ecological restoration or how to apply N and P fertilizers in karst areas which were with lower N and P contents.
Lianas contributed substantially to the diversity of the forest and were widely used for producing medicine.They also played a substantial role in forest regeneration,so they had a special superiority in ecological restoration(Bongers et al.2002).Both Pharbitis nil(Linn.)Choisy and Lonicera japonica Thunb.were excellent medicinal plants and afforestation plants widely cultivated in China(Jung et al. 2008;Stanturf et al.2004;Kumar et al.2005).Parthenocissus tricuspidata(Sieb.et Zucc.)Planch was a vertical virescence medicinal plant of the Vitaceae family that might climb 20 m or higher by adhesive tendrils attaching to supports(Wang et al.2009;Kim et al.2005).They were more superior in growth,breeding,adaptability and competition than woody and herbage plants.They had the developed root system,higher biomass,good for soil fxation and planting(Schnitzer and Bongers 2002),under drought stress conditions,these three climber plants were all with good adaptability and different mechanisms of photosynthetic response and adaptability respectively (Xing and Wu 2012).There was no research on the comparative studies of different adaptability in these three climber plants to low nutrition till now.
According to those studies,photosynthetic activities could be considered to represent the growth potential of a plant(Mooney 1972;Walters et al.1993).Changes in Chlorophyll fuorescence(ChlF)emission,arising mainly from photosystem II(PSII),provided information regarding almost all aspects of photosynthetic activity and therefore,refected plant tolerance to environmental stresses(Panda et al.2008).Photosynthesis of plant was affected by its photosynthetic activity.Photosynthesis was the important energy source and material basis for plant growth.Chlorophyll was an important pigment which participated in the absorption,transmission and transformation of light energy for photosynthesis(Wu et al.2008), its content was closely related with leaf photosynthetic rate and organic matter accumulation,growth and development, yield in plant.The higher the chlorophyll content in plant and higher the photosynthetic capacity(Gitelson et al. 2003).Meanwhile,Carbonic anhydrases(CA)were zinccontaining enzymes to transport carbon dioxide and protons across biological membranes and to retain inorganic carbon within the cell(Tavallali et al.2009).It was involved in diverse physiological processes including photosynthetic carbon dioxide fxation(Moroney et al. 2001).CA played an important role in photosynthesis process.Higher CA activity was more in favor of plant photosynthesis(Wu and Xing 2012).
This study selected P.nil,L.japonica and P.tricuspidata as experiment materials,simulated low nutrition environment of soil in southwestern karst areas and cultivated these three climber plants in different concentration nutrition.Determined their CA activities,ChlF parameters, chlorophyll contents,growth and N,P accumulation and compared the growth quality between P.nil,L.japonica and P.tricuspidata.Discussed the different adaptability of these three climber plants to low nutrition of soil,and provided general consideration for improving and repairing fragile karst ecological environment by biological method.
2 Materials and methods
2.1 Plant growth and treatment
The experiment was conducted in an artifcial climatic chamber at the Institute of Agricultural Engineering,Jiangsu University,Jiangsu Province,China(N 32°11′and E 119°27′).The seedlings of P.nil,L.japonica and P.tricuspidata were germinated and cultivated in 12-hole trays with quartz sand under a 12-h photoperiod(280±20 μmol/ (m2sec)PPFD),a day/night temperature cycle of 27/23°C, and 70%of relative humidity.Plants were irrigated daily with 1/4-strength Hoagland solution[6 mmol/L KNO3, 4 mmol/L Ca(NO3)2,2 mmol/L MgSO4,2 mmol/L Fe(Na)EDTA,1 mmol/L NH4H2PO4,2 μmol/L KCl, 50 μmol/L H3BO3,4 μmol/L MnSO4,4 μmol/L ZnSO4, 0.2 μmol/L CuSO4,and 0.2 μmol/L(NH4)6MO7O24](HoaglandandArnon1950).After2 monthsofgrowth,5differentnutrient concentration solutions(1/2,1/4,1/8,1/16,1/32-strength Hoagland solution)and original Hoagland solution as control were used to simulate six nutrition levels to seedlings that germinated healthily and uniformly.In this experiment,available N content in karst soil was equal to 1/2-strength Hoagland solution,available P content in karst soil was between 1/2 and 1/4-strength Hoagland solution,available potassium content in karst soil was between 1/4 and 1/8-strength Hoagland solution(Jiang 2000;Zhao et al.2007). The experiment was arranged in a completely randomized design,12healthyanduniformseedlingsfromeachspeciesof climber plants were used under each level.The treatment lasted for 30 days and then measurements were done.
2.2 Carbonic anhydrase activity measurement
Thefourthandffthyoungestfullyexpandedleavesfromthe top were chosen for CA activity measurement.Three plants from each treatment group were used for the measurement. Leaf tissues(0.3–0.8 g)were quickly frozen in liquid N and ground with 3 mL extraction buffer(0.01 mol/L barbitone sodium with 0.05 mol/L mercaptoethanol,pH 8.3).The homogenate was centrifuged at 13,000 r/min and 0°C for 5 min,and then placed on ice for 20 min.The supernatant was used to analyze the CA activity using the pH method described by Wilbur and Anderson(1948)with modifcations(Wu et al.2011).In brief,CA activity was assayed at 0–2°C in a mixture containing 4.5 mL,0.02 mol/L barbitone buffer(5,5-diethylbarbituric acid;pH 8.3),0.4 mL sample and 3 mL CO2-saturated water.CA activity was expressedinWilburandAnderson(WA)unitsasWA=(t0/ t)-1,where t0and t were the time(sec)measured for the pH change(8.2–7.2)with buffer alone(t0)and with sample(t).
2.3 Determination of ChlF
ChlF was measured with IMAGING-PAM modulated ChlF imaging system(IMAGING-PAM,Heinz Walz GmbH, Germany).Leaves were dark adapted for 30 min to ensure complete relaxation of all reaction centers before the measurements.As mentioned above,the fourth youngest fully expanded leaf from the top was selected for the measurement.Three plants from each treatment group were used for the measurement.The minimum ChlF(Fo)was determined using a measuring beam,whereas the maximum ChlF(Fm)was recorded after a 0.8 s saturating light pulse[6,000 μmol/(m2sec)].Maximal PSII photochemical effciency(Fv/Fm)was calculated as(Fm-Fo)/Fm.
2.4 Determination of chlorophyll content
Chlorophyll content was measured with SPAD-502 chlorophyll content measuring apparatus(SPAD-502,Konica Minolta,Tokyo).The fourth youngest fully expanded leaf from the top was selected for the measurement and three plants from each treatment group were used for the measurement.
2.5 Determination of growth status
Three plants from each treatment group were used for the measurement of stem diameter,leaf area and height respectively.Stem diameter was determined by vernier caliper(0–150,Chengliang,Chengdu),leaf area was measured with a portable leaf area measurement instrument (AM-200,ADC,UK).Height was measured with a ruler.
2.6 Determination of N and P content
Three plants from each treatment group were selected and dried in oven under 80°C,then the plant dry weight was weighed by BT125D electronic balance(BT125D,Sartorius,Germany).0.2–0.4 g drying of plant tissue were used for digesting with H2SO4·H2O2digestion method(Xu 2000),and N,P concentrations(mg/L)in the digestion solution were determined with AA3 continuous-fow analyzers(AA3,Seal Analytical,Germany).N and P%were calculated as follows:
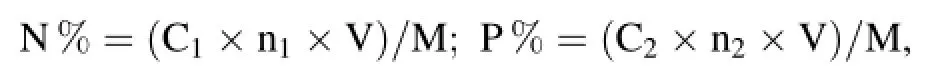
where C1and C2were the concentrations of N and P in the digestion solution,n1and n2were the dilution factor,V was the volume of the digestion solution,M was the quality of drying of plant tissue.
2.7 Statistical analysis
All measurements were subjected to analysis of variance (ANOVA)to discriminate signifcant differences(defned as P≤0.05)between group means.Data were shown as the mean±standard error(SE).These mean data were analyzed statistically using a factorial design by SPSS software(version 13.0,SPSS Inc),and mean results were compared by LSD post hoc test at 5%signifcance level (p<0.05).
3 Results
3.1 Carbonic anhydrase activity
CA activities varied with plant species and low nutrition levels.CA activity of P.nil was higher than the other two species.CA activity of P.tricuspidata was the lowest and nearly undetectable,P.tricuspidata had no CA activity (Table 1).CA activities of P.nil under 1/2 and 1/4-strengthHoagland solution were a little higher than that under control,while the values under 1/16 and 1/32-strength Hoagland solution were lower compared to control.CA activities of L.japonica under 1/4,1/8,1/16 and 1/32-strength Hoagland solution were higher than that under control or 1/2-strength Hoagland solution,the value under 1/4-strength Hoagland solution was the highest.
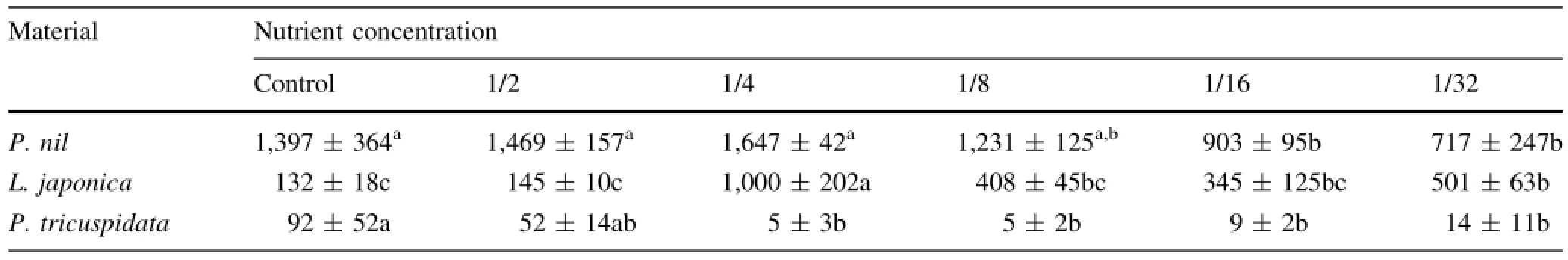
Table 1 Effects of low nutrition on carbonic anhydrase(CA)activity(WAU/gFW)of three climber plant species(samples n=5)
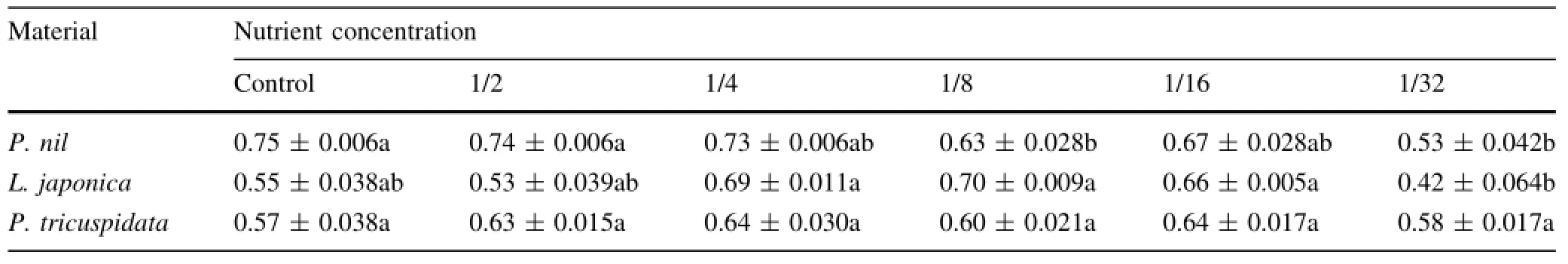
Table 2 Effects of low nutrition on maximal PSII photochemical effciency(Fv/Fm)of three climber plant species(samples n=5)
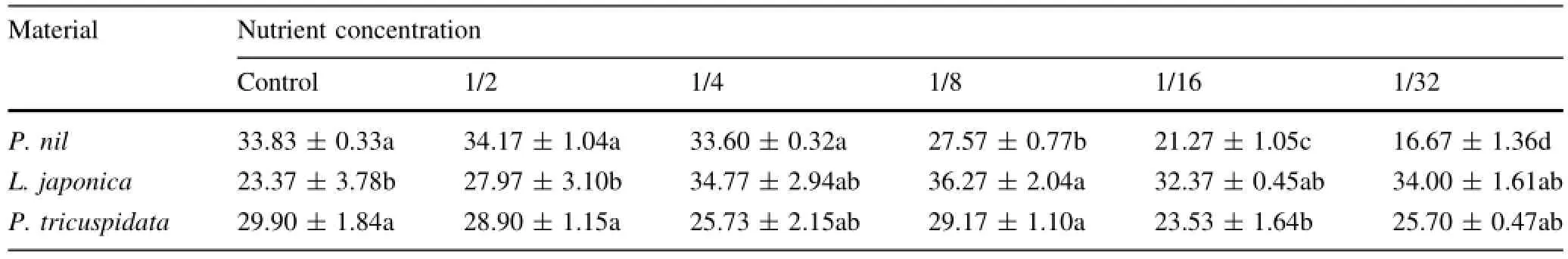
Table 3 Effects of low nutrition on chlorophyll content(SPAD/%)of three climber plant species(samples n=5)
3.2 Chlorophyll fuorescence
Maximal PSII photochemical effciency(Fv/Fm)of P.nil and L.japonica had the lowest value under 1/32-strength Hoagland solution,Fv/Fm value of P.nil under 1/8-strength Hoagland solution was also lower than that under control or 1/2-strength Hoagland solution(Table 2).Fv/Fm of P.tricuspidata did not change markedly with increasing low nutrition.
3.3 Chlorophyll content
Chlorophyll contents(SPAD/%)of P.nil under 1/8,1/16, 1/32-strength Hoagland solution were lower than that under 1/2,1/4-strength Hoagland solution and control(Table 3), chlorophyll content of P.nil under 1/32-strength Hoagland solution was only 48.8%of that under the 1/2-strength Hoagland solution.Whereaschlorophyllcontentsof L.japonica under 1/4,1/8,1/16 and 1/32-strength Hoagland solution were higher than that under control or 1/2 and chlorophyll content of L.japonica under 1/8 was 1.6 times of that under control.Chlorophyll content of P.tricuspidata had a lower value under 1/16-strength Hoagland solution compared to other levels.
3.4 Plant growth
Low nutrition(1/8,1/16,1/32-strength Hoagland solution) was associated with a lower stem diameter of P.nil, whereas stem diameters of L.japonica and P.tricuspidata were higher than that under control(Fig.1a).Leaf area of P.nil decreased signifcantly under low nutrition(1/4,1/8, 1/16,1/32-strength Hoagland solution),and the value under 1/32 was only 23.1%of that under control(Fig.1b).Leaf areas of L.japonica and P.tricuspidata had higher value under 1/4 or 1/8-strength Hoagland solution compared to other levels and leaf areas of L.japonica and P.tricuspidata under low nutrition were all not lower than that undercontrol respectively.Heights of P.nil under 1/16 and 1/32-strength Hoagland solution decreased compared to other levels(Fig.1c).Low nutrition was associated with a higher height of L.japonica and value of L.japonica under 1/8 was the highest.Height of P.tricuspidata appeared independent of low nutrition.Plant dry weight of P.nil under 1/4 was higher than that under other levels,but the value under 1/32 became lower than that under control(Fig.1d). Low nutrition(1/4,1/8,1/16,1/32-strength Hoagland solution)was also associated with higher plant dry weight of L.japonica,and the value of L.japonica under 1/8 was also the highest.Plant dry weight of P.tricuspidata increased under 1/4,1/8 and 1/32-strength Hoagland solution compared to other levels.
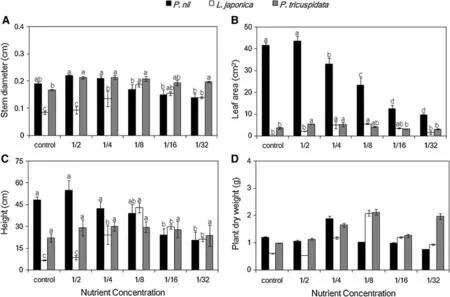
Fig.1 Effects of low nutrition on stem diameter,leaf area,height and plant dry weight in three climber plant species.a Stem diameter,b leaf area,c height,d plant dry weight.The mean±SE followed by different letters in the same species signifcantly atP≤0.05,according to oneway ANOVA andttest
3.5 Accumulation of total N and P
N/P ratio of P.nil and P.tricuspidata under low nutrition (1/4,1/8,1/16,1/32-strength Hoagland solution)was higher than that under control or 1/2-strength Hoagland solution(Table 4).N/P ratio of P.nil under 1/8 or 1/32 was higher than that under 1/2,1/4 and 1/16-strength Hoagland solution.N/P ratio of L.japonica under control was higher than that under 1/2 and 1/32.Value under 1/32 was the lowest and value under 1/8 was the highest.N/P ratio of P.tricuspidata had the highest value under 1/32-strength Hoagland solution,N/P ratio of P.tricuspidata under 1/8-strength Hoagland solution was higher than that under control,1/2,1/4,and 1/16-strength Hoagland solution.
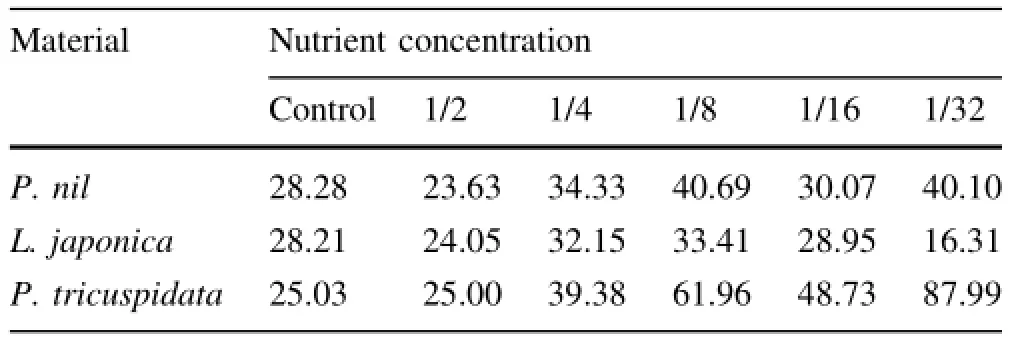
Table 4 Effects of low nutrition on N/P(mg/mg)ratio of three climber plant species
Figure 2a showed the total N accumulation of three species.Total N accumulation of P.nil and P.tricuspidata appeared independent of low nutrition.Low nutrition was associated with a little lower total N accumulation ofL.japonica compared to control,value under 1/8-strength Hoagland solution was the lowest.
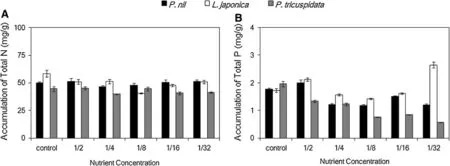
Fig.2 Effects of low nutrition on accumulation of total nitrogen(N),phosphorus(P)in three climber plant species.a.accumulation of total N, b accumulation of total P
Figure 2b showed the total P accumulation of three species.Low nutrition(1/4,1/8,1/16,1/32-strength Hoagland solution)was associated with a lower total P accumulation of P.nil and P.tricuspidata compared to control or 1/2-strength Hoagland solution.Total P accumulation of P.nil under 1/16 was higher than that under 1/4,1/8 and 1/32-strength Hoagland solution.Whereas total P accumulation of L.japonica decreased under 1/4,1/8 and 1/16-strength Hoagland solution compared to control,1/2 or 1/32-strength Hoagland solution,and total P accumulation of L.japonica under 1/32 became the highest.Total P accumulations of P.tricuspidata under 1/8 and 1/16 were higher than that under 1/32 but lower than that under 1/2 or 1/4-strength Hoagland solution.
4 Discussion
4.1 Photosynthetic activity
Changes in ChlF emission,arising mainly from PSII, provided information regarding almost all aspects of photosynthetic activity which could represent the plant growth potential(Panda et al.2008).In this study,the response of Fv/Fm of P.nil to increased low nutrition indicated that the reaction center of PSII could have been damaged under 1/8,1/16 and 1/32-strength Hoagland solution.Reaction center of PSII of L.japonica was damaged under 1/32-strength Hoagland solution.Effect of low nutrition on the photosynthesis of P.tricuspidata did not involve any damage to the PSII reaction centers.Under low nutrition conditions,CA played an important role in the photosynthesis process.CA was involved in diverse physiological processes(Badger and Price 1994;Sasaki et al.1998).Higher CA activity was in favor of plant photosynthesis.Activated by low nutrition,CA activities of P.nil under 1/2 and 1/4-strength Hoagland solution had some increase,which could prevent the reaction center of P.nil from damage.Lower CA activity of P.nil under 1/8, 1/16 and 1/32-strength Hoagland solution showed bad fexibility and worse regulatory ability.A substantial increase of CA activity of L.japonica occurred under 1/4-strength Hoagland solution,indicating that high CA activity was activated to respond low nutrition,and a stable photosynthetic activity could be maintained.Although CA activity of L.japonica under 1/32 had some increase compared to 1/16-strength Hoagland solution,regulation of CA could not prevent reaction center of L.japonica from damage.DifferentincreaseofCA activity between L.japonica and P.nil under 1/4 compared to 1/2-strength Hoagland solution indicated that CA of L.japonica was more sensitive to low nutrition and more fexibility than P.nil.CA activity of P.tricuspidata was the lowest and nearly undetectable,but P.tricuspidata could also maintain a stable photosynthetic activity under low nutrition.
4.2 Plant growth
When plant was under adversity stress conditions,its various physiological processes would be affected,and thus affected the chlorophyll content in plant directly or indirectly(Gitelson et al.2003).Leaf photosynthesis could be estimated from measurements of chlorophyll content.The decrease of chlorophyll content of P.nil under 1/8,1/16 and 1/32-strength Hoagland solution indicated that the photosynthesis of P.nil would decrease.Whereas theincrease of chlorophyll content of L.japonica under 1/4, 1/8,1/16 and 1/32-strength Hoagland solution was good for the photosynthesis of L.japonica.Chlorophyll content of P.tricuspidata was not affected by low nutrition expected for 1/16-strength Hoagland solution,but it could maintain a stable photosynthetic activity.
Growth analysis indicated that P.nil grew fast and could accumulate more dry matter under 1/4-strength Hoagland solution but it could not grow well under 1/8,1/16 and 1/32-strength Hoagland solution.Whereas L.japonica demonstrated the best adaptability to 1/8-strength Hoagland solution,L.japonica could grow well under 1/4,1/8 and 1/16-strength Hoagland solution.P.tricuspidata grew slowly,low nutrition had less infuence on its growth,but it accumulated less dry matter under 1/16-strength Hoagland solution compared to 1/4,1/8 and 1/32-strength Hoagland solution.
4.3 Accumulation of total N and P
The‘N/P tool’could predict the limiting nutrient for plant growth on a community level conceptually simple and accurately.It could be used in restoration projects aiming at a improving of site fertility(Koerselman and Meuleman 1996).Moreover,there were different N/P ratio thresholds for N or P limitation among species in a given area(Li et al.2011).In this study,the N/P ratio of three climber species under control was taken as threshold respectively.N/P ratio of P.nil or P.tricuspidata under low nutrition(1/4,1/8,1/16 and 1/32-strength Hoagland solution)was higher than that under control or 1/2-strength Hoagland solution respectively.The accumulation of total P in P.nil or P.tricuspidata under low nutrition(1/4,1/8,1/16 and 1/32-strength Hoagland solution)was lower than that under control or 1/2-strength Hoagland solution,but the accumulation of total N in P.nil or P.tricuspidata under low nutrition(1/4,1/8,1/16 and 1/32-strength Hoagland solution)was not signifcantly lower than that under control or 1/2-strength Hoagland solution respectively,so the nutrient limitation for growth of P.nil or P.tricuspidata under 1/4,1/8,1/16 and 1/32-strength Hoagland solution was P rather than N. N/P ratio of P.nil,L.japonica or P.tricuspidata under 1/8-strength Hoagland solution was higher than that under 1/4 and 1/16-strength Hoagland solution,which indicated that nutrient limitation for growth of P.nil,L.japonica or P.tricuspidata under 1/8-strength Hoagland solution was P rather than N compared to 1/4 and 1/16-strength Hoagland solution.While nutrient limitation for growth of P.nil or P.tricuspidata under 1/16-strength Hoagland solution was N rather than P compared to 1/8 and 1/32-strength Hoagland solution.N/P ratio of L.japonica under 1/32 waslowerthan thatunderotherlevelsand accumulation of total P in L.japonica under 1/32 was higher compared to other levels,so the nutrient limitation for growth of L.japonica under 1/32-strength Hoagland solution was N rather than P.N/P ratio of L.japonica under 1/8 was higher than that under other levels,nutrient limitation for growth of L.japonica under 1/8-strength Hoagland solution was P rather than N.But compared to 1/4,1/8,1/16-strength Hoagland solution and control, nutrient limitation for growth of L.japonica under 1/2-strength Hoagland solution was N rather than P.
Therefore nutrient limitation for growth varied with low nutrition level and plant species.When N/P ratio threshold for N or P limitation changed,nutrient limitation for growth of plants changed too.With the consideration of fertilizing N and P fertilizers in karst areas which were with lower N and P contents,plant species,N/P ratio threshold and low nutrition level should be taken into account synchronously.
5 Conclusions
We can conclude from discussions above,CA activity,Fv/ Fm,chlorophyll content and growth of P.nil decreased with decreasing nutrition concentrations.P.nil could not grow well when exposed to low nutrition conditions(1/8, 1/16 and 1/32-strength Hoagland solution)and P.nil was not adapt to low nutrition(1/8,1/16 and 1/32-strength Hoagland solution).Under low nutrition conditions(1/4, 1/8,1/16 and 1/32-strength Hoagland solution)especially 1/8-strength Hoagland solution,L.japonica had a higher value of chlorophyll content and better photosynthetic effciency,it could grow well and accumulate more organic matter,it had a good adaptability to low nutrition(1/4,1/8 and 1/16-strength Hoagland solution);For P.tricuspidata, its growth was not affected signifcantly.The nutrient elements in soil of karst areas were very low and not good for growth of many plants.On the other hand L.japonica and P.tricuspidata had good adaptability to low nutrition environments,P.tricuspidata could even grow on the wall. With a faster growth rate,P.nil consumed more nutrition (N and P),and its growth was mainly affected by P defciency under low nutrition.Although L.japonica suffered damage from N and P defciency simultaneously,but the nutrient defciency was not serious except for 1/32-strength Hoagland solution.P.tricuspidata grew slowly,so its requirement of N and P was the least.Even if it was mainly affected by the P defciency,it could still grow well under low nutrition.With the consideration of fertilizing N and P fertilizers in karst areas which were with lower N and P contents,plant species,N/P ratio threshold and low nutrition level should be taken into account synchronously.This study could provide a general consideration for planningand developing low nutrition resistant plants,and fertilizing the three climber plant species in the low nutrition environment.
AcknowledgmentsThis study was supported by the‘‘One Hundred Talents Program of The Chinese Academy of Sciences’’and the project of the National Natural Science Foundation of China(No. 31070365).
Alloush GA,Boyer DG,Belesky DP,Halvorson JJ(2003)Phosphorus mobility in a karst landscape under pasture grazing system[J]. Agronomie 23:593–600
Badger MR,Price GD(1994)The role of carbonic anhydrase in photosynthesis[J].Annu Rev Plant Physiol 45:369–392
Bongers F,Schnitzer SA,Traore´D(2002)The importance of lianas and consequences for forest management in west africa[J]. Bioterre:revue internationale scientifque de la vie et de la terre 2002:59–70
Chen R,Bi K(2011)Correlation of karst agricultural geo-environment with non-karst agricultural geo-environment with respect to nutritive elements in Guizhou[J].Chin J Chem 30:563–568
Doncheva S,Vassileva V,Ignatov G,Pandev S(2001)Infuence of nitrogen defciency on photosynthesis and chloroplats ultrastructure of pepper plants[J].Agric Food Sci 10:59–64
Du YX,Pan GX,Li LQ,Hu ZL,Wang XZ(2011)Leaf N/P ratio and nutrient reuse between dominant species and stands:predicting phosphorus defciencies in karst ecosystems,southwestern China [J].Environ Earth Sci 64:299–309
Foyer C,Spencer C(1986)The relationship between phosphate status and photosynthesis in leaves[J].Planta 167:369–375
Fredeen AL,Rao IM,Terry N(1989)Infuence of phosphorus nutrition on growth and carbon partitioning inGlycine max[J]. Plant Physiol 89:225–230
Gitelson AA,Gritz Y,Merzlyak MN(2003)Relationships between leaf chlorophyll content and spectral refectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves [J].J Plant Physiol 160:271–282
Hoagland DR,Arnon DI(1950)The water-culture method for growing plants without soil[J].Calif Agric Exp St 347:1–32
Jiang ZC(2000)Liable content of elements in ecological environments in karst mountains in south China[J].Carsologica Sin 19:123–128
Jung DY,Ha H,Lee HY,Kim C,Lee JH,Bae K,Kim JS,Kang SS (2008)Triterpenoid saponins from the seeds ofPharbitis nil[J]. Chem Pharm Bull 56:203–206
Kim HJ,Saleem M,Seo SH,Jin C,Lee YS(2005)Two new antioxidant stilbene dimers,parthenostilbenins A and B fromParthenocissus tricuspidata[J].Planta Med 71:973–976
Koerselman W,Meuleman AFM(1996)The vegetation N:P ratio:a new tool to detect the nature of nutrient limitation[J].J Appl Ecol 33:1441–1450
Kumar N,Singh B,Bhandari P,Gupta AP,Uniyal SK,Kaul VK (2005)Bifavonoids fromLonicera japonica[J].Phytochemistry 66:2740–2744
Li LJ,Zeng DH,Yu ZY,Fan ZP,Mao R,Peri PL(2011)Foliar N/P ratio and nutrient limitation to vegetation growth on Keerqin sandy grassland of North-east China[J].Grass Forage Sci 66:237–242
Liu XP,Fan YY,Long JX,Wei RF,Kjelgren R,Gong CM,Zhao J (2013)Effects of soil water and nitrogen availability on photosynthesis and water use effciency ofRobinia pseudoacaciaseedlings[J].J Environ Sci 25:585–595
Mooney HA(1972)The carbon balance of plants[J].Annu Rev Ecol Syst 3:315–346
Moroney JV,Bartlett SG,Samuelsson G(2001)Carbonic anhydrases in plants and algae[J].Plant,Cell Environ 24:141–153
Panda D,Sharma SG,Sarkar RK(2008)Chlorophyll fuorescence parameters,CO2photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent reemergence in rice(Oryza sativaL.)[J].Aquat Bot 88:127–133
Qiu SS,Lian B(2012)Weathering of phosphorus-bearing mineral powder and calcium phosphate by Aspergillus niger[J].Chin J Geochem 31:390–397
Rao IM(1996)The role of phosphorus in photosynthesis.In: Pessarakli M(ed)Handbook of photosynthesis.Marcel Dekker, New York,pp 173–194
Sasaki H,Hirose T,Watanabe Y,Ohsugi R(1998)Carbonic anhydrase activity and CO2-transfer resistance in Zn-defcient rice leaves[J].Plant Physiol 118:929–934
Schnitzer SA,Bongers F(2002)The ecology of lianas and their role in forest[J].Trends Ecol Evol 17:223–230
Stanturf A,Conner WH,Gardiner ES,Schweitzer CJ,Ezell AW (2004)Practice and perspective:recognizing and overcoming diffcult site conditions for afforestation of bottomland hardwoods[J].Ecol Restor 22:183–193
Tavallali V,Rahemi M,Maftoun M,Panahi B,Karimi S,Ramezanian A,Vaezpour M(2009)Zinc infuence and salt stress on photosynthesis,water relations,and carbonic anhydrase activity in pistachio[J].Sci Hortic 123:272–279
Vance CP,Uhde-Stone C,Allan DL(2003)Phosphorus acquisition and use:critical adaptations by plants for securing a nonrenewable resource[J].N Phytol 157:423–447
Walters MB,Kruger EL,Reich PB(1993)Relative growth rate in relation to physiological and morphological traits for northern hardwood tree seedlings:species,light environment and ontogenetic considerations[J].Oecologia 96:219–231
Wang WB,Kim YH,Lee HS,Kim KY,Deng XP,Kwak SS(2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stress[J].Plant Physiol Biochem 47:570–577
Wilbur KM,Anderson NG(1948)Electrometric and colorimetric determination ofcarbonic anhydrase [J].J BiolChem 176:147–154
Wu YY,Xing DK(2012)Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae[J].Photosynthetica 50:587–594
Wu CY,Niu Z,Tang Q,Huang WJ(2008)Estimating chlorophyll content from hyperspectral vegetation indices:modeling and validation[J].Agric For Meteorol 148:1230–1241
Wu YY,Shi QQ,Wang K,Li PP,Xing DK,Zhu YL,Song YJ(2011) An electrochemical approach coupled with Sb microelectrode to determine the activities of carbonic anhydrase in the plant leaves.In:Zeng D(ed)Future intelligent information systems. Springer,Berlin,pp 87–97
Xing DK,Wu YY(2012)Photosynthetic response of three climber plant species to osmotic stress induced by polyethylene glycol (PEG)6000[J].Acta Physiologiae Plant 34:1659–1668
Xu GH(2000)Determination of plant ash and diverse nutrient element.In:Bao SD(ed)Soil and agricultural chemistry analysis.China Agriculture Press,Beijing,pp 263–270
Yuan DX(2001)On the karst ecosystem[J].Acta Geologica Sini-Engl Ed 75:336–338
Zhao SH,Zhang C,Xia Q,Shen HG(2007)The primary analysis of soil organic matter and nitrogen in karst and non-karst areas of Maocun,Guilin[J].J Guangxi Acad Sci 23:36–38
Received:5 January 2014/Revised:8 July 2014/Accepted:15 July 2014/Published online:26 December 2014 ©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2014
D.Xing·Y.Wu
Key Laboratory of Modern Agricultural Equipment and Technology,Ministry of Education&Jiangsu Province,Institute of Agricultural Engineering,Jiangsu University, Zhenjiang 212013,China
D.Xing·Y.Wu(✉)
State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences, Guiyang 550002,China
e-mail:wuyanyou@mail.gyig.ac.cn
杂志排行
Acta Geochimica的其它文章
- Petrogenetic study of Mesoproterozoic volcanic rocks of North Delhi fold belt,NW Indian shield:implications for mantle conditions during Proterozoic
- Geochemical characteristics and families of the Paleozoic oil seepage and solid bitumen in the Southern Guizhou Depression, SW China
- Petrogenesis of the Xuanwoling mafc–ultramafc intrusion in the northeastern Tarim Block(Northwest China)
- Comprehensive diagnostic review of the13C-enriched crude oils exemplifed by TD2θand TZ62S in Tarim Basin,NW China
- Preparation of Mn3O4from low-grade rhodochrosite ore by chemical bath deposition method
- Some aspects of excellent marine source rock formation: implications on enrichment regularity of organic matter in continental margin basins
