Anther Dehiscence Disturbed by High Temperature and Water Stress Presented in OsDIR Gene Expression in Rice(Oryza sativa L.)
2015-02-06ZhongwenRANGQingmingZHOUXiqinFU
Zhongwen RANG,Qingming ZHOU,Xiqin FU
1.College of Agronomy,Hunan Agricultural University,Changsha 410128,China;
2.State Key Laboratory of Hybrid Rice,Hunan Hybrid Rice Research Center,Changsha 410125,China
Anther Dehiscence Disturbed by High Temperature and Water Stress Presented in OsDIR Gene Expression in Rice(Oryza sativa L.)
Zhongwen RANG1*,Qingming ZHOU1,Xiqin FU2
1.College of Agronomy,Hunan Agricultural University,Changsha 410128,China;
2.State Key Laboratory of Hybrid Rice,Hunan Hybrid Rice Research Center,Changsha 410125,China
In future climates,rice could more frequently be subjected to simultaneous high temperature(HT)and water stress(WS)during sensitive developmental stages such as flowering.In this study,two rice genotypes were exposed to HT,WS and combined high temperature and water stress(WS+HT)during flowering to quantify their response through anther dehiscence.Gene expression profiles of 15 selected OsDIRs revealed differences among stresses and between varieties.The targeted gene OsDIR-08,which was considered to be a HT stress candidate gene,was decreasingly expressed from the 1std to the 4thd under HT stress in Nagina 22 (N22)while increased in Moroberekan.Varies of the expression of OsDIR genes in stresses intuitively reflects on the lignin-staining at the anther dehisced sites,which implied a negative relationship between the lignin biosynthesis and OsDIRs’expression.Anther dehiscence disturbed by HT and WS+HT stress showed a negative cumulative effect in HT sensitive variety Moroberekan but not in N22.Higher level of anther dehiscence in N22 under HT and WS+HT stress indicated its true tolerance of HT and to WS+HT during anthesis.The differentially expressed of OsDIR(s)under various managed stresses caused the difference of the lignin-staining at the anther dehisced site in N22,and thus transformed anther dehiscence correspondingly might be one of the main reasons for the tolerance.
OsDIR gene;Anther dehiscence;Lignin-staining;High temperature;Water stress;Rice
H igh temperature(HT)stress is usually accompanied by inadequate water supply.Abiotic stresses such as HT,water stress (WS)are the major stresses subjected in a lot of regions during rice flowering and maturing period[1].Recent global climate models predict an increase in mean temperature by 2-4.5℃and the rice area affected by water stress to double by the end of this century[2]. Researchers around the world have done a large number of studies on HT stress or WS (or drought)during rice growth and reproduction,which were mainly perspective to physiology and concentrated on the effect of single stress during anthesis on rice produc tion[3-12].However,during the actual rice production process,various stress factors often appear simultaneously, especially in recent years with more frequent of climate change,HT stress, WS and their synergistic effects and other abiotic stresses might bring more serious effect[13].
The combination of HT and WS represents an excellent example of multiple abiotic stresses occurring concomitantly in the field.However,it was poorly understood in physiological and biochemical response to such combined stress in crops[14].It was found that combined stress had a significantly greater detrimental effect on growth and productivity than exposure to a single stress in Hordeum vulgare and Poa pratensis[15-16].Effects of this combination in Arabidopsis thaliana, Triticum aestivum and Nicotiana tabacum have been documented[14,17-18]. The studies of Arabidopsis thaliana found that the response to HT combined WS was different from the response to any single stress with interaction effect,especially in gene expression levels and expression patterns (up-regulation or down-regulation),and this differential expression of gene(s)under combined stress usually was considered to be relative to multiple abiotic stress resistance for organisms[14-18].Particularly in rice,different varieties with different tolerance/sensitivity to HT and/or WS,were used to study the physiological response and seed-setting under HT,WS,and the combined stress,which indicated the importance of the physiological processes of anther dehiscence response to each stress differently[19].
Dirigent protein was first found in F.intermedia by Davin et al.as a 26 kDa glycosylated protein and with the first description of the dirigent protein as rapidly induced transcripts in a variety of crop species[20-21].Recently,a large number of DIR gene homologues have been shown to exist in most plant tissues[22].Suggested by Ralph and coworkers,the DIR proteins are subdivided into five groups:the DIR-a, DIR-b,DIR-c,DIR-d and DIR-e subfamilies[21].With the increasing numbers of dirigent proteins found by researchers,the DIR-b and DIR-d subfamilies are combined together with the appearance of the DIR-f and DIR-g subfamilies[23].However,only members of DIR-a subfamily are studied for their biochemical functions,the others are referred to as DIR-like proteins[24].
Dirigent(DIR)genes,which encode dirigent proteins,have been identified playing a vital role in the for-mation of lignin and in enhancing stress resistance in differentcrop plants as well[25].For example,dirigent proteins mediate the formation of pinoresinol from coniferyl alcohol in developing sesame seeds,and catalyze the polymerization oflignin monomers for free radicals involved in plant defense response[26-27].Many dirigent and dirigent-like genes are regulated in a developmental-and tissuespecific way.Dirigent proteins in F.intermedia were specifically detected in the cell wall of vascular tissue,while mostly detected in the vascular bundle in Arabidopsis,and increasingly expressed under P deficiency in roots, and were temporal and spatial expressed in roots,stems,leaves,and florets from seedling to mature stages with different levels in rice(Oryza sativa subsp.Japonica)according to online MPSS[22,28-30].Dirigent proteins are also influenced by stress conditions with different expressions.For example,a gene fragment encoding a dirigent protein that is predicted to function in lignin biosynthesis was identified from leaves of the resurrection plant plays a role in drought tolerance, which demonstrated that the dirigent protein may play a role in the capture of monolignols and stereo-selective coupling,thus influencing the composition of monolignols in lignin,and affecting the physical properties in clouding the mechanical strength and flexibility of the cell wall;the upregulation of the dirigent protein that is encoded by a dirigent-like gene in heat sensitive variety Moroberekan under heat stress,followed by delayed degradation of the anther cell wall and obstruction of the normal process of anther dehiscence,due to increased lignification;dirigent proteins also catalyze the synthesis of a diverse group of polyphenolic substances(lignans) which may play a role in plant pathogen defense;and a sugarcane dirigent protein gene offered better protectionagainstPEG andNaCl stresses[21-22,28,31-32].These results demonstrate a possibility thatdirigent proteins may be involved in the response to a variety of stresses and play a common role in abiotic stress responses.
Most abiotic stresses result in the production of ROS that can damage the cell when produced in excess but can also act as stress sensor and signal transduction molecules[33].Moreover,ROS generated in the apoplast may react with cell wall aromatic compounds such as lignin to generate signaling molecules[34].Abiotic stresses also affect plant secondary metabolism and lignification,while examples of increased lignin synthesis were found foralmostallabiotic stresses without being able to confirm whether it is a general feature or whether it is highly species dependent[35-36].Although it is likely that stress-induced lignins play different roles such as signaling,defence and adjustmentduring the stress response,direct evidence of these roles are still necessary[34].In order to make clear the expression of OsDIR genes under HT,WS and combined stresses in rice,experiments were therefore carried out on one hand,to study the expression profiles of15 OsDIR genes,which were selected according to the results of MPSS(Massively Parallel Signature Sequencing online), under HT,WS,and the combined stress by using semi-quantitative RTPCR analysis;on the other hand,anther dehiscence and anther ligninstaining were observed in two rice varieties under managed stresses,accordingly to illuminate the possible relationship between OsDIR(s)gene expression profiles and lignin-staining, and anther dehiscence as well.
Materials and Methods
Crop husbandry
Rice varieties Nagina 22(O.sativaaus,originating from India,tolerant to HT and WS)and Moroberekan (O.sativa japonica,originating from Guinea,tolerant to WS and sensitive to HT)were used for this study[4,9,11-12,37-38]. Seeds offered by IRRI(the International Rice Research Institution)were sown in seeding trays with clay loam soil after breaking dormancy (2 d,50℃).Fourteen-day-old seedlings were transplanted into plastic pots with two holes at the bottom sealed with stoppers to facilitate water control.Each pot was filled with 6.0 kg of the same clay loam soil with 2.0 g(NH4)2SO4(urea),1.0 g muriate ofpotash (KCl)and 1.0 g single super phosphate(SSP).An additional 2.5 g of (NH4)2SO4was top dressed,25-30 d after transplanting. Cypermethrin (Cymbush)0.42 g/L was sprayed 30 d after transplanting to control white flies (Bemisia spp.).There were no other pest or disease problems.
Greenhouse and growth chamber tests
Plants were grown in a temperature-controlled greenhouse maintained at 29/21℃day/night temperature[actual:28.8℃(SD{standard deviation}= 0.84)/20.9℃(SD=0.27)]and day/night relative humidity (RH)of 75%-85% [actual 75.2%(SD=0.11)/86.7%(SD= 0.07)]under natural sunlight conditions at the International Rice Research Institute(IRRI),Philippines.Plants were placed on a bench spaced at 30 cm to avoid shading effects.Ambient air temperature and RH were measured using thermocouples (Chessell 392, USA)every 10 s and averaged over 10 min.
Indoor growth chambers(Thermoline,Australia)were used with temperatures automated to gradually increase from 29 to 39℃starting from 0730 to 0830 (2.5 h after dawn)and maintained at 39℃ (SD=1.23)until 1430,with an RH of 75%(SD=3.88). Both temperature (P>0.97)and RH (P>0.73)were maintained consistently to avoid any chamber effects on plant observations recorded.Plants were spaced at approximately 15-20 cm to avoid crowding.A thermocouple placed above the canopy in the growth chamber measured the ambient air temperature and RH every 10 s and averaged them over 10 min (Chessell 392,USA).Photosynthetic photon flux density was maintained at 640 mol/(m2·s).CO2concentration was not measured.
Stress treatments
Five replicate plants were used for four treatments(control,HT,WS and HT+WS)in this experiment.Plants of both genotypes were grown in temperature-controlled greenhouse conditions at 29/21℃and used as absolute controls.For HT,plants were exposed to high temperature (39℃)for 6 h (0830-1430),on the first day of anthesis (i.e.the appearance of anthers)and then moved back to thecontrol conditions(29/21℃).Similarly, transfer between the control and HT conditions was continued for four consecutive flowering days.For WS,main tillers at 5 d before heading(DBH) were selected and tagged.Stoppers at the bottom of these pots were unplugged for overnight draining to reach maximum water holding capacity by the following morning.The main-tiller flag leaf in both genotypes began to roll after 5 successive non-watering days before heading.Following maintiller rolling and based on continuously monitored by recording flag-leaf relative water content(RWC)using the following formula of RWC (%)=[(WDW)/(TW-DW)]×100,where W:fresh weight,TW:turgid weight and DW:dry weight[9].A different set of plants as identified at 5 DBH for HT+WS treatment and exposed to WS as described earlier.These selected plants were exposed to both WS and four days of high-temperature (39℃)starting on the first day of anthesis.
PCR primer design
Fifteen OsDIR genes were selected from 53 rice OsDIR genes(DIR or DIR-like gene family)(Table 1)based on the fragment electronic expression analysis by using online MPSS(Table 2)[30].Gene specific primers were designed corresponding to these selected gene DNA sequences(Primer 3.0), and BLAST process also used forprimer specificity detection[39].The annealing temperature of the each primer pair for PCR was standardized by gradient PCR.More primers information was shown in Table 3.
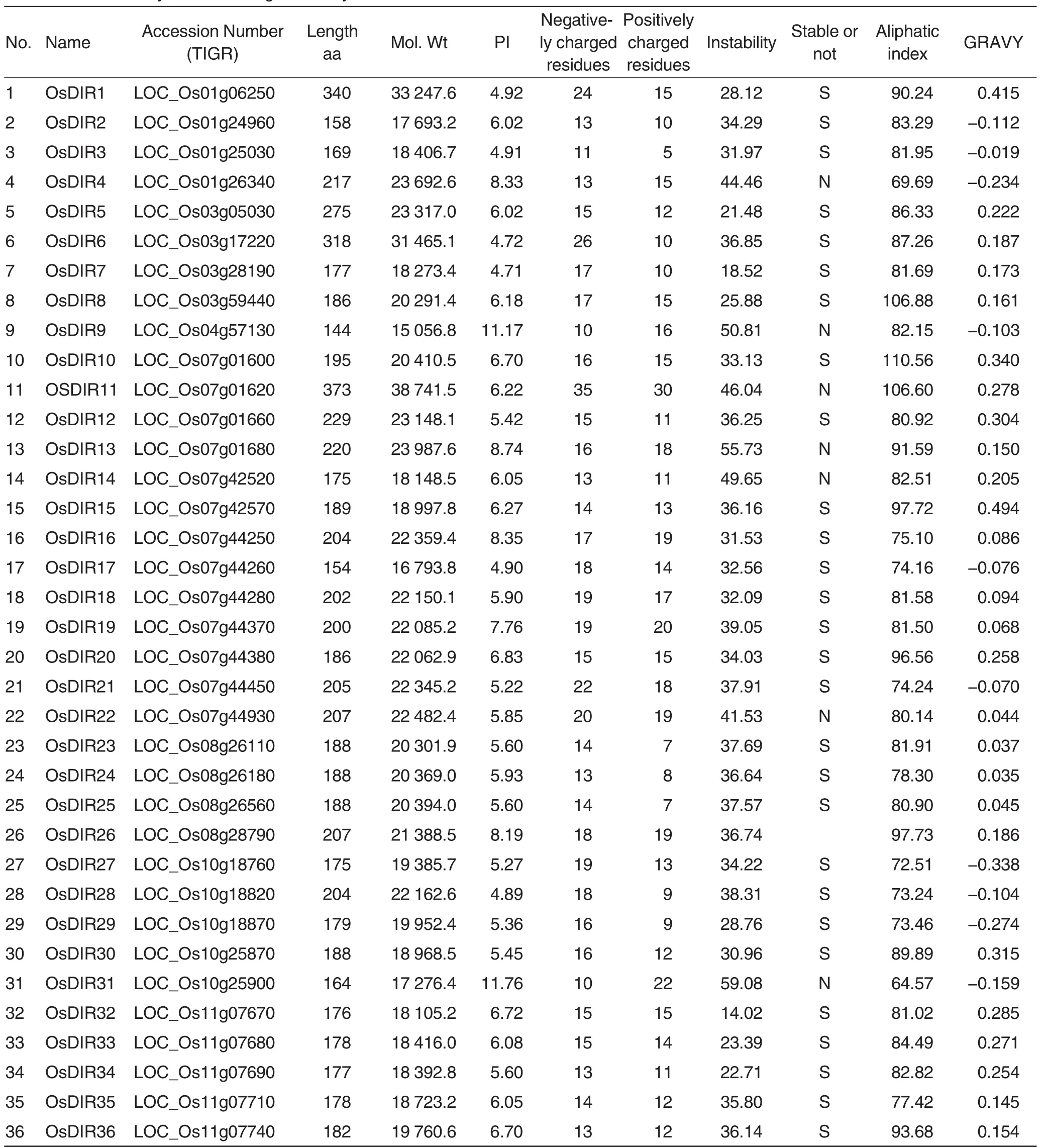
Table 1 Protein analysis of OsDIR gene family
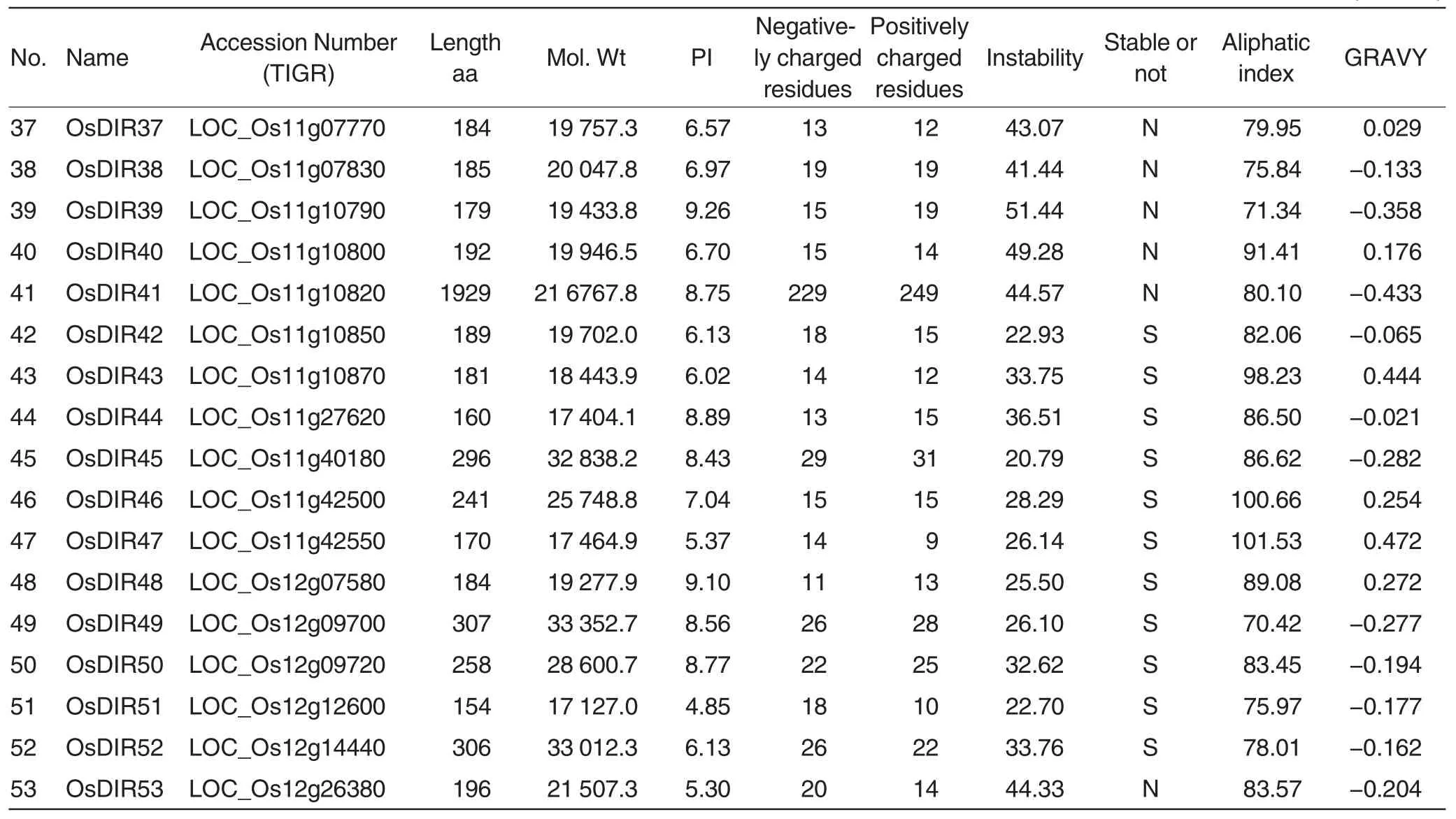
Continued(Table 1)
RNA isolation and cDNA synthesisSamples collected from different days (1stand 4thd)of flowering period in different stress conditions with three replicates.In order to prevent RNA degradation, liquid nitrogen and RNase-away was used throughout the sampling process.-80℃ refrigerator was also temporary used for samples deposited.Total RNA was isolated from spikelet(including palea,lemma, stamen and pistil)using TRIZOL reagent(Invitrogen,Germany)according to the manufacturer’s instructions. RNA concentration was measured in an Eppendorf Biophotometer,and 3 μg of total RNA was digested with RQ1 RNase-free (Cat.#M6101,promega corp.,USA).Absence of genomic DNA contamination was subsequently confirmed by PCR,using primers designed on intron sequence of a control gene (GAPDH).RNA integrity was checked on a 2.0% (w/v)agarose gel prior to,and after digestion(Fig.1). Ploy (A)+RNA was purified with an Oligotex mRNA Mini Kit(Qiagen,Germany),using the supplier’s batch protocol.RT reactions were performed with SuperScriptTMⅢ reverse transcriptase (Invitrogen GmbH),according to the manufacturer’s instructions. The efficiency of cDNA synthesis was assessed by PCR amplification of rice house keeping gene GAPDH.According to the detection of RT-PCR,which showed that the product of PCR amplification both in 25 and 30 cycles increased linearly, and the bands checked in agarose gel reacted in 25 cycles was clear seen with significant differences,in this experiment,therefore,the 25-cycle PCR process was utilized.
Observations
Lignin-stainingandmicroscopic analysisAnthers dissected from spikelets stressed by different treatments as mentioned before,were fixed by agarose gel,and the slicing machine was used to slice the samples with the thickness setting of 10 μm. Each full slice sample in each treatment was collected on the slide for lignin-staining,respectively.Staining reagents was mixed with 18%hydrochloric acid solution and saturated solution ofphloroglucinolwith the same volume.After 5 min,each slice sample was observed under stereomicroscope(Olympus SZX7,Olympus Corp.,Japan)with 50×magnification. Under this condition,the anther dehisced parts of the cross sectional where the lignin-containing region was dyed to be reddish purple.Microscope with DP70 digital camera was used for anther cross sectional diagram acquisition and analysis.
Anther dehiscenceAnther dehiscence of the main-tiller in two varieties under different treatment conditions was real-time observed from the first day to forth day was about 30 min after flowering(about the time interval needed for palea and lemma shut down after flowering).The high-power digital camera (DP70)was also applied for image acquisition that used for daily percentage of anther dehiscence statistics.Anthers were removed after detection in the testing day to avoid diffusing with the next day.
Statistic analysisData on RWC and anther dehiscence were analyzed as a two way completely randomized design using SPSS 13.0(Version 13, LEAD Technologies Inc.)with five replications.Tukey’s least significant difference (LSD)at a probability level of 5%was used to compare the differences between treatments and genotypes.
Results and Analysis
Relative water content of flag-leaf and water stress
Managed WS resulted in flag-leaf RWC.Before WS and WS+HT treatment,the flag-leaf RWC were more than 90%in both varieties,and there were differences (PV=0.13)in neither varieties nor treatments (PT=0.09). While,employed WS resulted in flagleaf RWC being maintained around 60%-70%throughout the stress period,which coincided with flag leaf rolling in two genotypes.After 4 days’WS treatment,the RWC of flag-leaf in two varieties were decreased by 26.78% and 28.98%,respectively. There were no differences between treatments managed by WS neither on the 1std (PD1=0.09)nor on the 4thd (PD4=0.21),and this provided evidence that plants in this experiment both in WS and WS+HT treatments were basically consistentthroughoutthe stress period(Fig.2).
OsDIRgeneexpressionprofiles under stresses
Gene expression profiles of selected 15 OsDIR genes under different treatments were obtained by using semi-quantitative RT-PCR,which was consistent with the online MPSS analysis.Results showed that only a few of these 15 selected OsDIR genes including OsDIR-08(LOC_Os03g594-40),OsDIR-12(LOC_Os07g01660), OsDIR-26 (LOC_Os08g28790),Os-DIR-43(LOC_Os11g10870),were expressed in floret(Fig.3A)due to difference of sampling from organs in rice. Moreover,these expressed genes in floret showed different profiles both in variety and in treatments:1)gene Os-DIR-08,which was expecting as rice candidate gene for HT stress tolerance,was increasingly expressed either in 25-clycles or in 30 cycles of PCR under normal condition in both varieties,decreasingly expressed under HT treatment from the 1stto 4thflowering day,and showed enhanced expression on the 1std of WS treat-ment,while decreased in WS+HT treatment only in N22;2)gene OsDIR-12 was expressed with low level in the floret under control conditions or HT stress and increasingly expressed when treated with WS or WS+HT on the 1std in N22;3)gene OsDIR-26 was only expressed in the HT tolerant variety N22 with relative low level;4)relative expression level of the gene Os-DIR-43 was not affected by stresses in both varieties.Furthermore,there was an interesting expression pattern on the target gene OsDIR-08 under HT stress,which was detected by using different cycles during the process of PCR(Fig.3B):during the linear amplification of the PCR (25-cycles),gene OsDIR-08 expression was already enhanced on the 1std of flowering,either in N22,or in Moroberekan.However, after 4 d of HT stress,although the expression level of this gene under normal conditions showed a proof of an increasing trend in both varieties,the expression level of gene OsDIR-08 in N22 was relatively decreased compared to both the first stressed day andcontrol,while enhanced along with the HT stress in Moroberekan.
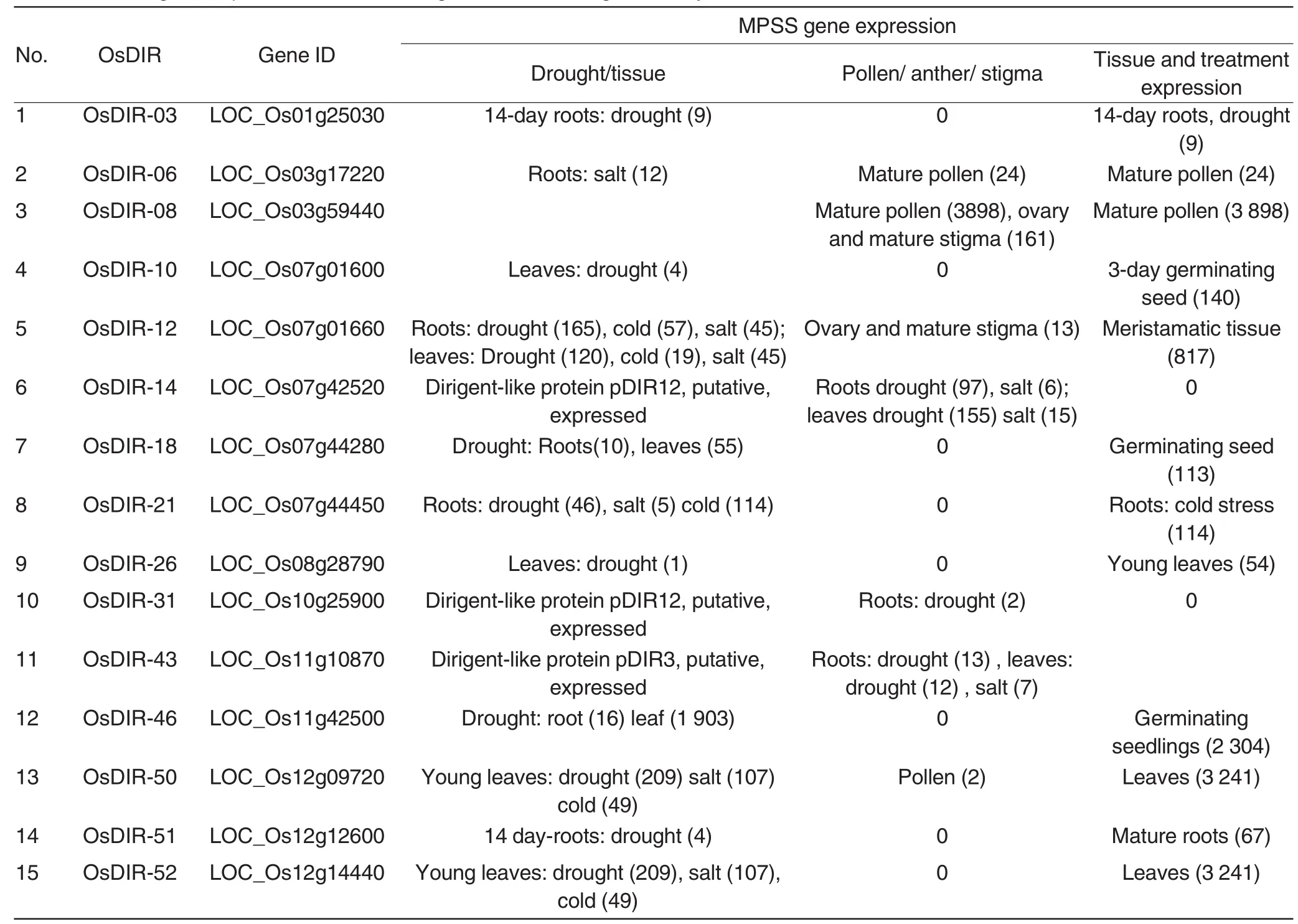
Table 2 MPSS gene expression of 15 OsDIR genes in rice DIR gene family
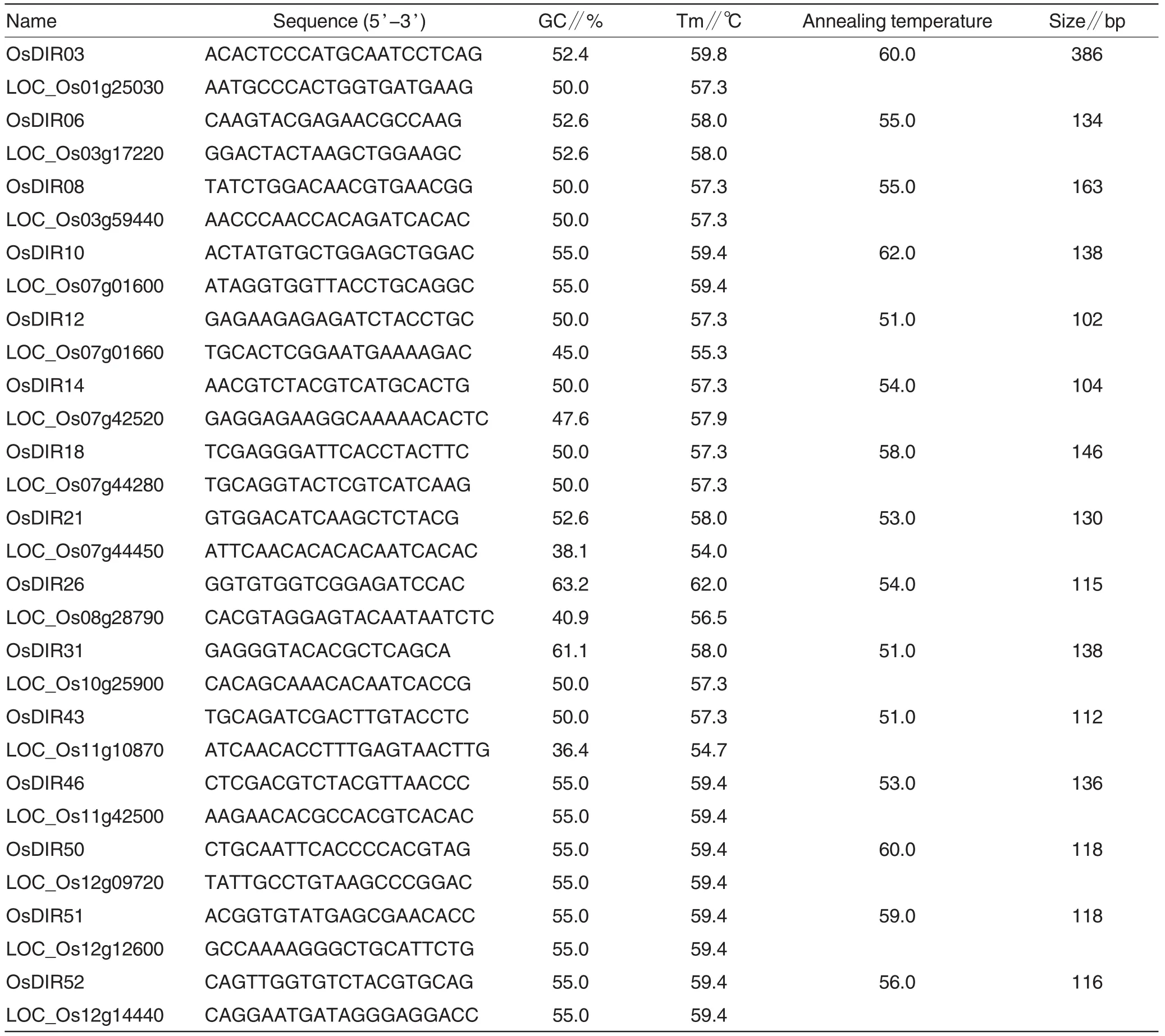
Table 3 Basic information of 15 OsDIR gene specific primers
Lignin-stainingandmicroscopic observation
In order to figure out the correlation between anther dehiscence and stresses,antherlignin-staining and microscopic observation were carried out under HT,WS,and WS+HT stresses using two rice genotypes. Results suggested that stress affected the lignin synthesis at the anther dehisced site according to the ligninstaining of the cross-sections of anther (Fig.4).Under normal conditions,the lignin synthesis at the anther dehisced site showed a cumulative trend during the flowering period,while on the 1std of flowering under HT stress,either in N22,or in Moroberekan,the stainedregion was obviously increased.However,there were differences between varieties presented in stained-region after four consecutive days of HT stress,that the stained-region was decreased at the dehisced site in N22 under the cumulated effect of HT stress compared to the control,and increased in Moroberekan compared to either control or N22.Additionally,the stained-region was increased at the dehisced site treated by WS compared to that under the normal condition inboth varieties,while decreased in the HT tolerant variety N22,compared either to control or to any single stress, under the combination of WS and HT stress.
Anther dehiscence
Although there was an uncertain relevance between the pollen number scattered on the stigma and anther dehiscence under specific stress treatment,such as HT,WS,and the combined stress with HT and WS,etc., the number of germinated pollen on the stigma was significantly related to the anther dehiscence,thus significantly affected the seed setting in rice[19].HT,WS,and the combined stress,especially the HT stress,could disturb anther dehiscence,the negative effect shown not only in the existing of cumulative effect,and there were differences between varieties and treatments.Under the normal conditions,there were no differences between genotypes during flowering period (from the 1std to the 4thd)in anther dehiscence (PN22=0.85,PMoroberekan= 0.71),while in the managed conditions for HT stress tolerance variety N22,although the anther dehiscence was significantly decreased in each treatment compared to the control(P<0.01),there were no differences between HT and WS,and between HT and WS+HT.However,there were significant differences between WS and WS+HT in anther dehiscence (P<0.01).For anther dehiscence in HT sensitive variety Moroberekan under different applied stresses,both between HT and WS and between WS and WS+HT showed significant differences(P<0.05),while no differences between HT and WS+HT(PHT/WS+HT= 0.700).These results suggested that WS was the main factor for HT tolerant variety and HT was the main factor for HT sensitive variety resulting in the different performances in anther dehiscence under different stresses (Fig.5).
Discussion
Dirigent protein plays an important role in lignin biosynthesis process[22].In rice(Oryza sativa subsp. japonica),there are at least 53 paralogs DIR genes,which were temporal and spatial expressed in organs such as roots,stems,leaves,and florets, from seedling to mature stages with different levels,under normal conditions.The hypothesis of this study was that there would be a correlation between anther dehiscence and lignin synthesis at the anther dehisced site region.Illumination of this question could provide a reference to interpret the phenomenon of anther dehiscence disturbed by abiotic stresses.
Anther dehiscence is one of the most sensitive physiological processes of rice production to high temperature stress[4,40].Under HT stress condition, the cell layers were closed at the anther dehisced site that led to the failure of anther dehiscence,followed by inadequate pollination and decreased seed-setting,in rice,especially in the varieties sensitive to HT[41-42].Jagadish et al.[12]have reported the up-regulation of dirigent protein in a heat-sensitive rice genotype,Moroberekan,in response to heat stress.In this study, the expression profiles of 15 selected OsDIR genes in rice anthers indicated that only a few of them were expressed with high level,most of the OsDIR genes were not expressed or expressed with low levelin rice spikelets under stress conditions.The expressed OsDIR genes showed different expression profiles under different stresses.Especially for the target gene OsDIR-08(LOC_Os03g59440), which was expected as rice candidate gene for HT tolerance,was increasingly expressed under normal condition in both varieties,while decreasingly expressed under HT treatment from the 1stto 4thflowering day,and showed enhanced expression on the 1st WS treated day,while decreasingly expressed in WS+HT treatment only in N22.In addition,these expressed genes could be classified to different sub-categories (OsDIR-12 and Os-DIR-26 were classified to DIR-d subcategories,OsDIR-08 for the DIR-e sub-categories,OsDIR-43 could not be classified to any sub-category).Diverse function of the OsDIRs was implied according to their expressed profiles:the down-regulated expression of the OsDIRs under high temperature and/or water stress,might be related to the lignin biosynthesis of the anther at the dehisced site;and the up-regulated expression of the OsDIRs under stress,might be reflected their function as stress defense proteins.
There was a negative cumulative effect on the level of rice anther dehiscence under HT stress,and such a negative cumulative effectcompetence to the HT resistant in varieties, which was consistent with the seed setting when rice was subjected to HT[19].Such effect reflected on the anther dehiscence and OsDIR gene expression profile of N22 indicating true tolerance of HT and to WS+HT during anthesis.Similar results were obtained from earlier reports of Mackill[3], Satake and Yoshida[42]and more recently it was clearly shown that N22 had a significantly higher pollen count and pollen germination on the stigma compared to the most sensitive Moroberekan.Further in comparison,N22 had a normal rate of pollen tube growth compared to significantly slower pollen tube growth rate with the moderate tolerant IR64[11].
Varies of the expression of target gene in stresses intuitively reflects on the lignin-staining at the anther dehisced sites,that the corresponding changesbetween geneexpression and lignin-staining implied an important interrelations,i.e.a negative correlation between the amount of lignin synthesis and gene (s)expression. However,there were incompletely correspondence relationships between anther dehiscence and gene expression under managed stresses.For example,the expression of the target gene visibly decreased in spikelets under WS+HT stress compared to control,while the percentage of the anther dehiscence was lower than that under control;another example was that the expression of the target gene enhanced expressed in the florets during the flowering period under normal conditions,while there were no differences in anther dehiscence among each flowering day under the same conditions (P>0.05).These examples,on the one hand,proved that the differences in the expression of a single gene or few genes might not sufficiently reflect or respond to the differences in physiological process;on the other hand,indirectly suggested that the effect of abiotic stresses such asHT,WS,and WS+HT,on anther dehiscence in rice was one aspect of stress response,while the complex physiological mechanisms response and the network regulation in mechanisms to abiotic stress(es)remain not clear.
Conclusions
Anther dehiscence was affected by varieties of abiotic stresses in rice. Specifically,HT stress disturbed anther dehiscence with a negative cumulative effect.The main reason of such effect might be that the differentially expressed of OsDIR under various managed stresses caused the difference of the lignin-staining at the anther dehisced site,and thus disturbed anther dehiscence correspondingly.
Acknowledgments
Sincere thanks to the International Rice Research Institution(IRRI)for the supplying of seeds and experimental facilities. Anthony and Raniare thanked for the excellent crop management.Aid program for science and technology innovative research team in higher educational institutions of Hunan province is appreciated.
[1]TESTER M,BACIC A.Abiotic stress tolerance in grasses.From model plants to crop plants [J].Plant Physiology, 2005,137:791-793.
[2]IntergovenmentalPanelonClimate Change(IPCC).Fourth assesement report of the intergovrnmental panel on climate change:the impacts,adaptation and vulberability [M].United Kingdom and New York,USA:Cambridge University Press,2007.
[3]MACKILL DJ.Studies on the mechanism and genetics of high temperature tolerance in rice[D].Los Banos:IRRI, 1981.
[4]YOSHIDA S.High temperature stress in rice[C]//IRRI Research Paper Series. Los Banos:IRRI,1981:67.
[5]YOSHIDA T,SAKUMA Y,TODAKA D, et al.Functional analysis of an Arabidopsis heat-shock transcription factor HsfA3 in the transcriptional cascade downstream of the DREB2A stressregulatory system[J].Biochemistry and BiophysicsReseachCommunication, 2008,368:515-521.
[6]MATSUI T,OMASA K J.Rice(Oryza sativa L.)cultivars tolerance to high temperature at flowering:anther characteristics[J].Ann Bot,2002,89:683-687.
[7]LAFITTE H R.Relationshop between leaf relative water content druing reproductive stage water deficit and grain formation in rice[J].Field Crops Reserch, 2002,76,165-174.
[8]LAFITTE HR,LI ZK,VIJAYAKUMAR CHM,et al.Improvement of rice drought tolerance through backcross breeding: Evaluation of donors and selection in drought nurseries[J].Field Crops Reserch,2006,97:77-86.
[9]LIU J X,LIAO D Q,OANE R,et al.Genetic variation in the sensitivity of anther dehiscence to drought stress in rice[J]. Field Crops Reserch,2006,97:87-100.
[10]JAGADISHSVK,CRAUFURDPQ,WHEELER TR.High temperature stress and spikelet fertility in rice(Oryza sativa L.)[J].JournalofExperimental Botany,2007,58(7):1627-1635.
[11]JAGADISH SVK,CRAUFURD PQ, WHEELER TR.Phenotyping parents of mapping populations of rice(Oryza sativa L.)for heat tolerance during anthesis[J].Crop Science,2008,48: 1140-1146.
[12]JAGADISH SVK,MUTHURAJAN R, OANE R,et al.Physiological and Proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.)[J].Journal of Experimental Botany,2010,61(1):143-156.
[13]MITTLER R.Abiotic stress,the field environment and stress combination [J].Trends of Plant Science,2006,11 (1):15-19.
[14]RIZHSKY L,LIANG HJ,SHUMAN J,et al.When defense pathways collide: the response of Arabidopsis to a combination of drougth and heat stress[J]. Plant Physiology,2004,134:1-14.
[15]SAVIN R,NICOLAS ME.Effects of short periods of droughtand high temperature on grain growth and starch accumulation of two malting barley cultivars[J].Australian Journal of Plant Physiology,1996,23,201-210.
[16]WANG Z,HUANG B.Physiological recovery of kentuchy bluegrass from simultaneous drought and heat stress [J].Crop Science,2004,44:1729-1736.
[17]RIZHSKY L,LIANG HJ,MITTLER R. The combined effect of drought stress and heat shock on gene expression in tobacco [J].Plant Physiology,2002, 130:1143-1151.
[18]Shah NH,Paulsen GM.Interaction of drought and high temperature on photosynthesis and grain-filling of wheat [J].Plant Soil,2003,257:219-226.
[19]RANG ZW,JAGADISH SVK,ZHOU QM,et al.Effect of high temperature and water stress on pollen germination and spikelet fertility in rice[J].Environmentaland ExperimentalBotany, 2011,70:58-65.
[20]DAVIN LB,WANG HB,CROWELL AL, et al.Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent)protein without an active center[J].Science,1997,275 (5298): 362-367.
[21]RALPH S,PARK JY,BOHLMANN J, et al.Dirigent proteins in conifer defense:gene discovery,phylogeny,and differential wound-and insect-induced expression of a family of Dir and Dirlike genes in spruce (Picea Spp.)[J]. Plant Molecular Biology,2006,60(1): 21-40.
[22]DAVIN LB,LEWIS NG.Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursorcoupling in lignan and lignin biosynthesis[J].PlantPhysiology,2000, 123(2):453-462.
[23]RALPH SG,JANCSIK S,BOHLMANN J.Dirigent proteins in conifer defense ii:extended gene discovery,phylogeny,and constitutive and stress-induced gene expression in spruce (Picea Spp.)[J].Phytochemistry,2007, 68(14):1975-1991.
[24]LI Q,CHEN JF,XIAO Y,et al.The Dirigent Multigene family in Isatis indigotica:gene discovery and differential transcript abundance[J].BMC Genomics,2014,15(1):388.
[25]GANG DR,COSTA MA,FUJITA M,et al.Regiochemical control of monolignol radical coupling:a new paradigm for lignin and lignan biosynthesis[J]. Chemistry and Biology,1999,6(3): 143-151.
[26]SUH MC,KIM M J,HUR CG,et al. Comparative Analysis of Expressed Sequence Tags From Sesamum Indicum and Arabidopsis Thaliana Developing Seeds[J].Plant Molecular Biology,2003,52(6):1107-1123.
[27]BHUIYAN NH,SELVARAJ G,WEI YD,et al.Role of lignification in plant defense[J].Plant Signal Behav,2009, 4(2):158-159.
[28]KIM MK,JEON JH,DAVIN LB,et al. Monolignol radical-radical coupling networks in western red cedar and Arabidopsis and their evolutionary implications[J].Phytochemistry,2002,61(3): 311-322.
[29]MISSON J,RAGHOTHAMA KG,JAIN A,et al.A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation[J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(33): 11934-11939.
[30]NAKANO M,NOBUTA K,VEMARAJU K,et al.Plant MPSS databases:signature-based transcriptional resources for analyses of mRNA and small RNA[J].Nucleic Acids Reserch,2006,34: 731-735.
[31]DAVIN L B,LEWIS NG.Lignin primary structures and Dirigent sites[J].Current Opinion in Biotechnology,2005, 16(4):407-415.
[32]YOO JH,LEE HJ,KANG K,et al.Lignans inhibit cell growth via regulation of Wnt/β-catenin signaling [J].Food and Chemical Toxicology,2010,48 (8):2247-2252.
[33]FOYER CH,NOCTOR G.Oxidant and antioxidant signaling in plants:a reevaluation of the concept of oxidative stress in a physiological context[J]. Plant,Cell and Environment,2005,28 (8):1056-1071.
[34]CABANE M,AFIF D,HAWKINS S. Lignins and abiotic stresses[J].Advances in Botanical Research,2012, 61:245-247.
[35]YOSHIMURA K,MASUDA A,KUWANO M,et al.Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon)under water deficits [J].Plant and Cell Physiology,2008, 49(2):226-241.
[36]ALVAREZ S,MARSH EL,SCHROEDER SG,et al.Metabolomic and proteomic changes in the xylem sap of maize under drought[J].Plant,Cell and Environment,2008,31(3):325-340.
[37]PRASAD PVV,BOOTE KJ,SHEEHY JE,et al.Species,ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress[J].Field Crops Reserch,2006,95:398-411.
[38]SELOTE DS,CHOPRA PK.Drought induced spikelet sterility is associated with an inefficent antioxidant defense in rice panicles[J].Plant Physiology, 2004,121:462-471.
[39]ROZEN S,SKALETSKY HJ.Primer3 on the WWW for general users and for biologist programmers[C]//In:Krawetz S,Misener S (eds)bioinformatics methods and protocols:methods in molecular biology.Totowa:Humana Press,2000:365-386.
[40]MATSUI T,OMASA K,HORIE T.High temperature-induced spikelet sterility of japonica rice at flowering in relation to air temperature,humidity and wind velocity conditions[J].Japense Journal of Crop Science,1997,66(3):449-445.
[41]HORIE T.Effect of elevated CO2 and blobal climate change on rice yield in Japan[C]//In:Omasa K,Kai K,Taoda H,Uchijima Z,Yoshino M,eds.Climate change and plants in east Aisa. Tokyo,Springer Japan,1996:39-56.
[42]SATAKE T,YOSHIDA S.High temperature-induced sterility in indica rice at flowering[J].Japanese Journal of Crop Science,1978,47:6-10.
Responsible editor:Qingqing YIN
Responsible proofreader:Xiaoyan WU
*Corresponding author.E-mail:rzwronger@126.com
Received:June 1,2015 Accepted:August 1,2015
杂志排行
Agricultural Science & Technology的其它文章
- Establishment of a Method for Determination of Anemoside B4 Content in Pulsatilla Water Extract
- Screening and Evaluation of Different Wheat Cultivars for Resistance to Sitobion avenae at Seedling and Adult-plant Stages
- Interspecific Cross Compatibility of Rhododendron in Changbai Mountain
- Variations of Frost-free Period and Its Impact on Grain Yields in Henan Province during 1961-2013
- Influences of Surface Drip Irrigation on the Growth,Yield and Quality of Several New Species of Guitang Sugarcane
- Fuzzy Analysis Method for Orthogonal Test on Seed Propagation of Eurya chinensis
