The Effect of Crop Rotation on Soil Nematode Community Composition in a Greenhouse
2015-02-05JingwenLUWeiSHENGQianYUZijingCHENQiangXUQianWANGLinlinDONG
Jingwen LU,Wei SHENG,Qian YU,Zijing CHEN,Qiang XU,Qian WANG*,Linlin DONG
1.China Agricultrual University,Beijing 100193,China;
2.Institute of Chinese Materia Medica,China Academy of Chinese Medical Sciences,Beijing 100700,China
The Effect of Crop Rotation on Soil Nematode Community Composition in a Greenhouse
Jingwen LU1,Wei SHENG1,Qian YU1,Zijing CHEN1,Qiang XU1,Qian WANG1*,Linlin DONG2*
1.China Agricultrual University,Beijing 100193,China;
2.Institute of Chinese Materia Medica,China Academy of Chinese Medical Sciences,Beijing 100700,China
[Objective] The aim was to identify changes in a nematode community in response to crop rotation and to determine the appropriate catch crop for a greenhouse.[Method]The experiment was carried out in a typical 6-year-old greenhouse, in which cucumber crops were cultivated twice each year(in spring and autumn), and catch crops were planted in summer.The total number of nematodes was counted and nematode community indices were calculated after collecting soil samples in different stages.[Result]Total nematode abundance was decreased in the soils of catch crop in contrast with former crops(cucumber crops).The abundance of the nematode community was reduced in the treatment of crop rotation compared to the soils of catch crop.In addition,the number of nematode taxa was significantly reduced by the treatment of crown daisy compared to the treatments of following crops.Crop rotation regulated the functional composition of the nematode community by increasing the omnivores-predators functional group and decreasing the relative abundance of root herbivores.[Conclusion]These results indicate that crop rotation affects the nematode community in abundance,diversity and functional composition of the nematode community and crown daisy can be served as the most appropriate catch crop in the greenhouse.
Nematode;Crop rotation;Catch crop;Continuous cropping obstacle
C ontinuous cropping in vegetable production systems causes a series of obstacles through poor plant growth and the promotion of soilborne diseases in the greenhouse[1-2].Among the economically important horticultural plants that are susceptible to continuous cropping obstacles,cucumber cultivation occupies an area of up to 1.55×107hm2in China,which accounts for 5.62%of the combined vegetable cultivation area[3].At present,an estimated 67.60%of the greenhouses in the main vegetable cultivation region are infected by soilborne diseases under continuous cropping conditions[4]. Therefore,continuous cropping obstacles are the primary restricting factors in the development of cucumber in the greenhouse.Recently,several effective methods have been developed to overcome continuous cropping obstacles in greenhouses.Among these measures,crop rotation plays an important role in enhancing soil organic matter and reducing soilborne disease, and the cultivation of catch crops in summer has proven to be a useful and common measure[5-6].
Soil nematodes are widespread, abundant,and highly diverse under many environmental conditions;they occupy multiple positions at several trophic levels in soil food webs[7].The overall nematode community,including root herbivores,bacterivores,fungivores,and omnivores-predators,is an effective indicator of soils that have been subjected to anthropogenic management,and the soil nematode community is used to indicate soil quality and health[8-10].Among soil nematodes,herbivores damage various crops and cause more than$100 billion annually in crop losses in the world;they are one of the most important factors that create continuous cropping obstacles in the greenhouse[11-12].Recent studies have shown that the functional composition of the soil nematode community is a model group for the soil animal food web[13-14], and changes in nematode community composition are generally indicative of shifts in environmental conditions[7,15-16]. In addition,the ratio between fungal and bacterial feeders can assign the major decomposition channels,and a decrease in herbivore infestation can contribute to over-yielding[17-18].Many studies have reported agricultural practices,such as crop rotation,can decrease the nematode populations and density in the field[19-20].Moreover, present study has reported that several cash crops such as crown daisy, common bean,and sweet corn act as catch crops that can alleviate nematode infections and reduce nematode abundance in the greenhouse[6].Nevertheless,rare studies have examined the composition and changes in the nematode community in response to crop rotation,in the greenhouse.And catch crops are lacking that can effectively improve soil quality and health in the greenhouse by mediating the composition of the nematode commu nity,and an urgent need exists to identify useful catch crops.
The objectives of this study were to characterize the effects of crop rotation on the nematode community in the greenhouse and to determine appropriate catch crops that can improve soil quality and health in the greenhouse.Understanding of the responses of and changes in nematode communities to crop rotation will help identify the mechanisms underlying this traditional method for overcoming continuous cropping obstacles.
Materials and Methods
Plant species
All plant species,cucumber(Cucumis sativus L.),sweet corn(Zea mays L.),crown daisy(Chrysanthemum segetum L.),green onion(Allium fistulosum L.),and kidney bean (Phaseolus vulgaris.L.)were produced by the Vegetable Research Institute,Beijing,China.Seeds of all species were surface sterilized with a solution of 15%w/w H2O2for 5 min, washed thoroughly in distilled water, and then placed on moist filter paper. Germinated seeds were planted following traditional practices.
Soil properties and plant growth conditions
This experiment was carried out in a typical 6-year-old greenhouse,in which cucumber crops were cultivated twice each year(in spring and autumn),and catch crops were planted in summer from 2005 to 2010 in Beijing(40°3′N,116°28′E).The soil samples in the greenhouse was a silty loam soil and had the following properties:pH(in water)6.36,EC 0.42 ms/cm;available nutrients:total N 1.32 g/kg,Olsen-P 241.4 mg/kg,and NH4OAc-K 202.5 mg/kg.Furrow irrigation systems were used to supply water in the greenhouse.The combination of NPK compound fertilizer(15-15-15,109.5 kg/hm2),organic fertilizer (164.3 kg/hm2),and chicken manure (164.3 kg/hm2)was used as the base fertilizer for the spring crop.No fertilizer was added during catch crop periods.The greenhouse conditions were 26/20℃and 14/10 h cycles with a relative humidity of 70%-75%.Soil water content was maintained at approximately 50%WHC(Water Holding Capacity)by the daily supply of water.
Experimental design
The experiment was conducted as a complete randomized block design with three replicates;each replicate plot was 5.80×1.05 m and maintained under the same management regime.Cucumber crops were cultivated from February 26 to June 9(spring crop),and autumn crops(cucumber) were planted from August 30 to December 11;in addition,catch crops (crown daisy,green onion,sweet corn, and kidney bean)were cultivated from June 13 to August 20 in 2010.
Collection of plant and soil samples
Soil samples were collected from five completely randomized cores per plot at depths of 0-30 cm(depths of cucumber rhizospheres)after harvest; samples were collected on June 9, August 26,and December 11.Nematode populations were extracted from 100 g soil according to the Baermann funnel method as described by Hooper[21],with minor modifications.Nematode populations were collected every 12 h and successively gathered four times.After counting the total number of nematodes,the first 100 individuals in each sample were identified to the genus level using an inverted compound microscope.If the total number of nematodes was less than 100,all nematodes were identified.
Calculations and indices
Nematode abundance was presented as the number of nematodes per 100 g dry soil.Nematodes were divided into four trophic groups according to the descriptions of Yeates et al[9].
The following nematode community indices were calculated.Nematode taxon richness,the number of genera identified,was determined according to Yeates et al[9].The Shannon diversity index(H’)was calculated as an indicator of the diversity of the nematode community[22].The maturity index(MI)and the plant parasite index (PPI)were calculated according to Bongers[23].
Statistical analyses
SPSS 11.0(SPSS Inc.,Chicago, IL)was used for all statistical analyses. Analysis of variance(ANOVA)was used to compare parameters among treatments.Mean values were compared by calculating least significant differences(LSD)at the 5%level.
Results and Discussions
Crop rotation affected the abundance of the nematode community
Crop rotation affects the total abundance of the nematode community in the greenhouse(Fig.1).Catch crop treatments,crown daisy,green onion,and sweet corn,reduced total nematode abundance by 58.2,45.4, and 18.2%compared to their former crops(cucumber crops),respectively, but kidney bean increased nematode abundance by 13.8%.Crop rotation exerted effects to reduce the abundance of the soil nematode community in the following crop.Nematode abundance was reduced by 74.1,22.4, 11.6,and 48.9%compared to the crown daisy,green onion,sweet corn, and kidney bean catch crop treatments,respectively.Among effects of crown daisy,as catch crop,were significant for the former and following crops.
Crop rotation affected the taxonomic diversity of the nematode community
Thirty-one genera were identified. Bacteriovorous taxa included Eucephalobus,Cephalobus,Acrobeles, Acrobeloides,Chiloplacus,Cervidellus,Rhabaditis,Mesorhabditis, Alaimus and Monhystera.Fungivorous genera consisted of Aphelenchus, Paraphelenchus,and Leptonchus. Herbivorous taxa comprised Ty-lenchus,Filenchus,Malenchus,Lelenchus,Rotylenchus,Pararotylenchus,Helicotylenchus,Hoplolaimus,Tylenchorhynchus,Meloidogyne,Boleodorus,andTrichodorus. Omnivores-predators genera includedDorylaimus,Mesodorylaimus,Eudorylaimus,Aporcelaimus,Mononchus, andChromadorita.
Crop rotation affected the taxonomic diversity of the nematode community(Fig.2).Nematode richness ranged from 11 to 21 genera(Fig.2A). The crown daisy treatment significantly reduced the number of nematode taxa compared to the following crop; however,nematode genera were not significantly affected by crop rotation with sweet corn,green onion,and kidney bean.Indices of nematode diversity(H’)did not differ significantly in systems with crop rotation(Fig.2B).
Crop rotation altered the functional composition of the nematode community
Crop rotation affected relative abundance within the functional composition of the nematode community (Table 1).Crop rotation increased the relative abundance of omnivorespredators compared with the former crop and improved the abundance of omnivores-predators in the following crop.Among the catch crops,crown daisy was the most effective at improving the abundance of omnivorespredators in the following crop.Crop rotation altered the relative abundance of plant feeders(herbivores).Crown daisy and sweet corn reduced the relative abundance of plant feeders,but green onion and kidney bean increased the relative abundance of plant feeders compared to the former crop.In addition,crown daisy and kidney bean catch crops suppressed the relative abundance of plant feeders. The relative abundance of fungal feeders(fungivores)was reduced by crown daisy and green onion catch crops, and the decreased relative abundance in fungal feeders persisted into following crops of crown daisy and kidney bean.However,fungal feeders are reclusive groups in nematode communities.Bacterial feeders(bacterivores)are ascendant groups in nematode communities,and increases in the relative abundance of bacterial feeders were found with crown daisy catch crops.However,decreases in the relative abundance of bacterial feeders were found with catch crops of green onion,sweet corn,and kidney bean.
Catch crops increased the maturity index(MI),which was higher in crops following crown daisy,sweet corn,and kidney bean compared to former crops(Fig.3A).And these effects were maintained for crown daisy in the subsequent cucumber season. A decrease in the plant parasite index (PPI)was found in crop rotation of cucumber-crown daisy-cucumber(Fig. 3B).PPI tended to be low in the cucumber-sweet corn-cucumber treatment.However,catch crops of green onion and kidney bean increased the PPI compared to former crops.
Discussions
These results show that catch rotation was useful for suppressing theabundance of the soil nematode community,and that crown daisy was the most effective catch crop.Crop rotation is a traditional method for altering nematode abundance to improve soil quality in greenhouses,and many studies have found that crop rotation can reduce nematode populations, change microbial activities,and alter soil organic matter content to increase crop yield[24-26].And crown daisy displayed strong potential as a catch crop or an intercrop to suppress nematode populations in greenhouse environments[6,27].Our results were very similar to the findings of these studies.
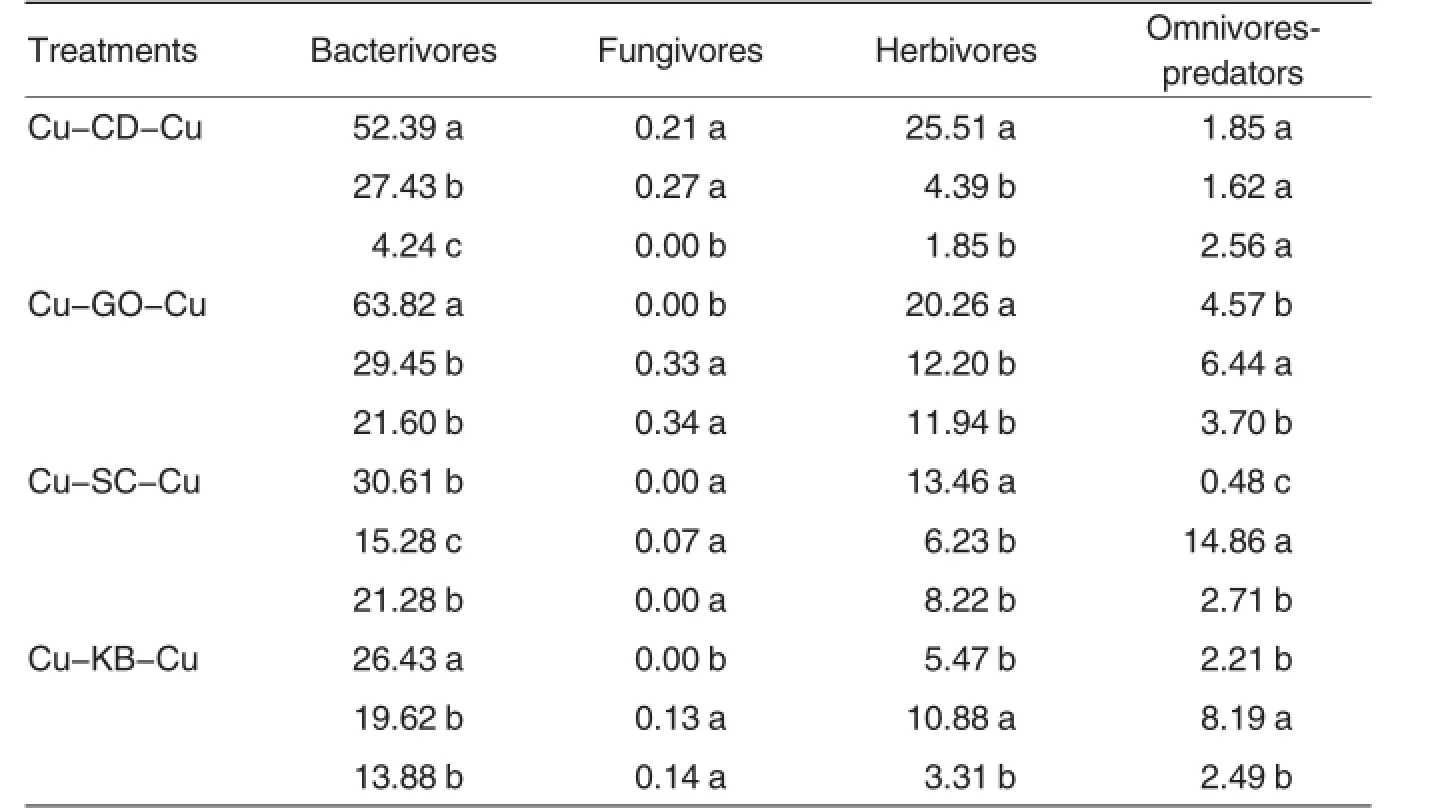
Table 1Effects of different catch crop on the relative abundance of trophic composition per 100 gram dry soil
Data on how crop rotation affects the functional composition of nematode communities are lacking at present.Our results showed that crop rotation altered trophic groups within the nematode community;catch crops increased the ratio of groups at higher trophic levels of the food chain and improved conditions for subsequent crops.These data indicate that crop rotation changed the functional structure of the soil food web and increased the number of omnivores-predators, which represented an increase in food web complexity,and that the effects of crown daisy were significant.These changes suggest that the background food web was more consolidated[17], which helps explain the positive effects in altered greenhouse soil quality.The maturity index provided information about the response of the environment to stress and served as an indicator of soil condition[28].An increase in MI can reflect a reduction of stress in the greenhouse environment due to crop rotation.In other words,crop rotation did not markedly affect the background energy pathways.
Moreover,the effects of crop rotation on nematode diversity depended on the species of the catch crop. Cucumber-sweet corn rotation and cucumber-kidney bean rotation increased the number of nematode genera,and an increase in diversity (H’)was found with cucumber-kidney bean rotation.However,a marked decrease in general richness was found with crown daisy-cucumber rotation, and the low numbers for richness were mainly caused by the suppression of herbivores.Meanwhile,decreases in the plant parasite index(PPI)are indicative of a reduced ability to reproduce and resist stress.Taken together,these results indicate that crop rotation mainly suppressed the abundance and diversity of herbivores.As catch crops,many plants showed potential to alleviate nematode damage on crops,such as Asteraceae and Zea plants,by reducing the root-knot index and soil nematode populations[6,29-30]. One of the most important reasons for these reductions was the lack of habitats and food resources due to crop rotation for herbivore species that rely on particular host plants[17].Moreover, many crops naturally release nematotoxic compounds into the environment, either from their roots or directly from plant tissues,to suppress nematode populations.One example is the phototoxin alpha-therthienyl which can be extracted from Asteraceae species;it is a major nematicidal compound[31-33]. The suppression of herbivores is an important effect that contributes to over-yielding in greenhouses.In this respect,crown daisy was the most appropriate catch crop in the greenhouse.
The present study found that crop rotation affected nematode abundance depending on the species of the catch crop,changed the functional composition of the nematode community by causing a shift to higher trophic groups,and significantly suppressed herbivores in the nematode community.In addition,crown daisy was the most effective catch crop for improving greenhouse environments.
Acknowledgments
This study was supported by a Key Grant from the Earmarked Fund for Beijing Leaf Vegetables Innovation Team of Modern Agro-industry Technology Research System(blvt-08),the Natural Science Foundation of Beijing (Project 6972014),and the National Science Foundation of China(Grant No.30972034).
[1]AHN T,OKE M,SCHOFIELD A,PALIYATH G.Effects of phosphorus fertilizer supplementation on antioxidant enzyme activities in tomato fruits[J].Agric. Food Chem.2005,53:1539-1545.
[2]LIN XG,SHEN WS,SHI WM,MIN J, GAO N,ZHANG HY,YIN R,HE XH. Higher rates of nitrogen fertilization decrease soil enzyme activities,microbial functional diversity and nitrification capacity in a Chinese polytunnel greenhouse vegetable land[J].Plant Soil. 2010,337:137-150.
[3]Changing regional cultivated areas and yields of vegetables[DB/OL].Chinese Ministry of Agriculture.2009.http://zzys. agri.gov.cn/shucai_cx_result.asp.
[4]DONG WB,SHI YM,LI RG,JIANG RD, ZHAO ZQ,LI Q.Species identification and occurrence investigation of vegetable root-knot nematodes under protected cultivation in Shandong Province [J].Laiyang Agric.2004,21:106-108.
[5]HICKMAN M.V.Long-term tillage and crop rotation effects on soil chemical and mineral properties[J].Plant Nutr. 2002,25:1457-1470.
[6]TIAN YQ,ZHANG XY,LIU J,GAO LH. Effects of summer cover crop and residue management on cucumber growth in intensive Chinese production system:soil nutrients,microbial properties and nematodes[J].Plant Soil.2011, 339:299-315.
[7]BONGERS T,FERRIS H.Nematode community structure as a bioindicator in environmental monitoring[J].Trends Ecol.Evol.1999,4:224-228.
[8]PORAZINSKA,DL,DUNCAN,LW, MCSORLEY,R,GRAHAM,JH.Nematode communities as indicators of status and processes of a soil ecosystem influenced by agricultural management practices[J].Appl.Soil Ecol.1999,13: 69-86.
[9]YEATES GW,BONGERS T,DE GOEDE RGM,FRECKMAN DW,GEORGIEVA SS.Feeding habits in soil nematode families and genera an outline for soil ecologists[J].Nematol.1993,25: 315-331.
[10]YEATES GW.Nematodes as soil indicators functional and biodiversity aspects[J].Biol.Fertil.Soils.2003,37: 199-210.
[11]BAKHETIA M,CHARLTON W,ATKINSON HJ,MCPHERSON MJ.RNA interference of dual oxidase in the plant nematode Meloidogyne incognita[J]. Mol.Plant-Microbe Interact.2005,18: 1099-1106.
[12]WU HY,SHI LB.Effects of continuous cropping duration on population dynamics of second-stage juvenile Meloidogyne spp.and free-living soil nematodes.Afric[J].Agric.Res.2011, 6:307-312.
[13]FERRIS H,BONGERS T.Nematode indicators of organic enrichment[J]. Nematol.2005,38:3-12.
[14]NEHER,DA.Ecology of plant and freeliving nematodes in natural and agricultural soil[J].Annu.Rev.Phytopathol.2010,48:371-394.
[15]FERRIS H,BONGERS T,DE GOEDE RGM.A framework for soil food web diagnostics:extension of the nematode faunal analysis concept[J].Appl. Soil Ecol.2001,18:13-29.
[16]KARDOL P,BEZEMER TM,VAN DER WAL A,VAN DE PUTTEN WH.Successional trajectories of soil nematode and plant communities in a chronosequence of ex-arable lands[J].Biol. Conserv.2005,126:317-327.
[17]EISENHAUER N,MIGUNOVA VD, ACKERMANN M,RUESS L,SCHEU S.Changes in plant species richness induce functional shifts in soil nematode communities in experimental grassland[J].PLoS ONE.2011,6: e24087.
[18]RUESS L,FERRIS H.Decomposition pathways and successional changes. In:Proceedings of the 4th International Congress of Nematology(Cook RC, Hunt DJ,editors eds),Nematology Monographs and Perspectives[M]. Leiden,Netherlands:E.J.Brill.2004,2: 1-10.
[19]JOHNSON AW,DOWLER CC,GLAZE NC,HANDOO ZA.Role of nematodes,nematicides,and crop rotation on the productivity and quality of potato,sweet potato,peanut,and grain sorghum[J].Nematol.1996,28: 389-399.
[20]MCSORLEY R,GALLAHER RN.Effect of crop rotation and tillage on nematode densities in tropical corn[J]. Nematol.1993,25:814-819.
[21]HOOPER D.J.Extraction of nematode from plant material[M].//Southey,J.F. (Ed.).Laboratory Methods for work with Plant and Soil Nematodes.Ministry of Agriculture,Fisheries and Food,London:HMSO,1984:59-80.
[22]WEI CZ,ZHENG HF,LI Q,L XT,YU Q,ZHANG HY,CHEN QS,HE NP, KARDOL P,LIANG WJ,HAN XG.Nitrogen addition regulates soil nematode community composition through ammonium suppression[J].PLoS ONE.2012,7:e43384.
[23]BONGERS T.The maturity index:an ecological measure of environmental disturbance based on nematode species composition[J].Oecologia. 1990,83:14-19.
[24]LAFOND GP,CAMPBELL CA, LEMKE R,MAY WE,HOLZAPFEL CB.Indian head long term crop rotation:Indian head Saskatchewan[J]. Prairie Soils Crops.2012,5:42-50.
[25]VAN DE PUTTEN WH,COOK R, COSTA S,DAVIES KG,FARGETTE M,FREITAS H,HOL WHG,KERRY BR,MAHER N,MATEILLE T,MOENS M,DE LA PE A E,PI KIEWICZ A, RAEYMAEKERS A,RODR GUEZECHEVERR A S,VAN DER WURFF AWG.Nematode interaction in nature: models for plants[J].Adv.Agron.2006, 89:228-260.
[26]YAO H,JIAO X,WU F.Effects of continuous cucumber cropping and alternative rotations under protected cultivation on soil microbial community diversity[J].Plant Soil.2006,284:195-203.
[27]DONG LL,HUANG CD,HUANG L,LI XL,ZUO YM.Screening plants resistant against Meloidogyne incognita and integrated management of plant resources for nematode control[J]. Crop Protect.2012,33:34-39.
[28]PAVAO-ZUCKERMAN MA,COLEMAN DC.Urbanization alters the functional composition,but not taxonomic diversity,of the soil nematode community[J].Appl.Soil Ecol.2007,35:329-339.
[29]HOOKS CRR,WANG KH,PLOEG A, MCSORLEY R.Using marigold (Tagetes spp.)as a cover crop to protect crops from plant-parasitic nematodes[J].Appl.Soil Ecol.2010,46: 307-320.
[30]TSAY TT,WU ST,LIN YY.Evaluation of Asteraceae plants for control of Meloidogyne incognita[J].Nematol. 2004,36:36-41.
[31]BAIS HP,PARK SW,WEIR TL, CALLAWAY RM,VIVANCO JM.How plants communicate using the underground information superhighway[J]. Trends Plant Sci.2004,9:26-32.
[32]BAIS HP,WEIR TL,PERRY LG, GILROY S,VIVANCO JM.The role of root exudates in rhizosphere interaction with plants and other organisms [J].Annu.Rev.Plant Biol.2006,57: 233-266.
[33]WANG KH,HOOKS CRR,PLOEG A. Protecting crops from nematode pests: using marigold as an alternative to chemical nematicides[J].Plant Dis. 2007,35:1-6.
Responsible editor:Xiaoxue WANG
Responsible proofreader:Xiaoyan WU
*Corresponding author.E-mail:wangq@cau.edu.cn;lldong@icmm.ac.cn
Received:May 3,2015 Accepted:June 14,2015
杂志排行
Agricultural Science & Technology的其它文章
- The Synergism of Chemical Herbicides and Aureobasidium pullulans for Control Cleavers (Galium aparine L.)in Wheat
- Experimental Study on Friction Characteristics of Caragana korshinskii Kom
- Changes of NF-κB,Bax and Caspase 3 in Apoptosis Induced by Ligustrazine Combined with Cis-dichlorodiamine Platinum in Human Gastric Carcinoma SGC-7901 Cell Lines
- Identification and Mutagenesis of Lactic Acid Bacteria from Chinese Sauerkraut
- Effects of Mechanical Sowing and Transplanting on Characteristics of Dry Matter Production in Middle-season Hybrid R ice
- Effect of Nitrogen Fertilizer on the Accumulation and Distribution of Dry Matter in Broomcorn Millet
