端粒酶活性检测方法研究进展
2015-02-02郭林燕阳明辉
郭林燕,阳明辉
(中南大学化学化工学院,湖南长沙410083)
端粒酶活性检测方法研究进展
郭林燕,阳明辉*
(中南大学化学化工学院,湖南长沙410083)
人体端粒酶是一种核糖核蛋白复合物,在生物体内,以其自身的RNA为模板,通过催化添加端粒重复序列TTAGGG至染色体末端。由于在绝大多数癌症中端粒酶活性与恶性肿瘤之间存在着较大的关联,其在肿瘤的发生、发展过程中起着重要作用,因而对端粒酶活性的检测可为癌症的诊断、预测以及临床治疗提供重要依据。该文总结了近几年来针对端粒酶活性几种不同的检测方法,重点强调了几种机体内原位检测方法,并适当的进行了分析比较。
端粒酶;G-四联体;恶性肿瘤;分析检测
0 引言
端粒,是一种核蛋白,由短的富含鸟嘌呤的串联重复序列组成,在人类细胞中真核染色体的末端形成帽状结构,以抑制不必要的降解、重组或端到端的融合[1]。在一些体细胞中,DNA复制循环后,因DNA聚合酶的不完全复制而使得端粒逐渐缩短,当长度达到一个临界值时,细胞便会停止分裂到达衰老阶段[2]。端粒作为维持染色体复制过程完整性必不可少的组成,由于其缩短或加帽蛋白缺乏会造成不利的后果,包括形成染色体异常,细胞衰老和细胞凋亡[3-5]。
端粒酶是一种核糖核蛋白逆转录酶,由一个基本的催化亚基和RNA模板以及端粒相关蛋白一起维持端粒长度和功能[6-8]。通过其逆转录酶活性及其内在的RNA作为模板催化添加端粒重复序列(TTAGGG)n至染色体DNA的3’端[9],由于其富含鸟嘌呤(Guanine,G)序列,在生理条件下,端粒DNA形成了一种分子间和分子内的鸟嘌呤四联结构。研究发现,在超过85%的人类恶性肿瘤中端粒酶具有异常高的活性,如乳腺癌,结肠癌,肺癌,前列腺癌,卵巢癌,胃癌和皮肤癌等。而在正常体细胞中,端粒酶的表达是被抑制或成缺失状态[10-12]。由于癌细胞中过度表达的端粒酶会维持端粒的长度,使得细胞无限制地增生而不会衰老,因此科学家认为端粒酶在肿瘤的形成过程中起着关键作用。端粒酶的这种特性使得其在癌症早期诊断、预测和治疗领域成为一种重要的生物标志物[13-16]。除此之外,端粒酶和端粒的结构在研究其它疾病、基因调节、细胞/机体老化和哺乳类动物的克隆方面也起到了至关重要的作用[17-18]。鉴于此,高灵敏度的检测端粒酶活性方法对于疾病机理研究和临床诊断显得尤为重要[19]。
1 端粒酶活性的传统检测方法
近年来,已经开发出多种测定端粒酶活性的方法[5,20-21],而 Kim开发的以聚合酶联反应(Polymerase Chain Reaction,PCR)为基础的端粒重复序列扩增法 (Telomeric Repeat Amplification Protocol,TRAP)因其超高的灵敏度已被发展为端粒酶活性的标准分析方法[22]。
1.1 TRAP分析法的原理
TRAP法是一个单管反应,首先,从细胞或组织中提取端粒酶合成端粒重复序列,接着从外源添加端粒链引物,然后将这些延伸后的产品作为模板进行PCR扩增,随后进行聚丙烯酰胺凝胶电泳鉴定,最后使用密度测定法进行定量[23],从而实现端粒酶活性的检测。
1.2 TRAP分析法的不足
TRAP分析法能够实现高通量高灵敏度的检测[24-25],无疑是一个功能强大的测定方法,但保留着PCR放大技术带来的缺点。此外,TRAP法需要使用昂贵的设备和试剂,相当耗时[26]。再加上抑制端粒酶活性已被提议作为人类癌症治疗的潜在方法,而TRAP在筛选有效的端粒酶抑制剂如G-四联体配体时易受PCR-派生产物的影响,因此,该方法存在很多局限性。
1.3 TRAP分析法的发展
为了克服TRAP分析法的上述缺点,不断有新的改善过的PCR步骤被提出来。包括采用TRAP法结合PCR后杂交协议的化学发光探针、荧光染料或生物素化的引物来测量PCR扩增双链DNA的数量[27-29]。例如2010年,Plaxco等利用引物修饰金纳米粒子来检测高浓度细胞溶解物中端粒酶的活性,这种方法虽然比传统的TRAP法在特异性和灵敏性方面有所提高,但是这种以凝胶为基础的方法仍然耗时且需要丰富的专业知识[30]。
2 端粒酶活性的最新检测方法
为替代传统的TRAP分析法,研究者们已经开发了许多PCR-free分析方法并将之应用到端粒酶活性的检测中,例如光学传感器、表面等离子共振、电致化学发光、电化学检测和指数等温扩增分析等[31-35]。
2.1 紫外吸收法
纳米金由于其自身较高的消光系数和粒径依赖型的光学性能在构建生物传感平台方面显示出极大的应用潜力,被广泛应用到众多领域的检测[36-39]。基于纳米金的分析方法主要优点就是分子识别行为可以转换成颜色改变,继而可以通过肉眼观察或者利用紫外可见光谱简单测定出来。因此,该文简要列举了两个例子来说明其在端粒酶活性检测方向的应用。
Willner等[40]开发了一种利用端粒酶产生的端粒-氯化血红素/G-四联体催化氧化L-半胱氨酸诱导纳米金聚集的方法来定量分析端粒酶活性,其反应原理如图1。端粒是富含鸟嘌呤核苷的折叠核酸链,在K+和氯化血红素的存在下,端粒单元自组装成氯化血红素/G-四联体结构,表现出辣根过氧化物酶催化活性[41]。端粒-氯化血红素/G-四联体结构能催化硫醇类物质 (例如L-半胱氨酸)氧化成二硫化物(例如胱氨酸)[42]。L-半胱氨酸能促进Au纳米颗粒的聚集,伴随着紫外吸收的变化,从红色(单个金纳米粒子)变为蓝色(团聚的金纳米粒子),该过程被用来定量分析端粒酶活性。从293T肿瘤细胞中提取端粒酶,当存在脱氧核糖核苷三磷酸混合物(dNTPs)和合适的引物探针时,所述调聚反应将催化端粒-氯化血红素/G-四联体链的生成,用以控制L-半胱氨酸介导的纳米金的聚集。因此,聚合的程度由端粒酶的浓度控制。该方法检测限能检测到27细胞/ μL中端粒酶活性。并且此方法是灵敏度高,检测时间相对较短(3h),以及无需进行复杂的处理和昂贵的仪器。
此外,Xia等[43]设计了一个拥有四个检测色状态(如蓝色、紫色、红色和沉淀)的双功能纳米金(GNP)探针,用于双向直接、准确简单的检测尿液中端粒酶活性,并成功地将其应用于膀胱癌的无痛诊断,反应机理如图2。根据数轴理论,首先定义红色GNP探针为原点,它代表尿提取物中端粒酶无活性。正方向对应于相对高浓度活性端粒酶,在该系统中GNP探针组装明显,产生沉淀。负方向对应于相对低浓度(紫色)和无端粒酶存在(蓝色)的样品。四个检测状态可以通过肉眼或紫外-可见光谱来区分。沉淀和紫色状态代表膀胱癌患者,而蓝色和红色状态代表正常个体。相比于传统比色法,具有优良的辨别能力,并且准确简洁,避免大量的操作和复杂的仪器。此外,该系统稳定性极强,探针保存一个月后依然可以区分。因此,这种具有良好的精度、成本、选择性、稳定性和适用性的检测系统在端粒酶活性和膀胱癌甚至其它癌症的诊断方面具有广阔的应用前景。

图1 基于氯化血红素/端粒-G四联体控制的L-半胱氨酸介导纳米金团聚检测端粒酶活性机理图Fig.1 Analysis of telomerase activity by following the hemin/telomere-G-quadruplex-controlled L-cysteine mediated aggregation of Au NPs
2.2 等温扩增法
2013年,Weizmann等[44]开发了一种新颖的指 数等温扩增 端粒重复序 列(exponential isothermal amplification of telomere repeat,EXPIATR)分析法,这是一种简单、灵敏、可靠的检测细胞提取物中端粒酶活性的方法。
如图3所示,此法基于DNA等温扩增这样一个策略,通过链置换扩增激发,使用限制性内切酶刻痕于一个识别位点,并利用聚合酶多次复制和重置目标,实现25 min的超快检测。这种方法不仅具有TRAP的优越性,还在此基础上有了一定的提升。它放弃了昂贵的热循环协议,使该法更加廉价且在临床上更加通用,此外其检测时间相较于其他方法大大缩短。由于高通量、等温、即用型等特点,EXPIATR在未来临床试验使用中显示出了巨大潜力。
此后,Zhang等[45]提出了一种利用端粒诱导两级等温扩增介导化学发光的分析方法来检测单细胞水平的端粒酶活性。如图4,存在端粒酶时,端粒重复序列 (TTAGGG)n被加到端粒酶基底引物的3’端,这可以转化成链置换扩增的模板[46-47],用来生成短寡核苷酸 、DNA 酶和(TTAGGG)n中的端粒重复序列。短寡核苷酸可以充当一个新的触发点来结合游离的端粒酶基底引物,从而启动一个恒温指数扩增反应[48-50],生成大量的催化DNA酶。无论是DNA酶还是富含G的端粒重复单元都可以与血红素相互作用,形成催化血红素-G-四联体纳米结构,在鲁米诺与过氧化氢共存时,可以催化产生化学发光信号。而在缺乏端粒酶时,两阶段等温扩增不能启动,所以观察不到化学发光信号。

图2 (a)膀胱癌的无痛临床估计数轴理论;(b)端粒酶提取尿样;(c)端粒酶检测原理图Fig.2 (a)The number axis theory for clinical estimate of bladder cancer without pain.(b)Telomerase extraction from urine samples.(c)The scheme for telomerase detection

图3 EXPIATR测定的原理图Fig.3 Schematic diagram of the EXPIATR assay
该方法与TRAP相比,更简单、更灵敏、更快速,而且不需要热循环、洗涤和分离步骤[27]。而与EXPIATR分析相比,该方法有一个突出的优点就是成本低,它不需要使用昂贵的荧光标记核苷酸[44]。由于两阶段等温扩增的扩增效率高、灵敏度高以及化学发光测定法的动态范围宽等优点,该方法能够灵敏地检测到从单一的HeLa癌细胞中提取的端粒酶活性,而不需要任何标记的DNA探针。上述方法可进一步用于筛选抗癌药物,并且可能提供一种有前途的方法来发现新的抗癌药物。

图4 端粒诱导两级等温扩增介导化学发光检测端粒酶活性原理图Fig.4 Schematic illustration for the detection of telomerase activity using telomeres-induced two-stage isothermal amplification-mediated chemiluminescence assay
2.3 电化学分析法
电化学法由于其自身的高灵敏度、操作简单、反应迅速和成本低廉等优点在分析端粒酶活性方面正越来越备受关注[51-54]。
Ozsoz等[31]开发了一种基于鸟嘌呤氧化信号来检测端粒酶活性的免标记电化学分析法,如图5所示,这种分析技术利用一次性电极——碳石墨电极作为电化学传感器。通过以端粒重复序列扩增为基础的PCR分析法,将鸟嘌呤的氧化信号作为端粒酶活性的量度来进行电化学检测。此分析法是第一个免标记的电化学分析法,相较于其他检测方法,如表面等离子共振和石英晶体微天平等具有快速,简单,廉价而且无放射性等优点。并且无需标记,使其适用于临床样品的定量测定。然而这种方法也存在PCR分析法的固有局限性,为用于常规分析,有必要开发一种不依赖于易产生误差的PCR的分析方法。
研究表明,生物传感器中使用电化学阻抗法(Electrochemical Impedance Spectroscopy,EIS)有着诸多优点,如高灵敏度、高精密度、检测速度快以及可实现实时监测等[55-58],Lin等[59]开发了一种基于免标记的电化学阻抗法来检测端粒酶活性,反应原理如图6所示。该方法检出限能够测定1000 HeLa细胞中端粒酶的活性。首先将硫醇化的DNA引物固定到金表面上,然后在dNTPs的存在下与端粒酶一起孵育进行延伸反应。随着端粒酶活性的增强,延长的DNA引物可阻断Au电极表面Fe(CN)63-/Fe(CN)64-电子的转移,导致阻抗逐渐增大。该方法没有涉及PCR和任何信号放大技术,因此不存在和这些技术相关的缺陷。同时还发现,阻抗值与103~105范围内的HeLa细胞中端粒酶活性呈对数线性相关。由于其简单的和方便的操作,在癌症的临床诊断方面显示出潜在的应用。
Cunci等[60]利用免标记电化学阻抗法也制备了生物传感芯片实现原位检测癌细胞中端粒酶的活性。首先将能与端粒酶特异性结合的单链DNA探针修饰在金电极阵列表面,接着利用EIS实现端粒酶活性检测。

图5 分别来自端粒酶阳性细胞提取的热灭活的阴性(A)、中度(B)和强端粒酶活性(C)的PCR产物的鸟嘌呤氧化信号示意图Fig.5 Schematic illustration of guanine oxidation signals of PCR products from(A)heat inactivated negative controls,telomerase positive cell extracts with(B)moderate,and(C)strong telomerase activity

图6 基于EIS方法的端粒酶活性分析原理图Fig.6 Illustration for the protocol of telomerase activity analysis based on EIS method
为了进一步提高电化学检测的灵敏度,通常会考虑降低背景或放大信号,因而核酸外切酶III被用来减少背景信号[61-62],但蛋白酶本身具有检测相对昂贵和复杂的缺点。因此,仍然急需合适的方法来放大信号。杂交链反应(Hybridization Chain Reaction,HCR)是一种无酶、室温线性放大的方法,它操作简单,而且只使用了DNA单链,成本效益高[63],两个DNA发夹探针可以稳定地共存于杂交溶液中。但引入的引发剂会触发两个探针的交替杂交形成有缺口的双螺旋。因此,它已被广泛应用在DNA、miRNA、小分子,蛋白质和细胞的放大检测[64-67],并已取得了一系列令人满意的结果。然而,HCR尚未用于端粒酶的检测。对此,Zhang等[68]开发了一种基于金纳米粒子触发模拟HCR来产生无酶双信号扩增以检测端粒酶活性的电化学传感器。如图7,在该检测方法中,使用了AuNPs和两个发夹探针。AuNPs作为初始放大元件,不仅在电极上与端粒重复序列杂交以扩增信号,而且还启动了随后的二次扩增,利用两个发夹探针模拟HCR[69]。如果细胞提取物的端粒酶活性呈阳性,AuNPs就可以被捕获到电极上,从而触发两个发夹探针产生有缺口双螺旋交替杂交反应,导致大量的三氯化六铵合钌通过静电相互作用被插入到双螺旋DNA的长链,从而在合适的电位产生电化学信号。用这种方法,不仅可以将检测限降低至两个HeLa细胞,能实现10~10000个细胞范围内的端粒酶活性检测,同时还能成功地评估不同细胞内端粒酶的活性。该方法具有较高的灵敏性,而且使用了两步信号扩增,操作简单,可用于临床细胞提取物中端粒酶活性的检测。

图7 基于SNAs AuNPs触发模拟HCR双信号扩增的电化学检测端粒酶活性原理图Fig.7 Schematic illustration of SNAs AuNPs triggered mimic-HCR dual signal amplification electrochemical assay for telomerase activity detection
另一方面,均相电化学是一种免固定化的方法,其中,探针DNA与靶DNA之间的杂交,以及酶的识别都发生在溶液相,而不是在电极的表面上。因此具有简单,响应快速,识别效率高的优点[70-74]。利用这些优点,已经开发了许多均相电化学法用于各种目标,如DNA,小生物分子和金属离子的检测[71,73-74]。 Li等提出了一种基于T7核酸外切酶辅助目标循环扩增的均相电化学检测法,实现端粒酶活性的简单、快速、高灵敏度的测定[75]。如图8所示,在此方法中,设计了一个5’端亚甲基蓝(MB)标记的发夹探针,它可以与端粒酶反应产物杂交,然后被T7核酸外切酶切割,释放大量的MB标记的单核苷酸以引起电化学信号的显著增强。充分利用了T7启动辅助目标循环的高扩增效率优势,该测定法能够检测单细胞水平的端粒酶活性,这是优于一般检测方法的。此外,测定是在均匀的溶液中进行,无需复杂的修饰或固定过程,其具有操作简单,反应快速和识别效率高的优点,这对基于端粒酶的癌症早期诊断具有极大的临床应用前景。

图8 基于T7核酸外切酶辅助目标回收扩增的电化学均相分析法检测端粒酶活性的原理图Fig.8 Principle of the homogeneous electrochemical strategy for the detection of telomerase activity based on T7 exonuclease-aided target recycling amplification
2.4 电化学发光检测法
电化学发光(ECL)作为一个高灵敏度的检测技术,集合了电化学方法与化学发光法两者的优点,已在药物分析,临床诊断,环境和食品分析,免疫测定法以及DNA检测方面引起了相当大的关注[76-82]。
鲁米诺因其电化学发光性能在生物传感检测方面应用广泛[83-84],而早期已有报道显示纳米金能够增强鲁米诺的电化学发光强度[85-86]。鉴于鲁米诺与纳米金结合形成的复合物的特殊性质,Xu等研究了一种可见电化学发光分析方法来分析端粒酶的活性[87]。 利用端粒酶反应后生成的G-四联体脱氧核酶和鲁米诺在微阵列芯片上共同修饰纳米金,作为催化放大信号来提高鲁米诺-双氧水系统的化学发光信号,从而实现端粒酶活性的多通路检测。该方法通过一种简便灵敏的视觉方式来达到检测313~10000范围内的HL-60癌细胞的目的。
此后,Xu等又设计了一种新颖的双电位ECL信号检测方法来检测癌细胞中端粒酶的活性[88]。首先将CdS量子点修饰在玻碳电极表面,接着将巯基修饰的端粒酶引物经由Cd—S作用固定到CdS量子点上,这种引物在端粒酶和dNTPs的存在下可以被延伸,而延伸部分则与鲁米诺-纳米金标记的捕获DNA发生杂交,从而导致硫化镉量子点的ECL发光强度增强,这种增强的发光强度来源于两处,一处是硫化镉量子点被纳米金表面等离子共振诱导产生的-1.25 V,另一处则是来自于鲁米诺的一个新的电致化学发光信号-0.45 V。这双电位信号检测方法可以检测100~9000 cell范围内的HL-60细胞提取的端粒酶活性。这种方法不同于ECL比例感测方法,两个激发电位的ECL强度之比可用于灵敏地检测目标DNA的浓度[89-90]。
2.5 荧光法
Majerska等[91]设计了一种新颖的不需要使用放射性材料和高纯度端粒酶样品的免PCR端粒酶分析法—一种基于等温循环链置换聚合反应的荧光分析法,反应原理如图9。该方法以端粒酶引物在端粒酶存在时的延伸反应、发夹荧光探针的固有信号转导机制和聚合酶的链置换特性为基础,采用放大荧光法来检测癌细胞中端粒酶活性。此处的发夹荧光探针不仅可以用作荧光信号载体,还可成为端粒延伸反应的模板。当端粒酶存在时,发夹探针的“茎”被打开,从而根据荧光的增强来确定端粒酶活性。用此方法可检测到HeLa细胞中的提取的端粒酶活性相当于 40~ 1000个细胞。打开的探针退火后,引发聚合反应,该方法能检出从细胞提取的端粒酶活性当量下降至4个HeLa细胞。这表明目前荧光战略在癌症的早期诊断生化分析方面具有巨大潜力。
众所周知SYBR Gree(SG)是用于DNA染色的敏感荧光染料。利用SG与单、双链DNA的不同相互作用可鉴别单链DNA和双链DNA的结构[84]。大量的研究表明,在K+存在时富含鸟嘌呤的单链DNA可以折叠成一个G-四联体结构[92-94]。 也有其他报告表明,SG可以插入这些G-四联体结构中使荧光强度显著增加[95]。基于上述特点,Chung等[96]开发了一种利用SG将端粒酶活性转换成荧光信号的免PCR分析法。该法是将TS寡核苷酸引物与肿瘤细胞中的端粒酶提取物在dNTPs的存在下一起孵育,接着TTAGGG重复单元在TS引物的3’端连续合成,以形成一个更长的单链DNA分子。在K+存在下,被端粒酶延伸的端粒重复单元能形成G-四联体。此时加入SG会导致荧光强度的急剧增强。然而,在细胞提取物中没有端粒酶活性或有端粒酶抑制剂的存在下,TS引物是不伸长的,因此,加入的SG不能使荧光强度发生变化。
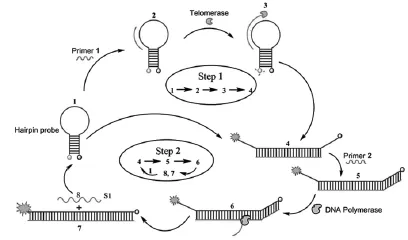
图9 端粒酶活性的荧光放大分析Fig.9 Fluorescence amplification assay of telomerase activity
近来,随着纳米技术的不断成熟,基于纳米材料的探针在生物相关领域的应用逐渐增加。由于纳米材料特殊的物理和化学性能,许多荧光检测法均采用纳米粒子作为荧光探针来实现端粒酶的检测,例如,介孔二氧化硅纳米粒子(Mesoporous Silica Nanoparticle,MSN)具有独特的孔隙结构,生物相容性,且易于功能化等优点[97-98]。此外,MSN还拥有较大的孔体积和表面积,可以负载大量的分子[99]。

图10 基于MSN探针的细胞内端粒酶分析示意图Fig.10 Schematic illustration of MSN probe-based intracellular analysis of telomerase
因此,Ju等[100]利用MSN负载荧光素作为荧光探针实现细胞内端粒酶活性原位“开-关”的成像技术。如图10,用MSN组装特殊设计的DNA作为探针的生物门。当存在端粒酶时,在DNA的3’端原位合成了端粒重复序列,致使DNA从MSN表面脱离释放负载在MSN孔隙中的荧光素,从而触发荧光的释放,实现了原位检测细胞内端粒酶活性的目的。当前,大多数检测方法都使用细胞提取物来分析端粒酶活性,因此很难在原位检测和提供单细胞水平的端粒酶信息。该方法的问世正弥补了这一缺陷。因MSN无毒、无干扰、特异性强,可以用来作为细胞内传递工具,当细胞吞入MSN后,细胞内端粒酶能激活荧光,通过观察信号,即可获得端粒酶活性的原位轨道,以监测药物对端粒酶活性的影响,从而探索出合适的抑制药物。但是,该方法也存在其固有缺陷,引物DNA的表面吸附易受周围环境影响,导致荧光素的非特异性释放,以产生一定程度的背景。
近来,Ju等[101]又针对上述不足,设计了一个智能囊泡试剂盒实现原位成像和检测细胞质内端粒酶活性,机理如图11所示。
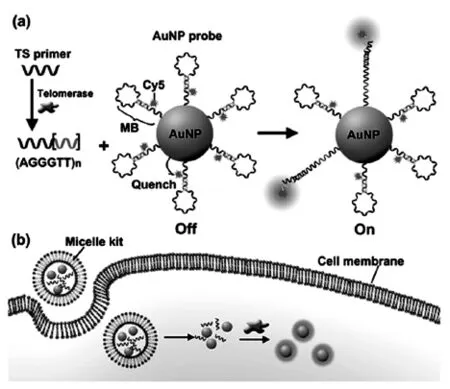
图11 (a)端粒酶触发TSP伸长引起探针的荧光恢复;(b)内化囊泡试剂盒对端粒酶的原位检测Fig.11 (a)Telomerase-triggered TSP elongation and following fluorescence recovery of probe.(b)Internalization of vesicle kit for in situ detection of telomerase
试剂盒中含有TS引物和Cy5标记的分子信标(MB)功能化的纳米金[102-106],通过将其联合封装在脂质体内,从而实现细胞内递送[107]。之后,囊泡试剂盒转染到细胞质中,释放的TS引物可以在端粒酶存在下得到一定的延伸,在DNA的3’末端产生端粒重复序列,它与组装在探针表面的MB环互补。因此,一旦杂交,MB就会被打开,将荧光状态从“关”切换为“开”。荧光信号强弱取决于端粒酶活性,从而达到了原位追踪细胞质内端粒酶活性的目的。在每个HeLa细胞,BEL肿瘤细胞和QSG正常细胞内,用该方法评估得到的细胞质内端粒酶活性分别为 3.2×10-11,2.4×10-11,8.6×10-13IU,这充分证明该方法能够在单细胞水平区分肿瘤细胞和正常细胞。相较于当前存在的其他方法,这种囊泡试剂盒可以通过“一步孵化”实现原位检测,并且,由于纳米金的高效率荧光共振能量转移,从而具有较低的荧光背景。因此上述方法是一种成本低廉、操作简便、灵敏度高的方法,还可以用于动态监测细胞质内端粒酶活性,在癌症的诊断、治疗和端粒酶相关药物的发现与筛选方面成为一种良好的分析工具。
3 结语
关于端粒酶活性的检测方法还有很多未详细列举出来,例如,Mirkin等开发出一种基于寡核苷酸功能化的金纳米粒子的生物条形码,以改善端粒酶的检测[108]。Willner等使用Zn(II)-卟啉化合物作为荧光团结合到G-四联体上,以检测端粒酶活性[109]。
然而,上述大多数策略因为TS引物的固定化、成本高、灵敏度低和操作复杂等问题在应用时或多或少受到了限制。此外,当前的大多数检测方法都使用细胞提取物来分析端粒酶活性,于临床应用较为不符,而能够实现原位分析和动态监测细胞内端粒酶活性的检测方法则凤毛麟角。端粒酶活性的检测对癌症的早期诊断具有重要的生物学意义,对临床上癌症的预警和治疗这一复杂科学问题的研究起到促进作用。因此,对端粒酶活性分析的简单、灵敏、低成本的原位实时监测和抑制剂筛查技术仍然是当前亟待解决的问题。
[1]Blackburn E H,Szostak J W.The molecular structure of centromeres and telomeres[J].Annual review of biochemistry,1984,53(1):163-194.
[2]Rodier F,Campisi J.Four faces of cellular senescence [J].The Journal of cell biology,2011,192(4):547-556.
[3]Harley C B,Futcher A B,Greider C W.Telomeres shorten during ageing of human fibroblasts[J].Nature,1990, 345(6274):458-460.
[4]Hastie N D,Dempster M,Dunlop M G,et al.Telomere reduction in human colorectal carcinoma and with ageing [J].Nature,1990,346(6287):866-873.
[5]Counter C M,Avilion A A,LeFeuvre C E,et al.Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity [J].The EMBO Journal,1992,11(5):1921-1929.
[6]Bodnar A G,Ouellette M,Frolkis M,et al.Extension of life-span by introduction of telomerase into normal human cells[J].Science (New York,N.Y.),1998,279 (5349):349-352.
[7]Perry P J,Jenkins T C.Recent advances in the development of telomerase inhibitors for the treatment of cancer [J].Expert Opinion on Investigational Drugs,1999, 8(12):1981-2008.
[8]Ulaner G A,Hu J F,Vu T H,et al.Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT)transcription and by alternate splicing of hTERT transcripts[J].Cancer research, 1998,58(18):4168-4172.
[9]Cech T R.Life at the end of the chromosome:Telomeres and telomerase[J].Angewandte Chemie-International Edition,2000,39(1):34-43.
[10]Holt S E,Wright W E,Shay J W.Regulation of telomerase activity in immortal cell lines[J].Molecular and cellular biology,1996,16(6):2932-2939.
[11]Shay J W,Bacchetti S.A survey of telomerase activity in human cancer[J].European journal of cancer(Oxford, England:1990),1997,33(5):787-791.
[12]Greider C W.Telomerase activity,cell proliferation,and cancer[J].Proceedings of the National Academy of Sciences of the United States of America,1998,95(1):90-102.
[13]Agrawal A,Dang S,Gabrani R.Recent Patents on Anti-Telomerase Cancer Therapy[J].Recent Patents on Anti-Cancer Drug Discovery,2012,7(1):102-117.
[14]Shay J W,Zou Y,Hiyama E,et al.Telomerase and cancer [J].Human Molecular Genetics,2001,10(7):677-685.
[15]Williams S C P.No end in sight for telomerase-targeted cancer drugs[J].Nature Medicine,2013,19(1):6-6.
[16]Zhou X M,Xing D.Assays for human telomerase activity: progress and prospects[J].Chemical Society Reviews, 2012,41(13):4643-4656.
[17]Artandi S E,DePinho R A.Telomeres and telomerase in cancer[J].Carcinogenesis,2010,31(1):9-18.
[18]Cong Y S,Shay J W.Actions of human telomerase beyond telomeres[J].Cell Research,2008,18(7):725-732.
[19]De Cian A,Lacroix L,Douarre C,et al.Targeting telomeres and telomerase[J].Biochimie,2008,90(1):131-155.
[20]Nemos C,Rémy-Martin J P,Adami P,et al.Improved TRAP-silver staining versus conventional radioactive TRAP assays:quantification of telomerase activity during immortalization and in pathological human endometrium [J].Clinical Biochemistry,2003,36(8):621-628.
[21]Wen J M,Sun L B,Zhang M,et al.A non-isotopic method for the detection of telomerase activity in tumour tissues:TRAP-silver staining assay[J].Molecular Pathology,1998,51(2):110-112.
[22]Savoysky E,Akamatsu K,Tsuchiya M,et al.Detection of telomerase activity by combination of TRAP method and scintillation proximity assay(SPA)[J].Nucleic acids research,1996,24(6):1175-1176.
[23]Piatyszek M,Kim N,Weinrich S,et al.Detection of telomerase activity in human cells and tumors by a telomeric repeat amplification protocol(TRAP)[J].Method incell science,1995,17(1):1-15.
[24]Herbert B S,Hochreiter A E,Wright W E,et al.Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol[J].Nature Protocols, 2006,1(3):1583-1590.
[25]Zuo X L,Xia F,Patterson A,et al.Two-Step,PCR-Free Telomerase Detection by Using Exonuclease III-Aided Target Recycling[J].Chembiochem,2011,12(18):2745-2747.
[26]Krupp G,Kühne K,Tamm S,et al.Molecular basis of artifacts in the detection of telomerase activity and a modified primer for a more robust‘TRAP’assay[J].Nucleic acids research,1997,25(4):919-921.
[27]Herbert B-S, Hochreiter A E,Wright W E, et al. Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol[J].Nature Protocols,2006,1(3):1583-1590.
[28]Hirose M,Abe-Hashimoto J,Ogura K,et al.A rapid, useful and quantitative method to measure telomerase activity by hybridization protection assay connected with a telomeric repeat amplification protocol[J].Journal of Cancer Research and Clinical Oncology,1997,123(6): 227-344.
[29]Wu Y-Y,Hruszkewycz A M,Delgado R M,et al. Limitations on the quantitative determination of telomerase activity by the electrophoretic and ELISA based TRAP assays[J].Clinica Chimica Acta,2000,293(1-2): 199-212.
[30]Xiao Y, Dane K Y,Uzawa T,et al.Detection of Telomerase Activity in High Concentration of Cell Lysates Using Primer-Modified Gold Nanoparticles[J]. Journal of the American Chemical Society,2010,132 (43):15299-15307.
[31]Eskiocak U,Ozkan-Ariksoysal D,Ozsoz M,et al.Labelfree detection of telomerase activity using guanine electrochemical oxidation signal[J].Analytical chemistry, 2007,79(22):8807-8811.
[32]Takata M,Kerman K,Nagatani N,et al.Label-free bioelectronic immunoassay for the detection of human telomerase reverse transcriptase in urine[J].Journal of Electroanalytical Chemistry,2006,596(2):109-116.
[33]Wu L,Wang J,Ren J,et al.Ultrasensitive Telomerase Activity Detection in Circulating Tumor Cells Based on DNA Metallization and Sharp Solid-State Electrochemical Techniques[J].Advanced FunctionalMaterials, 2014,24(18):2727-2733.
[34]Sharon E,Freeman R,Riskin M,et al.Optical,Electrical and Surface Plasmon Resonance Methods for Detecting Telomerase Activity[J].Analytical Chemistry,2010,82 (20):8390-8397.
[35]Tian L,Weizmann Y.Real-Time Detection of Telomerase Activity Using the Exponential Isothermal Amplification of Telomere Repeat Assay[J].Journal of the American Chemical Society,2013,135(5):1661-1664.
[36]Liu J,Lu Y.Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes[J].Nature Protocols,2006,1(1):246-252.
[37]Zhang X,Servos M R,Liu J.Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route[J].Journal of the American Chemical Society, 2012,134(17):7266-7269.
[38]Li H,Huang J,Lv J,et al.Nanoparticle PCR:Nanogold-Assisted PCR with Enhanced Specificity [J]. Angewandte Chemie International Edition,2005,44(32): 5100-5103.
[39]Shen H,Hu M,Yang Z,et al.Polymerase chain reaction of Au nanoparticle-bound primers[J].Chinese Science Bulletin,2005,50(18):2344-2350.
[40]Sharon E,Golub E,Niazov-Elkan A,et al.Analysis of telomerase by the telomeric hemin/G-quadruplexcontrolled aggregation of au nanoparticles in the presence of cysteine[J].Analytical Chemistry,2014,86(6):3153-3158.
[41]Freeman R,Sharon E,Teller C,et al.DNAzyme-Like Activity of Hemin-Telomeric G-Quadruplexes for the Optical Analysis of Telomerase and its Inhibitors[J]. Chembiochem,2010,11(17):2362-2367.
[42]Golub E,Freeman R,Willner I.Hemin/G-Quadruplex-Catalyzed Aerobic Oxidation of Thiols to Disulfides: Application of the Process for the Development of Sensors and Aptasensors and for Probing Acetylcholine Esterase Activity[J].Analytical Chemistry,2013,85 (24):12126-12133.
[43]Duan R,Wang B,Zhang T,et al.Sensitive and Bidirectional Detection of Urine Telomerase Based on the Four Detection-ColorStatesofDifunctionalGold Nanoparticle Probe[J].Analytical Chemistry,2014,86 (19):9781-9785.
[44]Tian L,Weizmann Y.Real-time detection of telomerase activity using the exponential isothermal amplification of telomere repeat assay[J].Journal of the American Chemical Society,2013,135(5):1661-1665.
[45]Yuefei W,Renliang H,Wei Q,et al.Kineticallycontrolled self-assembly of redox-active ferrocenediphenylalanine:from nanospheres to nanofibers[J]. Nanotechnology,2013,24(46):465603.
[46]Connolly A R,Trau M.Isothermal detection of DNA by beacon-assisted detection amplification[J].Angewandte Chemie,2010,49(15):2697-2863.
[47]Guo Q,Yang X,Wang K,et al.Sensitive fluorescence detection of nucleic acids based on isothermal circular strand-displacement polymerization reaction[J].Nucleic Acids Research,2009,37(3):1-6.
[48]Jia H,Li Z,Liu C,et al.Ultrasensitive Detection of microRNAs by Exponential Isothermal Amplification[J]. Angewandte Chemie International Edition,2010,49(32): 5498-5501.
[49]Van Ness J,Van Ness L K,Galas D J.Isothermal reactions for the amplification of oligonucleotides[J].Procceedings of the National Academy of Sciences of the U-nited States of America,2003,100(8):4504-4509.
[50]Zhang Y,Hu J,Zhang C-y.Sensitive Detection of Transcription Factors by Isothermal Exponential Amplification-Based Colorimetric Assay[J].Analytical Chemistry, 2012,84(21):9544-9549.
[51]Hou T,Liu X,Wang X,et al.DNAzyme-guided polymerization of aniline for ultrasensitive electrochemical detection of nucleic acid with bio-bar codes-initiated rolling circle amplification[J].Sensors and Actuators B:Chemical,2014,190:384-388.
[52]Liu X,Chen M,Hou T,et al.A novel electrochemical biosensor for label-free detection of uracil DNA glycosylase activity based on enzyme-catalyzed removal of uracil bases inducing strand release[J].Electrochimica Acta,2013,113:514-518.
[53]Sato S,Kondo H,Nojima T,et al.Electrochemical Telomerase Assay with Ferrocenylnaphthalene Diimide as a Tetraplex DNA-Specific Binder[J].Analytical Chemistry,2005,77(22):7304-7309.
[54]Eskiocak U,Ozkan-Ariksoysal D,Ozsoz M,et al.Label-Free Detection of Telomerase Activity Using Guanine Electrochemical Oxidation Signal[J].Analytical Chemistry,2007,79(22):8807-8811.
[55]Gao Z,Deng H,Shen W,et al.A Label-Free Biosensor for Electrochemical Detection of Femtomolar MicroRNAs [J].Analytical Chemistry,2013,85(3):1624-1630.
[56]Bonanni A,Pumera M.Graphene Platform for Hairpin-DNA-Based Impedimetric Genosensing[J].ACS Nano, 2011,5(3):2356-2361.
[57]Guo S,Wen D,Zhai Y,et al.Platinum Nanoparticle Ensemble-on-Graphene Hybrid Nanosheet:One-Pot, Rapid Synthesis,and Used as New Electrode Material for Electrochemical Sensing[J].ACS Nano,2010,4(7): 3959-3968.
[58]Basuray S,Senapati S,Aijian A,et al.Shear and AC Field Enhanced Carbon Nanotube Impedance Assay for Rapid,Sensitive,and Mismatch-Discriminating DNA Hybridization[J].ACS Nano,2009,3(7):1823-1830.
[59]Yang W,Zhu X,Liu Q,et al.Label-free detection of telomerase activity in HeLa cells using electrochemical impedance spectroscopy[J].Chemical Communications, 2011,47(11):3129-3131.
[60]Cunci L,Vargas M M,Cunci R,et al.Real-Time Detection of Telomerase Activity in Cancer Cells using a Label-Free Electrochemical Impedimetric Biosensing Microchip[J].RSC Advances,2014,4(94):52357-52365.
[61]Wu L,Wang J,Ren J,et al.Ultrasensitive Telomerase Activity Detection in Circulating Tumor Cells Based on DNA Metallization and Sharp Solid-State Electrochemical Techniques[J].Advanced Functional Materials, 2014,24(18):2727-2733.
[62]Zhang Z,Wu L,Wang J,et al.A Pt-nanoparticle electrocatalytic assay used for PCR-free sensitive telomerase detection[J].Chemical Communications,2013,49(85): 9986-9988.
[63]Dirks R M,Pierce N A.Triggered amplification by hybridization chain reaction[J].Procceedings of the National Academy of Sciences of the United States of America,2004,101(43):15275-15278.
[64]Huang J,Wu Y,Chen Y,et al.Pyrene-Excimer Probes Based on the Hybridization Chain Reaction for the Detection of Nucleic Acids in Complex Biological Fluids [J].Angewandte Chemie International Edition,2011,50 (2):401-404.
[65]Huang J,Gao X,Jia J,et al.Graphene Oxide-Based Amplified Fluorescent Biosensor for Hg2+Detection through Hybridization Chain Reactions[J].Analytical Chemistry, 2014,86(6):3209-3215.
[66]Zhang B,Liu B,Tang D,et al.DNA-Based Hybridization Chain Reaction for Amplified Bioelectronic Signal and Ultrasensitive Detection ofProteins[J].Analytical Chemistry,2012,84(12):5392-5399.
[67]Choi J,Routenberg Love K,Gong Y,et al.Immuno-Hybridization Chain Reaction for Enhancing Detection of Individual Cytokine-Secreting Human Peripheral Mononuclear Cells[J].Analytical Chemistry,2011,83 (17):6890-6895.
[68]Wang W J,Li J J,Rui K,et al.Sensitive electrochemical detection of telomerase activity using spherical nucleic acids gold nanoparticles triggered mimic-hybridization chain reaction enzyme-free dual signal amplification[J]. Analytical Chemistry,2015,87(5):3019-3026.
[69]Zhang J,Song S,Zhang L,et al.Sequence-Specific Detection of Femtomolar DNA via a Chronocoulometric DNA Sensor(CDS):Effects of Nanoparticle-Mediated Amplification and Nanoscale Control of DNA Assembly at Electrodes[J].Journal of the American Chemical Society,2006,128(26):8575-8580.
[70]Liu S,Lin Y,Wang L,et al.Exonuclease III-Aided Autocatalytic DNA Biosensing Platform for Immobilization-Free and Ultrasensitive Electrochemical Detection of Nucleic Acid and Protein[J].Analytical Chemistry, 2014,86(8):4008-4015.
[71]Luo X,Lee T M-H,Hsing I M.Immobilization-Free Sequence-Specific Electrochemical Detection of DNA Using Ferrocene-Labeled Peptide Nucleic Acid[J].Analytical Chemistry,2008,80(19):7341-7346.
[72]Wang X,Liu X,Hou T,et al.Highly sensitive homogeneous electrochemical assay for methyltransferase activity based on methylation-responsive exonuclease III-assisted signal amplification[J].Sensors and Actuators B: Chemical,2015,208:575-580.
[73]Xuan F,Luo X,Hsing I M.Ultrasensitive Solution-Phase Electrochemical Molecular Beacon-Based DNA Detection with Signal Amplification by Exonuclease III-Assisted Target Recycling[J].Analytical Chemistry,2012, 84(12):5216-5220.
[74]Xuan F,Luo X,Hsing I M.Conformation-Dependent Exonuclease III Activity Mediated by Metal Ions Reshuffling on Thymine-Rich DNA Duplexes for an Ultrasensitive ElectrochemicalMethod for Hg2+Detection[J]. Analytical Chemistry,2013,85(9):4586-4593.
[75]Liu X,Li W,Hou T,et al.Homogeneous electrochemical strategy for human telomerase activity assay at singlecell level based on t7 exonuclease-aided target recycling amplification[J].Analytical Chemistry,2015,87(7): 4030-4036.
[76]Richter M M.Electrochemiluminescence (ECL)[J]. Chemical Reviews,2004,104(6):3003-3036.
[77]Miao W J.Electrogenerated chemiluminescence and its biorelated applications[J].Chemical Reviews,2008,108 (7):2506-2553.
[78]Zhang H-R,Xu J-J,Chen H-Y.Electrochemiluminescence Ratiometry:A New Approach to DNA Biosensing [J].Analytical Chemistry,2013,85(11):5321-5325.
[79]Zhou H,Zhang Y Y,Liu J,et al.Efficient quenching of electrochemiluminescence from K-doped graphene-CdS: Eu NCs by G-quadruplex-hemin and target recyclingassisted amplification for ultrasensitive DNA biosensing [J].Chemical Communications,2013,49(22):2246-2258.
[80]Zhang H-R,Xia X-H,Xu J-J,et al.Sensitive cancer cell detection based on Au nanoparticles enhanced electrochemiluminescence of CdS nanocrystal film supplemented by magnetic separation[J].Electrochemistry Communications,2012,25:112-115.
[81]Tian C Y,Xu J J,Chen H Y.A novel aptasensor for the detection of adenosine in cancer cells by electrochemiluminescence of nitrogen doped TiO2nanotubes[J].Chemical Communications,2012,48(66):8234-8236.
[82]Wang J,Shan Y,Zhao W-W,et al.Gold Nanoparticle Enhanced Electrochemiluminescence of CdS Thin Films for Ultrasensitive Thrombin Detection[J].Analytical Chemistry,2011,83(11):4004-4011.
[83]Rose A L,Waite T D.Chemiluminescence of Luminol in the Presence of Iron(II)and Oxygen:Oxidation Mechanism and Implications for Its Analytical Use[J].Analytical Chemistry,2001,73(24):5909-5920.
[84]Chai Y,Tian D,Wang W,et al.A novel electrochemiluminescence strategy for ultrasensitive DNA assay using luminol functionalized gold nanoparticles multi-labeling and amplification of gold nanoparticles and biotin-streptavidin system[J].Chemical Communications,2010,46 (40):7560-7562.
[85]Cui H,Zou G-Z,Lin X-Q.Electrochemiluminescence of Luminol in Alkaline Solution at a Paraffin-Impregnated Graphite Electrode[J].Analytical Chemistry,2003,75 (2):324-331.
[86]Tian D,Duan C,Wang W,et al.Ultrasensitive electrochemiluminescence immunosensor based on luminol functionalized gold nanoparticle labeling[J].Biosensors and Bioelectronics,2010,25(10):2290-2295.
[87]Zhang H R,Wang Y Z,Wu M S,et al.Visual electrochemiluminescence detection oftelomerase activity based on multifunctional Au nanoparticles modified with G-quadruplex deoxyribozyme and luminol[J].Chemical Communications,2014,50(83):12575-12579.
[88]Zhang H R,Wu M S,Xu J J,et al.Signal-on dual-potential electrochemiluminescence based on luminol-gold bifunctional nanoparticles for telomerase detection[J]. Analytical Chemistry,2014,86(8):3834-3840.
[89]Branchini B R,Rosenberg J C,Ablamsky D M,et al.Sequential bioluminescence resonance energy transferfluorescence resonance energy transfer-based ratiometric protease assays with fusion proteins of firefly luciferase and red fluorescent protein[J].Analytical Biochemistry, 2011,414(2):239-245.
[90]Takeuchi M,Nagaoka Y,Yamada T,et al.Ratiometric Bioluminescence Indicators for Monitoring Cyclic Adenosine 3′,5′-Monophosphate in Live Cells Based on Luciferase-Fragment Complementation[J].Analytical Chemistry,2010,82(22):9306-9313.
[91]Majerska J,Sykorova E,Fajkus J.Non-telomeric activities of telomerase[J].Molecular Biosystems,2011,7(4): 1013-1023.
[92]Hu K,Huang Y,Zhao S,et al.Ultrasensitive detection of potassium ions based on target induced DNA conformational switch enhanced fluorescence polarization[J].Analyst,2012,137(12):2770-2773.
[93]Qin H,Ren J,Wang J,et al.G-Quadruplex-Modulated Fluorescence Detection of Potassium in the Presence of a 3500-Fold Excess of Sodium Ions[J].Analytical Chemistry,2010,82(19):8356-8360.
[94]Huang C-C,Chang H-T.Aptamer-based fluorescence sensor for rapid detection of potassium ions in urine[J]. Chemical Communications,2008,12:1461-1463.
[95]Xu H,Gao S,Yang Q,et al.Amplified Fluorescent Recognition of G-Quadruplex Folding with a Cationic Conjugated Polymer and DNA Intercalator[J].ACS Applied Materials&Interfaces,2010,2(11):3211-3216.
[96]Quach Q H,Jung J,Kim H,et al.A simple,fast and highly sensitive assay for the detection of telomerase activity [J].Chemical Communications,2013,49(59):6596-6598.
[97]Climent E,Martínez-Máñez R,Sancenón F,et al.Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles[J].Angewandte Chemie, 2010,122(40):7439-7441.
[98]Niu D,Ma Z,Li Y,et al.Synthesis of Core-Shell Structured Dual-Mesoporous Silica Spheres with Tunable Pore Size and Controllable Shell Thickness[J].Journal of the American Chemical Society,2010,132(43):15144-15147.
[99]Slowing I,Trewyn B G,Lin V S Y.Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells[J].Journal of the American Chemical Society, 2006,128(46):14792-14793.
[100]Qian R,Ding L,Ju H.Switchable fluorescent imaging of intracellular telomerase activity using telomerase-responsive mesoporous silica nanoparticle[J].Journal of the American Chemical Society,2013,135(36):13282-13285.
[101]Qian R,Ding L,Yan L,et al.Smart vesicle kit for in situ monitoring of intracellular telomerase activity using a telomerase-responsive probe[J].Analitical Chemistry, 2014,86(17):8642-8648.
[102]Llevot A,Astruc D.Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer [J].Chemical Society Reviews,2012,41(1):242-257.
[103]Duncan B,Kim C,Rotello V M.Gold nanoparticle platforms as drug and biomacromolecule delivery systems [J].Journal of Controlled Release,2010,148(1):122-127.
[104]Li F,Zhang H,Dever B,et al.Thermal Stability of DNA Functionalized Gold Nanoparticles[J].Bioconjugate Chemistry,2013,24(11):1790-1797.
[105]Giljohann D A,Seferos D S,Patel P C,et al.Oligonucleotide Loading Determines Cellular Uptake of DNAModified Gold Nanoparticles[J].Nano Letters,2007,7 (12):3818-3821.
[106]Seferos D S,Giljohann D A,Hill H D,et al.Nano-Flares:Probes for Transfection and mRNA Detection in Living Cells[J].Journal of the American Chemical Society,2007,129(50):15477-15479.
[107]Dalby B,Cates S,Harris A,et al.Advanced transfection with Lipofectamine 2000 reagent:primary neurons, siRNA,and high-throughput applications[J].Methods, 2004,33(2):95-103.
[108]Zheng G,Daniel W L,Mirkin C A.A New Approach to Amplified Telomerase Detection with Polyvalent Oligonucleotide Nanoparticle Conjugates[J].Journal of the American Chemical Society,2008,130(30):9644-9645.
[109]Zhang Z,Sharon E,Freeman R,et al.Fluorescence Detection of DNA,Adenosine-5′-Triphosphate(ATP), and Telomerase Activity by Zinc(II)-Protoporphyrin IX/ G-Quadruplex Labels[J].Analytical Chemistry,2012, 84(11):4789-4797.
Recent research on monitoring telomerase activity
Guo Lin-yan,Yang Ming-hui*
(College of Chemistry and Chemical Engineering,Central South University,Changsha 410083,China)
Human telomerase is a ribonucleoprotein complex,which functions as a telomere terminal transferase by catalytic adding telomere repeat TTAGGG to the end of chromosome using its RNA as the template.Due to a very strong association between telomerase activity and malignancy in nearly all types of cancer,telomerase plays a key role in the tumor occurrence as well as development.Monitoring telomerase activity is believed to be important for cancer diagnosis,prediction and provides an important basis for clinical treatment.This review summarizes recent development for detection of telomerase activity,with emphasis placed on in situ detection.
telomerase;G-quadruplex;in situ;analysis detection
*通信联系人,E-mail:yangminghui@csu.edu.cn,Tel:0731-88836356
