Dorsal root ganglion neurons promote proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells
2015-01-18PeixunZhangXiaoruiJiangLeiWangFangminChenLinXuFeiHuang
Pei-xun Zhang, Xiao-rui Jiang, Lei Wang Fang-min Chen Lin Xu, Fei Huang
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Orthopedics, the Affliated Yantai Hospital of Binzhou Medical University, Yantai, Shandong Province, China
3 Department of Human Anatomy, Binzhou Medical University, Yantai, Shandong Province, China
Dorsal root ganglion neurons promote proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells
Pei-xun Zhang1,#, Xiao-rui Jiang2,#, Lei Wang2, Fang-min Chen2, Lin Xu2,*, Fei Huang3,*
1 Department of Trauma and Orthopedics, Peking University People’s Hospital, Beijing, China
2 Department of Orthopedics, the Affliated Yantai Hospital of Binzhou Medical University, Yantai, Shandong Province, China
3 Department of Human Anatomy, Binzhou Medical University, Yantai, Shandong Province, China
Preliminary animal experiments have confrmed that sensory nerve fbers promote osteoblast differentiation, but motor nerve fbers have no promotion effect. Whether sensory neurons promote the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells remains unclear. No results at the cellular level have been reported. In this study, dorsal root ganglion neurons (sensory neurons) from Sprague-Dawley fetal rats were co-cultured with bone marrow mesenchymal stem cells transfected with green fuorescent protein 3 weeks after osteogenic differentiation in vitro, while osteoblasts derived from bone marrow mesenchymal stem cells served as the control group. The rat dorsal root ganglion neurons promoted the proliferation of bone marrow mesenchymal stem cell-derived osteoblasts at 3 and 5 days of co-culture, as observed by fuorescence microscopy. The levels of mRNAs for osteogenic differentiation-related factors (including alkaline phosphatase, osteocalcin, osteopontin and bone morphogenetic protein 2) in the co-culture group were higher than those in the control group, as detected by real-time quantitative PCR. Our fndings indicate that dorsal root ganglion neurons promote the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells, which provides a theoretical basis for in vitro experiments aimed at constructing tissue-engineered bone.
nerve regeneration; bone marrow mesenchymal stem cells; bone; osteoblasts; ganglion; spine; neurons; co-culture techniques; proliferation; differentiation; real-time quantitative PCR; NSFC grants; neural regeneration
Funding:This study was supported by grants from the National Program on Key Basic Research Project of China (973 Program), No. 2014CB542200; the National Natural Science Foundation of China, No. 31271284, 81301570; Program for New Century Excellent Talents in University of Ministry of Education of China, No. BMU20110270; the Natural Science Foundation of Shandong Province of China, No. Y2008C18; Yantai Science and Technology Development Program of China, No. 2011207, 2011209.
Zhang PX, Jiang XR, Wang L, Chen FM, Xu L, Huang F (2015) Dorsal root ganglion neurons promote proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Neural Regen Res 10(1):119-123.
Introduction
Although our understanding of the mechanisms underlying bone regeneration method has greatly improved, the clinical treatment of large bone defects remains difficult. Autogenous bone graft is regarded as the gold standard surgical treatment, but this approach induces some complications such as bone nonunion and additional damage (Mahendra and Maclean, 2007).
Construction of tissue-engineered bone tissue is a potential way to treat bone defects. Bone is not only a kind of hard tissue, but also a complex body containing abundant nerves, blood vessels and fascia (Togari et al., 2005). Nerves play an extensive and crucial role in the development, formation and metabolism of bone tissue (Imai et al., 1997). Nerve cells function to regulate bone formation and resorption. Growing evidence from animal experiments has shown that sensory nerves promote bone formation, but this effect was not observed with motor nerves (Dai et al., 2008). Therefore, both bone tissue and surrounding tissue (such as blood vessels and nerve tissue) require surgical repair prior to reconstruction (Haastert et al., 2006).
In the present study, we aimed to verify whether dorsal root ganglion (DRG) neurons promote the proliferation and differentiation of bone marrow mesenchymal stem cell (BMSC)-derived osteoblasts at the cellular level. We co-cultured DRG neurons with the osteoblasts in a broader attempt to explore the correlation between the two and to lay the foundation for further in vivo experiments.
Materials and Methods
Experimental animals
Female Sprague-Dawley rats at pregnant 15 days and healthymale 2-week-old Sprague-Dawley rats, weighing 80 g, were obtained from the Experimental Animal Center of Binzhou Medical University (license No. SYXK (Lu) 20130020) in China. All investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of China in 2006.
Isolation, culture, identifcation and osteogenic differentiation of rat BMSCs
Healthy male 2-week-old Sprague-Dawley rats were killed by decapitation under anesthesia and soaked in 75% ethanol for 15 minutes. The rat femur and tibia were then harvested aseptically, and soft tissue attached to the skeleton was removed. After the medullary cavity was rinsed twice with sterile normal saline, bone marrow was directly collected and placed in a tube, and centrifuged at 300 × g for 10 minutes. The supernatant was discarded and the BMSCs were resuspended in standard culture medium (Cyagen Biosciences Inc., Sunnyvale, CA, USA) and counted. Cells were then cultured in 25 cm2culture fasks at 37°C, in a 5% CO2incubator. The culture medium was replenished 24 hours later and then changed every 2-3 days. When the cells reached 80-90% confluence (at 3-5 days of primary culture), cells were digested and subcultured using 0.25% trypsin. Passage 3 BMSCs were observed under an inverted phase contrast microscope and identifed by fow cytometry analysis. After identifcation, passage 3 BMSCs were induced for 14 days in an osteogenic induction liquid, which consisted of high-glucose DMEM (HyClone, Logan, UT, USA), 10% fetal bovine serum (HyClone), 1 × 10-8M dexamethasone, 10 mM β-glycerophosphate and 50 µg/mL vitamin C (Sigma, St. Louis, MO, USA). The induced cells were then detected using fow cytometry and alkaline phosphatase staining (Nanjing Jiancheng Bioengineering Co., Ltd., Nanjing, Jiangsu Province, China). The morphology of BMSCs was observed under an inverted phase contrast microscope (Nikon, Tokyo, Japan).
Isolation, culture and identifcation of rat DRG neurons
Female Sprague-Dawley rats at pregnant 15 days were anesthetized and sacrifced by cervical dislocation. After abdominal skin was disinfected with 75% ethanol, an axial incision was made, the uterus was harvested and placed in a dish containing D-Hank’s solution. Fetal rats were removed and fetal spine was isolated, followed by removal of the DRG attached to the spine. DRG samples were rinsed with D-Hank’s solution, separated under a microscope, placed in a 15 mL centrifuge tube, digested with 3-4 mL of 0.25% trypsin and 3-4 mL of 0.02% EDTA, and incubated at 37°C for 50 minutes. After digestion was terminated, cells were triturated and a cell suspension was collected. The obtained suspension was incubated with Neurabasal + B27 medium on cover slips. Half of the culture medium was changed every 24-48 hours and 1 × 10-5nM cytarabine was added to the culture medium; cytarabine treatment was terminated 24-48 hours later. DRG neurons were identifed using microtubule-associated protein 2 (MAP2) and 4′,6-diamidino-2-phenylindole (DAPI) double staining, and observed under a fuorescence microscope (Nikon, Tokyo, Japan).
Proliferation-promoting effect in a 24-well co-culture test
Osteoblasts differentiated from rat BMSCs (Cyagen) that were previously transfected with green fluorescent protein (GFP) were used in this study. After selection, the transfection effciency was up to 90%. The 24-well culture plate was divided into nine regions at the bottom and cells were counted under an inverted phase contrast microscope (Nikon).
Osteoblasts were divided into two groups as follows. (1) DRG neurons group: DRGs previously cultured on cover slips were transferred to 24-well culture plates, and GFP-transfected osteoblasts were prepared into cell suspensions at a density of 1 × 104/mL. Then, 80 µL of the cell suspension was placed in the 24-well plates and cells were cultured with high-glucose DMEM culture medium. (2) Blank group: GFP-transfected rat BMSCs were prepared into cell suspensions at a density of 1 × 104/mL. Then, 80 µL of the cell suspension was placed in the 24-well plates and cells were cultured with high-glucose DMEM culture medium. Each well contained nine regions. At 1, 3, and 5 days of co-culture, cells were counted under a fluorescence microscope (Nikon, Tokyo, Japan). The experiments were repeated three times.
Real-time fuorescent quantitative PCR detection
Co-culture of DRG neurons and BMSC-derived osteoblasts: The following three groups were used: co-culture 3 days, co-culture 5 days, and control groups. In the co-culture groups, DRG neurons were cultured on cover slips and placed in 6-well plates. The osteoblasts were prepared into cell suspensions at 1 × 104/mL, and 100 µL of the suspension was added to the 6-well plates; the cells were then co-cultured for 3 or 5 days. In the control group, osteoblasts were prepared into cell suspensions at 1 × 104/mL and 100 µL of the suspension was added to the 6-well plates. There were three wells for each group. All cells were cultured in high-glucose DMEM culture medium to induce osteogenic differentiation. The experiment was repeated three times.
The levels of mRNAs for osteocalcin, osteopontin, alkaline phosphatase and bone morphogenetic protein 2 were detected using real-time fuorescent quantitative PCR (Bio-Rad, Hercules, CA, USA) (Table 1). Total RNA was extracted according to the instructions of the Trizol Isolation Reagent kit. During the experiment, all containers and pipettes were treated by diethylpyrocarbonate under high pressure. The optical density at 260 and 280 nm was read on a DU800 UV spectrophotometer, to determine total RNA purity and concentration. The 2 × AllinOneTMQ-PCR Mix was thawed at room temperature, and the PCR reaction mix was prepared on ice with the addition of the following: 2 × AllinOneTMQ-PCR Mix, 10 µL; AllinOneTMQ-PCR Primer at a final concentration of 2 µM, 2 µL; cDNA (1:5), 2 µL; ddH2O. The PCR reaction mix was placed into 96-well plates and centrifuged. A standard three-step method was adopted for the PCR procedure: denaturation at 95°C for 10minutes; 40 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 15 seconds. Real-time quantitative PCR data were analyzed using the 2-ΔΔCtmethod (Livak and Schmittgen, 2001).

Table 1 Real-time fuorescent quantitative PCR primer sequences

Table 3 Osteocalcin, osteopontin, alkaline phosphatase and bone morphogenetic protein 2 mRNA expression (2−ΔΔCt) after co-culture of rat dorsal root ganglion neurons with bone marrow mesenchymal stem cell-derived osteoblasts
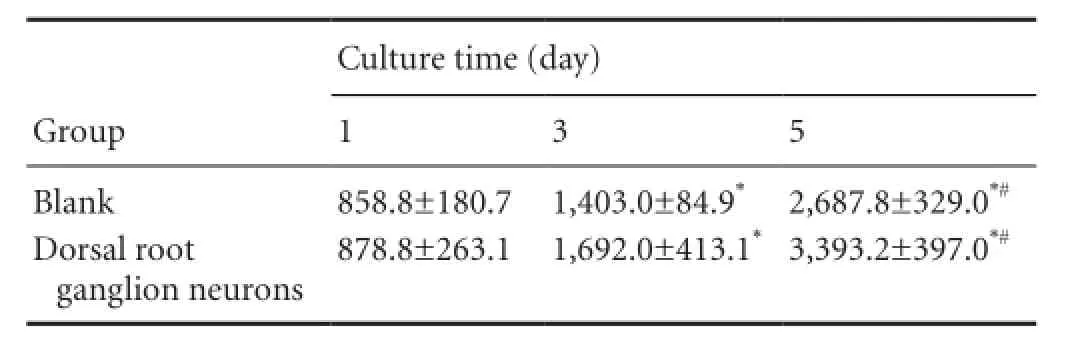
Table 2 Effect of rat dorsal root ganglion neurons on the proliferation of osteoblasts derived from bone marrow mesenchymal stem cells
Statistical analysis
Measurement data are expressed as the mean ± SD and were statistically analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Differences among groups were compared using one-way analysis of variance and intergroup comparisons were performed using the Student-Newman-Keuls method. Data from the co-culture test were analyzed using repeated measures analysis of variance, and intragroup pairwise comparisons were performed using the Bonferroni test. A P level < 0.05 was considered to indicate a signifcant difference.
Results
Identifcation of rat DRG neurons culturedin vitro
The results of MAP2 and DAPI double staining are shown inFigure 1. After 60 days in culture, rat DRG neurons positive for both MAP2 and DAPI were visible under a fuorescence microscope.
Morphology and osteogenic identifcation of rat BMSCs
At 2 weeks in culture, passage 3 BMSCs were long and fusiform and had a clear boundary under an inverted fluorescence microscope. Alkaline phosphatase staining and flow cytometry results showed that the rat BMSCs were successfully differentiated(Figure 2A-F).
Effect of rat DRG neurons on the proliferation of BMSC-derived osteoblasts
The results of the proliferation promotion test of DRG neurons are shown inTable 2. DRG neurons showed no obvious effect in terms of promoting the proliferation of BMSC-derived osteoblasts at 1 and 3 days after co-culture (P > 0.05); DRG neurons obviously promoted the proliferation of BMSC-derived osteoblasts at 5 days after co-culture (P < 0.05).
Osteocalcin, osteopontin, alkaline phosphatase and bone morphogenetic protein 2 mRNA expression after co-culture of rat DRG neurons and BMSC-derived osteoblasts
Real-time fuorescent quantitative PCR analysis showed that DRG neurons promoted the osteogenic differentiation of BMSC-derived osteoblasts after 3 and 5 days in co-culture. The co-culture groups showed higher expression levels of osteocalcin, osteopontin, alkaline phosphatase and bone morphogenetic protein 2 mRNA than did the control group (P < 0.05;Table 3).
Discussion

Figure 1 Primary cultured rat dorsal root ganglion neurons, identifed by MAP2 and DAPI double staining (fuorescence microscopy, × 200).
Bone contains abundant nerves, blood vessels and fascia (Takeda et al., 2002). Bone and nerve tissue are adjacent and closely linked to each other. In the past decade, several research groups have confrmed the presence of a variety of neurotransmitter receptors and peptidergic nerve fbers in bone cells (Goto and Tanaka, 2002; Togari, 2002, 2005), indicating that nerve cells regulate bone formation and resorption. The ultimate goal of ongoing research in the feld is to construct a skeleton of normal physiological state using tissue engineering techniques; that is, tissue-engineered bone. For this purpose, we need to explore the relationship between nerve cells and osteoblasts at the cellular level. Sensory nerves have been shown to promote osteogenesis, while no significant effect of motor nerves was observed. The effect of sensory nerve bundles on promoting bone formation was similar to that of single vascular transplantation: both sensory nerve fbers and vascular bundles promoted the regeneration and restoration of tissue-engineered bone (Chen et al., 2010). In the process of constructing tissue-engineered bone, calcitonin gene-related peptide (CGRP), substance P (SP) and neuropeptide Y were dynamically monitored, and it was found that the levels of these neuropeptides increase during bone formation and modeling (Goto et al., 2007). This highlights the contribution of nerve tissue to bone growth. However, these findings were obtained in animal experiments, and verifcation at the cellular level is rare. Whether sensory neurons promote the proliferation and osteogenic differentiation of BMSCs remains an unanswered question. In this study, we verifed this hypothesis through the co-culture of two kinds of cells.

Figure 2 Morphology, identifcation (fow cytometry) and osteogenic differentiation of rat bone marrow mesenchymal stem cells after 2 weeks of culture.
In this study, the proliferation of BMSC-derived osteoblasts co-cultured with DRG neurons was more pronounced than that in the control group. This indicated that DRG neurons promoted the proliferation of BMSCs. How to effectively co-culture DRG neurons with BMSC-differentiated osteoblasts has been a diffcult issue in previous studies, and no relevant experimental support was obtained. The culture medium for DRG neurons and BMSC induction are different, and DRG neurons do not survive well; if osteoblast culture medium is applied in the co-culture process, the survival of DRG neurons is sharply decreased, and the co-culture is prone to failure after 7 days. In addition, osteoblasts proliferate rapidly and are easy to count. After 3 and 5 days in co-culture, DRG neurons promoted the proliferation of BMSC-derived osteoblasts. This effect was mediated by the secretion of CGRP and SP by DRG neurons. CGRP and SP are the two main neuropeptides secreted by sensory nerve fbers (Quartu et al., 2014) and play a signifcant role in promoting bone formation. Among them, CGRP is the neuropeptide that provides the strongest dilation of blood vessels, and CGRP-positive nerve fbers are abundant at the growth plate and epiphyseal connection site, where bone metabolism is active (Bjurholm et al., 1988; Hill and Elde, 1991; Li et al., 2007). These nerve fbers also promote bone proliferation and reconstruction (Tuo et al., 2013) and have potential promotion effects in the construction of tissue-engineered bone. This finding is consistent with preliminary animal experiments by our research group, aiming to construct tissue-engineered bone in rabbits and showing the contribution of sensory nerves to bone formation. The majority of studies to date have demonstrated that DRG neurons promote the osteoblast proliferation through releasing neuropeptides. However, the mechanism of action and signaling pathways by which DRG-secreted neuropeptides promote the proliferation of BMSC-derived osteoblasts remain unclear and deserve further exploration.
To verify the effect of DRG neurons on the osteogenic differentiation of BMSCs, we co-cultured DRG neuronsand BMSCs on 6-well plates and measured the expression levels of various factors by real-time quantitative PCR. The expression levels of osteocalcin, osteopontin and bone morphogenetic protein-2 mRNA were significantly higher in the co-culture group than in the control group. This fnding suggests that DRG neurons promote the differentiation of BMSC-derived osteoblasts via the bone morphogenetic protein 2 pathway. However, this study only examined whether the co-cultured DRG neurons promote the proliferation and osteogenic differentiation of BMSCs at the cellular level, and there is no clear illustration of the underlying mechanism. DRG neurons can secrete a variety of neurotrophic factors, and these factors exert different effects on osteoblasts. The functions of DRG neurons at different stages of BMSC proliferation and differentiation are still unknown. In-depth studies with large sample sizes are needed. As part of our research topic (Construction Vascularized Blood Vessels Through In Vivo Experiments), the present study was designed to verify whether DRG neurons promote the proliferation and differentiation of BMSC-derived osteoblasts in the simulated in vivo environment at the cellular level, in an attempt to reveal the theoretical basis for the construction of tissue-engineered bone. Studies are needed to further explore the mechanism underlying the promotion of osteoblast proliferation and differentiation, and to examine the roles of vascular factors and neural factors in this effect on osteoblasts.
Author contributions:PXZ was responsible for writing the paper, performing statistical analysis and instructing the study. XRJ implemented experiment. LW integrated the data and performed the experiment. FMC provided information support. LX designed the study. PXZ, LX and XRJ were responsible for the funds. FH supervised the study. All authors approved the final version of the manuscript.
Conficts of interest:None declared.
Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M (1988) Substance P-and CGRP-immunoreactive nerves in bone. Peptides 9:165-171.
Chen SY, Qin JJ, Wang L, Mu TW, Jin D, Jiang S, Zhao PR, Pei GX (2010) Different effects of implanting vascular bundles and sensory nerve tracts on the expression of neuropeptide receptors in tissue-engineered bone in vivo. Biomed Mater 5:055002.
Dai J, GuoXian P, Yong L (2008) Effects of nerve implantation on osteogenesis of large tissue-engineered bone: one-year observation. Zhonghua Chuangshang Guke Zazhi 10:354-358.
Goto T, Tanaka T (2002) Tachykinins and tachykinin receptors in bone. Microsc Res Tech 58:91-97.
Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T (2007) Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 41:25-31.
Haastert K, Semmler N, Wesemann M, Rucker M, Gellrich NC, Grothe C (2006) Establishment of cocultures of osteoblasts, Schwann cells, and neurons towards a tissue-engineered approach for orofacial reconstruction. Cell Transplant 15:733-744.
Hill EL, Elde R (1991) Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264:469-480.
Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S (1997) Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res 15:133-140.
Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M (2007) Site-specifc CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J Orthop Res 25:1204-1212.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCtmethod. Methods 25:402-408.
Mahendra A, Maclean AD (2007) Available biological treatments for complex non-unions. Injury 38 Suppl 4:S7-12.
Quartu M, Carozzi VA, Dorsey SG, Serra MP, Poddighe L, Picci C, Boi M, Melis T, Del Fiacco M, Meregalli C, Chiorazzi A, Renn CL, Cavaletti G, Marmiroli P (2014) Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed Res Int 2014:180428.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305-317.
Togari A (2002) Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech 58:77-84.
Togari A, Arai M, Kondo A (2005) The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets 9:931-940.
Tuo Y, Guo X, Zhang X, Wang Z, Zhou J, Xia L, Zhang Y, Wen J, Jin D (2013) The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J Recept Signal Transduct Res 33:114-123.
Copyedited by McGowan D, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to: Lin Xu, M.D., Yantaixulin@126.com. Fei Huang, M.D., hfei22518@163.com.
# These authors contributed equally to this work.
10.4103/1673-5374.150717
http://www.nrronline.org/
Accepted: 2014-12-15
杂志排行
中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research (NRR) Instructions for Authors (2015)
- Hypersensitivity of vascular alpha-adrenoceptor responsiveness: a possible inducer of pain in neuropathic states
- Neural regeneration after peripheral nerve injury repair is a system remodelling process of interaction between nerves and terminal effector
- Acute carbon monoxide poisoning and delayed neurological sequelae: a potential neuroprotection bundle therapy
- Prediabetes and type 2 diabetes implication in central proliferation and neurogenesis
- Clinical strategies to enhance nerve regeneration
