疣孢漆斑菌发酵液的化学成分及其农药活性的研究
2015-01-11郭大乐丁立生顾玉诚
康 敏,郭大乐,胡 佳,万 波,周 燕,丁立生,顾玉诚,邓 赟*
1 成都中医药大学药学院 中药材标准化教育部重点实验室,四川省中药资源系统研究与开发利用重点实验室—省部共建国家重点实验室培育基地,成都 611137;2 中国科学院成都生物研究所 山地生态恢复与生物资源利用重点实验室,成都 610041;3Syngenta Jealott’s Hill International Research Centre,Berkshire RG42 6EY,UK
疣孢漆斑菌(Myrothecium verrucaria)为半知菌亚门丝抱纲瘤座抱目瘤座抱科漆斑菌属真菌。研究发现,疣孢漆斑菌对几种重要的杂草物种(如野葛)具有较高的毒性[1],且可直接作用于杂草或害虫目标,而不引起继发感染,实现对病虫害的有效控制,因此其在生物防治方面具有潜在的开发价值。疣孢漆斑菌的主要代谢产物为单端孢霉烯族化合物(Trichothecenes)[2],这类化合物属于倍半萜环氧化合物,其C-12 和C-13 位之间形成的氧桥是一个活性基团。当C-4 和C-15 成酯后活性则增强。单端孢霉烯是疣孢漆斑菌的主要活性成分可与核糖体的S60 亚基结合,从而抑制蛋白的合成。这种抑制真核细胞蛋白质合成的特性,显示出广泛的生物活性,包括抗真菌[3]、抗微生物[4]、抗白血病[5]、抗肿瘤活性[6]以及用于农药防治的生物除草剂和杀虫剂[7]。
本文从羌活中得到一株内生菌,对其发酵液乙酸乙酯部位进行初步活性测试,结果表现出良好的杀虫和除草活性。该菌经鉴定为疣孢漆斑菌,对其进行扩大培养,发酵,提取,分离,鉴定,得到得到一类共计14 个单端孢霉烯族化合物(Trichothecenes),经MS 及NMR 波谱解析技术分别鉴定了它们的结构,最后对每个化合物做了除草及杀虫的活性测试,旨在研究出疣孢漆斑菌除草杀虫作用的化学成分基础,对利用单端孢霉烯开发成为生物除草剂和杀虫剂具有指导意义。
1 材料与仪器
1.1 菌种
由羌活体内分离得到一株真菌(编号QH025),通过形态鉴定和18S rDNA 比对分析,经成都生物研究所万波副研究员鉴定为疣孢漆斑菌Myrothecium verrucaria,样品目前存放于中国科学院成都生物所。
1.2 仪器与试剂
Micromass Xevo Triple-quadrupole 型质谱仪(Waters 公司);Bruker Ascend 400 型核磁共振仪,TMS 为内标;制备型HPLC 为Waters 2545 HPLC、Waters 2489 检测器;色谱柱:kromasil RP-C18(19×250 mm,5 μm)、kromasil RP-C18(10×250 mm,5 μm);薄层色谱GF254和柱色谱硅胶(300~400 目)(青岛海洋化工厂);实验所用试剂(分析纯,成都科龙化工厂)。
1.3 培养基
马铃薯葡萄糖琼脂培养基(PDA);发酵培养基:按可溶性淀粉0.8%,蛋白胨0.5%,NaCl 0.2%,CaCO30.2%,MgSO4·7 H2O 0.05%,K2HPO40.05%配制。
2 实验方法
2.1 真菌发酵
将疣孢漆斑菌Myrothecium verrucaria QH025 斜面菌种接种于30 瓶锥形瓶中(200 mL PDA 培养基/500 mL 锥形瓶),培养3 d 得种子液,再接入200 瓶发酵培养基(400 mL/1 L 锥形瓶),在28 ℃、250 rpm 振摇培养14 d 得到发酵液。
2.2 发酵液的提取与分离
取发酵结束后的发酵液(80 L),过滤,除去菌丝体,合并滤液,用等体积乙酸乙酯萃取,重复三次,合并萃取液,减压蒸干浓缩得到乙酸乙酯部分浸膏25 g。
将乙酸乙酯部分浸膏25 g 经正相硅胶柱层析,以石油醚:丙酮(10∶0→9.5∶0.5→9∶1→……→1∶1,v/v)梯度洗脱,收集洗脱液,TLC 指引合并,得到组份A(1.2 g)、B(0.7 g)、C(2.6 g)、D(2.3 g)、E(1.8 g)、F(0.4 g)、G(0.9 g)、H(1.5 g)。组份B经反复制备HPLC,以甲醇-水洗脱,得到化合物1(23 mg)、2(17 mg)。组份C 继续用硅胶柱色谱纯化,石油醚:丙酮(30∶1~10∶1)梯度洗脱,得到3 个亚组份C1、C2、C3,3 个亚组份经反复制备HPLC 纯化,从C1 中得到化合物3(11 mg)、4(13 mg),C2 中得到化合物5(12 mg),C3 中得到化合物6(10 mg)。组份D 经硅胶柱层析,石油醚:丙酮(20∶1~10∶1)梯度洗脱,得到5 个亚组份D1、D2、D3、D4、D5。经制备HPLC 纯化,从D1 中得到化合物7(9 mg)、8(16 mg),从D3 中得到化合物9(14 mg),从D4 中得到化合物10(12 mg)。组份E 经硅胶柱层析,石油醚:丙酮(20∶1~10∶1)梯度洗脱,得到化合物3个亚组份E1、E2、E3,再经反复制备HPLC 纯化,从E2 中得到化合物11(10 mg),E3 中得到化合物12(18 mg)、13(11 mg)。组份F 经反复制备HPLC 纯化,得到化合物14(19 mg)。
2.3 分离成分农药活性测定
2.3.1 除草活性测试
根据文献[8]中的方法,对14 个化合物样品进行除草活性测试。分别用10 ppm、32 ppm 测试样品对杂草植物拟南芥、早熟禾的除草作用,测试板在特定条件下保存7 d。每个样品平行做两次,取其平均值。
2.3.2 杀虫活性测试
根据文献[8]中的方法,对14 个化合物样品做了杀虫活性测试。采用小叶块测试法,用1000 ppm 对绿棉铃虫进行测试。每个样品平行做三次,取其平均值。
3 实验结果
3.1 化合物结构鉴定
化合物1 白色无定型粉末;ESI-MS m/z:501([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.91(1H,dd,J=15.8,11.4 Hz,H-8'),6.65(1H,t,J=11.3 Hz,H-9'),6.19(1H,d,J=11.2 Hz,H-7'),6.08(1H,d,J=15.8 Hz,H-10'),5.86(1H,dd,J=8.6 Hz,4.2 Hz,H-4),5.45(1H,d,J=5.2 Hz,H-10),4.52(1H,d,J=12.4 Hz,H-15β),4.29-4.43(3H,m,H-15α,5'),3.87(1H,d,J=5.0 Hz,H-2),3.61(1H,d,J=5.3 Hz,H-11),3.41(1H,s,H-2'),3.16(1H,d,J=3.9 Hz,H-13β),2.85(1H,d,J=3.9 Hz,H-13α),2.50(1H,dd,J=13.2 Hz,8.3 Hz,H-3β),2.33(1H,td,J=15.5,6.0,2.7 Hz,H-4'β),2.22(1H,m,H-4'α),1.76(3H,s,H-16),1.72(1H,m,H-3α),1.70~2.20(4H,m,H-7,8),1.60(3H,s,H-12'),0.90(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:167.3(C-1'),166.0(C-11'),165.0(C-6'),140.4(C-9),138.3(C-8'),138.3(C-9'),127.5(C-10'),125.5(C-7'),117.9(C-10),78.9(C-2),75.5(C-4),67.1(C-11),64.8(C-12),63.8(C-15),61.3(C-3'),60.5(C-5'),58.2(C-2'),49.2(C-5),47.6(C-13),43.8(C-6),37.0(C-4'),35.0(C-3),27.5(C-8),23.0(C-16),20.0(C-7),15.9(C-12'),7.2(C-14)。以上核磁数据与文献[9,10]报道的疣孢菌素B(verrucarin B)的碳谱核磁数据基本一致,故确定该化合物为疣孢菌素B(verrucarin B)。
化合物2 白色无定型粉末;ESI-MS m/z:571([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.66(1H,m,H-8'),6.58(1H,t,J=11.4 Hz,H-9'),5.97(1H,dd,J=15.3,2.2 Hz,H-7'),5.92(1H,dd,J=8.3,4.4 Hz,H-4),5.81(1H,d,J=11.1 Hz,H-10'),5.73(1H,d,J=5.3 Hz,H-10),5.65(1H,s,H-2'),5.54(1H,dd,J=8.6,3.4 Hz,H-5'),5.20(1H,d,J=5.4 Hz,H-8),4.42(1H,d,J=12.2 Hz,H-15β),4.37(1H,d,J=12.2 Hz,H-15α),4.08(1H,d,J=8.6 Hz,H-6'),3.85(1H,d,J=5.0 Hz,H-2),3.77(1H,d,J=5.5 Hz,H-11),3.68(1H,dq,J=12.1,5.9 Hz,H-13'),3.12(1H,d,J=3.9 Hz,H-13β),2.85(1H,d,J=4.0 Hz,H-13α),2.67(1H,dd,J=12.3 Hz,H-4'β),2.50(1H,dd,J=15.4 Hz,8.3 Hz,H-3β),2.29(3H,s,H-12'),2.10~2.27(4H,m,H-3α,7,4'α),1.94(3H,s,H-18),1.77(3H,s,H-16),1.36(3H,d,J=6.0 Hz,H-14'),0.85(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:170.9(C-17),166.2(C-11'),165.8(C-1'),155.2(C-3'),143.2(C-9'),136.4(C-9),134.9(C-7'),126.0(C-8'),123.9(C-10),118.6(C-2'),118.4(C-10'),100.6(C-5'),81.9(C-6'),79.1(C-2),76.6(C-13'),73.4(C-4),68.7(C-8),67.2(C-11),65.4(C-12),64.5(C-15),49.0(C-5),47.9(C-13),47.7(C-4'),42.0(C-6),34.6(C-3),26.2(C-7),20.9(C-18),20.5(C-16),18.2(C-12'),16.4(C-14'),7.2(C-14)。以上核磁数据与文献[11]报道的8-乙酰基漆斑菌素H(8-acetoxyroridin H)的核磁数据基本一致,故确定该化合物为8-乙酰基漆斑菌素(8-acetoxyroridin H)。
化合物3 白色无定型粉末;ESI-MS m/z:501([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.99(1H,dd,J=15.7,11.7 Hz,H-8'),6.65(1H,t,J=11.7 Hz,H-9'),6.18(1H,d,J=11.7 Hz,H-10'),6.03(1H,d,J=15.7 Hz,H-7'),5.84(1H,s,H-2'),5.76(1H,d,J=3.2 Hz,H-4),5.58(1H,d,J=5.1 Hz,H-10),4.46(1H,m,H-3),4.42(1H,d,J=12.7 Hz,H-15β),4.40~4.50(1H,m,H-5'β),4.22(1H,d,J=5.5 Hz,H-11),4.13~4.40(1H,m,H-3),4.04(1H,d,J=12.7 Hz,H-15α),3.72(1H,d,J=4.9 Hz,H-2),3.11(1H,d,J=3.9 Hz,H-13β),2.82(1H,d,J=3.9 Hz,H-13α),2.40-2.60(2H,m,H-8β,4'β),2.28(3H,s,H-12'),2.00~2.10(3H,m,H-7β,8α,4'α),1.75(3H,s,H-16),1.72(1H,m,H-7α),0.83(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.5(C-6'),166.2(C-1'),165.6(C-11'),157.1(C-3'),140.3(C-9),139.5(C-9'),139.1(C-8'),127.7(C-7'),125.6(C-10'),118.9(C-2'),118.3(C-10),84.0(C-4),79.3(C-2),77.1(C-3),68.3(C-11),64.7(C-12),63.6(C-15),60.7(C-5'),49.1(C-5),47.5(C-13),43.9(C-6),40.4(C-4'),27.8(C-8),23.5(C-16),21.2(C-7),17.5(C-12'),7.1(C-14)。以上核磁数据与文献[12]报道的疣孢菌素M(verrucarin M)的核磁数据基本一致,故确定该化合物为疣孢菌素M(verrucarin M)。
化合物4 白色无定型粉末;ESI-MS m/z:485([M+H]+);1H NMR(CDCl3,400 MHz)δ:8.06(1H,dd,J=15.2,11.5 Hz,H-8'),6.62(1H,dd,J=11.5,11.5 Hz,H-9'),6.18(1H,d,J=11.5 Hz,H-10'),6.11(1H,d,J=15.2 Hz,H-7'),6.10(1H,m,H-4),5.82(1H,dd,J=8.2,4.3 Hz,H-4),5.45(1H,d,J=5.1 Hz,H-10),4.82(1H,d,J=12.2 Hz,H-15β),4.52(1H,m,H-5'β),4.23(1H,d,J=12.2 Hz,H-15α),4.16(1H,s,H-2'),4.00(1H,m,H-5'α),3.88(1H,d,J=5.2 Hz,H-2),3.59(1H,d,J=5.1 Hz,H-11),3.14(1H,d,J=4.1 Hz,H-13β),2.82(1H,d,J=4.1 Hz,H-13α),2.48~2.60(3H,m,H-3β,4'),2.18(1H,m,H-3α),1.80~
2.10 (4H,m,H-7,8),1.73(3H,s,H-16),0.90(3H,s,H-12'),0.83(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.1(C-6'),165.8(C-1'),165.5(C-11'),156.6(C-3'),140.5(C-9),139.5(C-9'),139.1(C-8'),127.4(C-7'),125.5(C-10'),118.6(C-2'),118.1(C-10),79.0(C-2),75.3(C-4),67.3(C-11),65.5(C-12),63.3(C-15),60.4(C-5'),48.8(C-5),48.2(C-13),43.0(C-6),40.2(C-4'),35.1(C-3),27.6(C-8),23.3(C-16),20.7(C-7),17.2(C-12'),7.0(C-14)。以上核磁数据与文献[13]报道的疣孢菌素J(verrucarin J)的核磁数据基本一致,故确定该化合物为疣孢菌素J(verrucarin J)。
化合物5 白色无定型粉末;ESI-MS m/z:531([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.54(1H,dd,J=15.4,11.2 Hz,H-8'),6.62(1H,t,J=11.2 Hz,H-9'),5.98(1H,dd,J=15.7,3.0 Hz,H-7'),5.85(1H,m,H-4),5.81(1H,m,H-10'),5.45(1H,d,J=4.5 Hz,H-10),4.39(1H,d,J=12.4 Hz,H-15β),4.28(1H,d,J=12.4 Hz,H-15α),3.87(1H,d,J=6.2 Hz,H-2),3.81(1H,m,H-6'),3.60-3.70(3H,m,H-11,5'),3.50(1H,s,H-2'),3.33(1H,m,H-13'),3.14(1H,d,J=3.9 Hz,H-13β),2.83(1H,d,J=3.9 Hz,H-13α),2.43~2.54(1H,m,H-3β),2.00-2.24(2H,m,H-4'),1.79~1.98(5H,m,H-3α,7,8),1.75(3H,s,H-16),1.63(3H,s,H-12'),1.22(3H,d,J=6.0 Hz,H-14'),0.85(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:167.89(C-1'),166.34(C-11'),143.10(C-9'),140.06(C-9),138.17(C-7'),126.28(C-8'),118.44(C-10),118.11(C-10'),85.71(C-6'),79.01(C-2),74.42(C-4),70.91(C-13'),67.40(C-5'),66.96(C-11),65.33(C-12),64.54(C-15),63.22(C-3'),58.19(C-2'),49.19(C-5),47.77(C-13),43.23(C-6),39.62(C-4'),35.11(C-3),27.55(C-8),23.27(C-16),20.53(C-7),18.20(C-14'),17.41(C-12'),6.99(C-14)。以上核磁数据与文献[9]报道的漆斑菌素D(roridin D)的核磁数据基本一致,故确定该化合物为漆斑菌素D(roridin D)。
化合物6 白色无定型粉末;ESI-MS m/z:543([M+H]+),565([M+Na]+);1H NMR(CDCl3,400 MHz)δ:8.02(1H,dd,J=15.9,11.2 Hz,H-8'),6.64(1H,t,J=11.2 Hz,H-9'),6.09(1H,d,J=11.2 Hz,H-7'),6.03(1H,d,J=15.9 Hz,H-10'),5.94(1H,dd,J=8.2,4.3 Hz,H-4),5.78(1H,s,H-2'),5.72(1H,d,J=5.0 Hz,H-10),5.21(1H,m,H-8),4.57(1H,d,J=12.5 Hz,H-15β),4.52(1H,td,J=11.2,4.0 Hz,H-5'β),4.23(1H,d,J=12.5 Hz,H-15α),4.13(1H,m,H-5'α),3.86(1H,d,J=5.1 Hz,H-11),3.81(1H,d,J=5.6 Hz,H-2),3.13(1H,d,J=3.9 Hz,H-13β),2.87(1H,d,J=3.9 Hz,H-13α),2.40~2.60(3H,m,H-3α,4'),2.30(3H,s,H-12'),2.10~2.30(3H,m,H-3β,7),1.97(3H,s,H-18),1.78(3H,s,H-16),0.83(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:170.9(C-13'),165.8(C-11'),165.6(C-6'),165.5(C-1'),157.0(C-3'),140.0(C-8'),138.7(C-9'),136.4(C-9),127.8(C-7'),125.1(C-10'),123.8(C-10),117.7(C-2'),78.9(C-2),74.7(C-4),68.8(C-8),66.9(C-11),65.3(C-12),64.4(C-15),60.4(C-5'),48.9(C-5),47.9(C-13),42.1(C-6),40.1(C-4'),34.8(C-3),26.3(C-7),21.8(C-16),20.5(C-14'),17.1(C-12'),7.0(C-14)。以上核磁数据与文献[14]报道的乙酰基疣孢菌素L(verrucarin L acetate)的核磁数据基本一致,故确定该化合物为乙酰基疣孢菌素L(verrucarin L acetate)。
化合物7 白色晶体;ESI-MS m/z:515([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.60(1H,dd,J=15.8,11.0 Hz,H-8'),6.63(1H,t,J=11.0 Hz,H-9'),6.39(1H,dd,J=8.0,4.1 Hz,H-4),5.88(1H,s,H-2'),5.85(1H,d,J=4.6 Hz,H-10'),5.73(1H,dd,J=15.8,6.3 Hz,H-7'),5.53(1H,d,J=5.18 Hz,H-10),4.05~4.20(3H,m,H-11,15),3.87(1H,d,J=5.0 Hz,H-2),3.85(1H,m,H-6'),3.70-3.80(2H,m,H-5'α,13'),3.47-3.55(1H,m,H-5'β),3.20(1H,d,J=3.9 Hz,H-13β),2.85(1H,d,J=3.9 Hz,H-13α),2.55~2.65(3H,m,H-3β,4'),2.25(3H,s,H-12'),1.98~2.57(5H,m,H-3α,7,8),1.75(3H,s,H-16),1.19(3H,d,J=5.8 Hz,H-14'),0.82(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.3(C-1'),166.3(C-11'),158.0(C-3'),142.0(C-9'),140.2(C-9),135.1(C-7'),131.1(C-8'),119.5(C-2'),118.8(C-10),117.0(C-10'),83.1(C-6'),79.2(C-2),75.3(C-4),69.7(C-13'),67.0(C-5'),66.7(C-11),65.7(C-12),64.5(C-15),48.5(C-5),47.6(C-13),42.5(C-6),39.8(C-4'),36.6(C-3),27.6(C-8),23.2(C-16),22.6(C-7),19.8(C-12'),18.5(C-14'),6.4(C-14)。以上核磁数据与文献[15]报道的异漆斑菌素E(isororidin E)的核磁数据基本一致,故确定该化合物为异漆斑菌素E(isororidin E)。
化合物8 针状白色晶体;ESI-MS m/z:515([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.50(1H,dd,J=15.8,11.4 Hz,H-8'),6.56(1H,t,J=11.3 Hz,H-9'),6.18(1H,dd,J=8.0,3.9 Hz,H-4),5.93(1H,s,H-2'),5.89(1H,d,J=4.0 Hz,H-10'),5.72(1H,dd,J=11.3,4.7 Hz,H-7'),5.46(1H,d,J=5.1 Hz,H-10),4.30(1H,d,J=12.54 Hz,H-15α),3.91(1H,d,J=12.54 Hz,H-15β),3.88(1H,d,J=12.54 Hz,H-2),3.82(1H,d,J=5.1 Hz,H-11),3.60~3.69(2H,m,H-5'α,13'),3.51-3.58(1H,m,H-5'β),3.12(1H,d,J=4.2 Hz,H-13β),2.80(1H,d,J=4.2 Hz,H-13α),2.45~2.55(3H,m,H-3β,4'),2.25(3H,s,H-12'),1.95-2.60(5H,m,H-3α,7,8),1.69(3H,s,H-16),1.17(3H,d,J=6.0 Hz,H-14'),0.77(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.4(C-1'),165.8(C-11'),159.2(C-3'),143.7(C-9'),140.2(C-9),138.2(C-7'),126.5(C-8'),118.8(C-10),117.6(C-10'),117.1(C-2'),83.7(C-6'),79.2(C-2),74.1(C-4),70.4(C-13'),69.7(C-5'),67.2(C-11),65.5(C-12),63.7(C-15),48.4(C-5),48.1(C-13),42.7(C-6),41.2(C-4'),35.7(C-3),27.7(C-8),23.2(C-16),21.5(C-7),20.2(C-12'),18.2(C-14'),6.7(C-14)。以上核磁数据与文献[16]报道的漆斑菌素E(Roridin E)的核磁数据相吻合,故确定该化合物为漆斑菌素E(Roridin E)。
化合物9 白色无定型粉末;ESI-MS m/z:513([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.71(1H,m,H-8'),6.58(1H,t,J=11.3 Hz,H-9'),5.97(1H,dd,J=13.2,0.8 Hz,H-7'),5.94(1H,m,H-4),5.83(1H,d,J=11.2 Hz,H-10'),5.70(1H,s,H-2'),5.56(1H,dd,J=8.4,3.2 Hz,H-5'),5.46(1H,J=0.8 Hz,H-10),4.35(1H,d,J=12.4 Hz,H-15β),4.03~4.11(2H,m,H-15α,H-6'),3.87(1H,d,J=4.9 Hz,H-2),3.69(2H,m,H-11,13'),3.15(1H,d,J=4.0 Hz,H-13β),2.85(1H,d,J=3.9 Hz,H-13α),2.67(1H,d,J=12.7 Hz,H-4'β),2.50(1H,dd,J=15.2,8.3 Hz,H-3β),2.30(3H,s,H-12'),2.15-2.27(2H,m,H-3α,4'α),1.86~2.09(4H,m,H-7,8),1.73(3H,s,H-16),1.36(3H,d,J=6.1 Hz,H-14'),0.88(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.2(C-1'),166.2(C-11'),154.7(C-3'),142.7(C-9'),140.3(C-9),134.8(C-7'),126.2(C-8'),119.0(C-2'),118.8(C-10'),118.7(C-10),100.8(C-5'),81.9(C-6'),79.0(C-2),77.0(C-13'),74.0(C-4),67.7(C-11),65.5(C-12),63.2(C-15),49.0(C-5),47.8(C-4'),47.5(C-13),43.2(C-6),34.8(C-3),27.6(C-8),23.2(C-16),20.5(C-7),18.3(C-12'),16.5(C-14'),7.2(C-14)。以上核磁数据与文献[17]报道的漆斑菌素H(Roridin H)的核磁数据相吻合,故确定该化合物为漆斑菌素H(Roridin H)。
化合物10 片状晶体;ESI-MS m/z:533([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.67(1H,dd,J=15.4,11.7 Hz,H-8'),6.66(1H,dd,J=11.3,11.3 Hz,H-9'),6.01(1H,dd,J=15.5,3.1 Hz,H-7'),5.82(1H,m,H-10'),5.79(1H,t,J=4.7 Hz,H-4),5.44(1H,d,J=5.2 Hz,H-10),4.44(2H,s,H-15),4.11(1H,s,H-2'),3.86(1H,d,J=5.1 Hz,H-2),3.50~3.70(5H,m,H-11,5',6',13'),3.12(1H,d,J=4.0 Hz,H-13β),2.81(1H,d,J=4.0 Hz,H-13α),2.64(1H,s,H-13'),2.46(1H,m,H-3β),2.22(1H,m,H-3α),1.80~2.10(7H,m,H-7,8,3',4'β),1.75(3H,s,H-16),1.58~1.69(1H,m,H-4'α),1.21(3H,d,J=7.0 Hz,H-14'),1.10(3H,d,J=6.1 Hz,H-12'),0.82(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:174.9(C-1'),166.5(C-11'),143.9(C-9'),141.0(C-9),139.2(C-7'),126.1(C-8'),118.2(C-10),117.5(C-10'),84.0(C-6'),79.1(C-2),75.6(C-2'),74.3(C-4),70.8(C-13'),69.9(C-5'),67.2(C-11),65.3(C-12),64.6(C-15),49.4(C-5),47.8(C-13),43.8(C-6),37.1(C-3'),34.8(C-3),33.0(C-4'),27.7(C-8),23.3(C-16),20.3(C-7),18.2(C-14'),14.7(C-12'),7.5(C-14)。以上核磁数据与文献[18]报道的漆斑菌素A(Roridin A)的核磁数据相吻合,故确定该化合物为漆斑菌素A(Roridin A)。
化合物11 白色无定型粉末;ESI-MS m/z:557([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.48(1H,dd,J=15.4,11.3 Hz,H-8'),6.59(1H,t,J=11.2 Hz,H-9'),6.27(1H,dd,J=8.1,4.1 Hz,H-4),5.88(1H,s,H-2'),5.81~5.87(2H,m,H-7',10'),5.51(1H,d,J=5.0 Hz,H-10),5.09(1H,m,H-13'),4.28(1H,d,J=12.5 Hz,H-15β),3.98~4.05(3H,m,H-11,15α,6'),3.87(1H,d,J=5.1 Hz,H-2),3.65~3.82(2H,m,H-5'),3.17(1H,d,J=4.0 Hz,H-13β),2.85(1H,d,J=4.0 Hz,H-13α),2.47-2.60(2H,m,H-4'),2.35~2.43(1H,m,H-3β),2.26(3H,s,H-12'),2.08(3H,s,H-16'),2.00-2.10(4H,m,H-3α,7β,8),1.73(3H,s,H-16),1.62(1H,m,H-7α),1.19(3H,d,J=6.0 Hz,H-14'),0.82(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:170.5(C-15'),166.4(C-11'),166.3(C-1'),159.2(C-3'),143.2(C-9'),140.3(C-9),137.6(C-7'),127.7(C-8'),119.1(C-10),117.7(C-10'),117.4(C-2'),79.5(C-2),79.2(C-6'),74.3(C-4),70.5(C-13'),69.1(C-11),67.6(C-5'),65.6(C-12),63.8(C-15),48.4(C-5),48.1(C-13),42.7(C-6),41.5(C-4'),35.7(C-3),27.7(C-8),23.3(C-16),21.5(C-7),21.3(C-16'),19.5(C-12'),14.8(C-14'),6.7(C-14)。以上核磁数据与文献[19]报道的乙酰基漆斑菌素E(roridin E acetate)的核磁数据相吻合,故确定该化合物为乙酰基漆斑菌素E(roridin E acetate)。
化合物12 黄色胶质状;ESI-MS m/z:533([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.58(1H,t,J=12.8 Hz,H-4'),6.63(1H,t,J=11.6 Hz,H-3'),6.24(1H,dd,J=15.5,7.6 Hz,H-4),6.11(1H,dd,J=15.5,4.7 Hz,H-5'),5.89(1H,d,J=1.0 Hz,H-2''),5.72(1H,d,J=11.5 Hz,H-2'),5.50(1H,d,J=5.0 Hz,H-10),4.07(1H,m,H-6'),4.03(1H,d,J=5.0 Hz,H-11),3.88(1H,d,J=5.1 Hz,H-2),3.82(1H,d,J=12.3 Hz,H-15β),3.60~3.92(3H,m,H-7',5''),3.65(1H,d,J=12.3 Hz,H-15α),3.19(1H,d,J=4.0 Hz,H-13β),2.87(1H,d,J=4.0 Hz,H-13α),2.60(2H,m,H-3),2.45(2H,m,H-4''),2.22(3H,d,J=1.0 Hz,H-6''),1.56~2.08(4H,m,H-7,8),1.74(3H,s,H-16),1.19(3H,d,J=6.3 Hz,H-8'),0.83(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.0(C-1'),165.9(C-1''),157.1(C-3''),143.8(C-3'),142.2(C-5'),140.5(C-9),127.0(C-4'),118.5(C-10),118.1(C-4'),116.9(C-2''),79.1(C-2),76.1(C-6'),75.0(C-4),70.6(C-7'),66.6(C-11),65.8(C-12),63.4(C-15),59.6(C-5''),48.6(C-5),48.2(C-13),43.9(C-4''),43.6(C-6),36.9(C-3),27.9(C-8),23.2(C-16),21.8(C-7),19.1(C-6''),18.9(C-8'),6.7(C-14)。以上核磁数据与文献[20]报道的Isotrichoverrin A 的核磁数据相吻合,故确定该化合物为Isotrichoverrin A。
化合物13 黄色胶质状;ESI-MS m/z:533([M+H]+);1H NMR(CDCl3,400 MHz)δ:7.56(1H,dd,J=15.5,11.3 Hz,H-4'),6.63(1H,dd,J=11.4,11.3 Hz,H-3'),6.11-6.21(1H,m,H-5'),6.10(1H,m,H-4),5.85(1H,d,J=1.0 Hz,H-2''),5.70(1H,d,J=11.3 Hz,H-2'),5.49(1H,d,J=5.4 Hz,H-10),4.25(1H,m,H-6'),3.98(1H,d,J=5.4 Hz,H-11),3.93(1H,m,H-7'),3.85(1H,d,J=5.2 Hz,H-2),3.81(1H,d,J=12.3 Hz,H-15β),3.75-3.80(2H,m,H-5''),3.64(1H,d,J=12.3 Hz,H-15α),3.17(1H,d,J=4.0 Hz,H-13β),2.86(1H,d,J=4.0 Hz,H-13α),2.58(1H,dd,J=7.8,15.4 Hz,H-3β),2.42(2H,t,J=6.0 Hz,H-4''),2.19(3H,d,J=1.1 Hz,H-6''),2.18(1H,m,H-3α),1.91~2.16(3H,m,H-7β,8),1.72(3H,s,H-16),1.59(1H,m,H-7α),1.14(3H,d,J=6.5 Hz,H-8'),0.81(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:166.1(C-1'),166.0(C-1''),157.2(C-3''),144.1(C-3'),141.4(C-5'),140.5(C-9),127.5(C-4'),118.5(C-10),117.8(C-2'),116.8(C-2''),79.1(C-2),75.4(C-6'),75.1(C-4),70.2(C-7'),66.6(C-11),65.8(C-12),63.4(C-15),59.5(C-5''),48.5(C-5),48.2(C-13),43.6(C-4''),42.8(C-6),36.9(C-3),27.8(C-8),23.2(C-16),21.9(C-7),19.2(C-6''),17.9(C-8'),6.7(C-14)。以上核磁数据与文献[20]报道的Isotrichoverrin B 的核磁数据基本一致,故确定该化合物为Isotrichoverrin B。
化合物14 无色块状晶体;ESI-MS m/z:503([M+H]+);1H NMR(CDCl3,400 MHz)δ:8.06(1H,dd,J=15.5,11.7 Hz,H-8'),6.69(1H,dd,J=11.3,11.3 Hz,H-9'),6.18(1H,d,J=11.05 Hz,H-10'),6.07(1H,d,J=15.6 Hz,H-7'),5.82(1H,dd,J=8.1,4.1 Hz,H-4),5.45(1H,d,J=5.2 Hz,H-10),4.82(1H,d,J=12.1 Hz,H-15β),4.52(1H,dq,J=11.45,2.33 Hz,H-5'β),4.23(1H,d,J=12.1 Hz,H-15α),4.16(1H,d,J=1.8 Hz,H-2'),4.00(1H,td,J=11.9,3.3 Hz,H-5'α),3.88(1H,d,J=5.08 Hz,H-2),3.59(1H,d,J=5.2 Hz,H-11),3.14(1H,d,J=3.9 Hz,H-13β),2.82(1H,d,J=3.9 Hz,H-13α),2.50(1H,m,H-3β),2.20~2.40(4H,m,H-3α,2',3'),1.90~2.00(4H,m,H-7β,8,4'β),1.75(3H,s,H-16),1.70-1.80(2H,m,H-7α,4'α),0.90(3H,d,J=6.75,H-12'),0.87(3H,s,H-14);13C NMR(CDCl3,100 MHz)δ:174.7(C-1'),166.1(C-11'),165.4(C-6'),141.1(C-9),138.9(C-9'),138.8(C-8'),127.5(C-7'),125.8(C-10'),117.9(C-10),78.9(C-4),75.5(C-2),74.2(C-2'),66.9(C-11),65.2(C-12),63.5(C-15),61.1(C-5'),49.5(C-5),47.8(C-13),44.2(C-6),34.9(C-3),33.2(C-3'),32.2(C-4'),27.5(C-8),23.3(C-16),20.0(C-7),10.0(C-12'),7.3(C-14)。以上核磁数据与文献[18]报道的疣孢菌素A(verrucarin A)的核磁数据基本一致,故确定该化合物为疣孢菌素A(verrucarin A)。
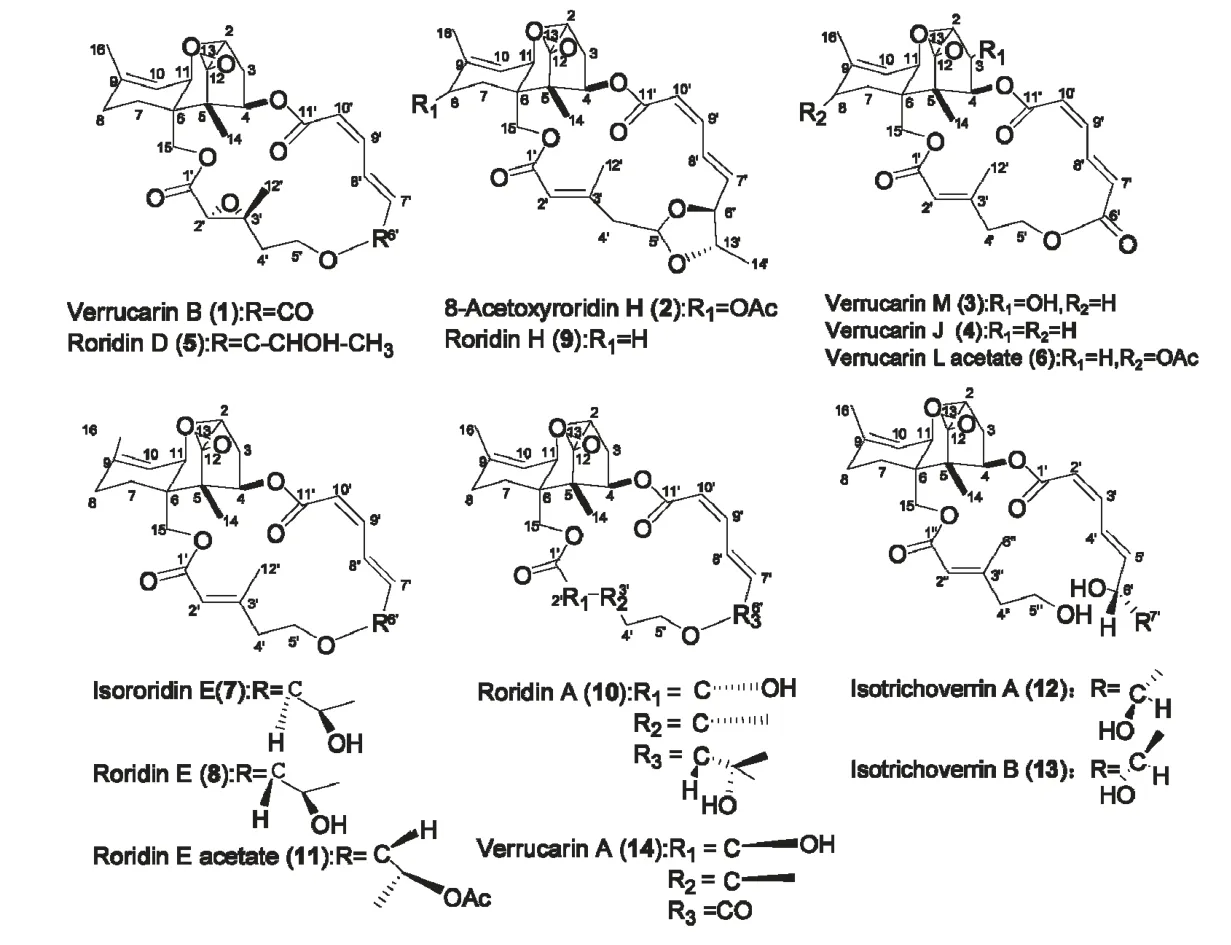
图1 化合物1~14 的化学结构Fig.1 Chemical structures of compounds 1-14
3.2 农药活性
3.2.1 除草活性测试
测试了14 个化合物样品的除草活性。测试结果以测试值99 或0 表示,其中,99 表示测试样品能完全抑制杂草的生长,具有显著的除草活性,0 表示该样品没有除草活性。测试结果见表1。试验表明,除化合物11 外,其余化合物以10 ppm 的浓度对拟南芥都有显著的生长抑制作用;大多数化合物以32 ppm 的浓度对早熟禾有生长抑制作用。
3.2.2 杀虫活性测试
测试了14 个化合物样品的杀虫活性。相对于对照组,样品的测试结果以死亡率评估,采用99 或0 来表示,其中99 表明样品对害虫的死亡率超过70%,0 表明死亡率不显著或没有死亡,测试结果见表1。试验表明,除化合物12 外,其余化合物在1000 ppm 的浓度下,对绿棉铃虫致死率大于70%。
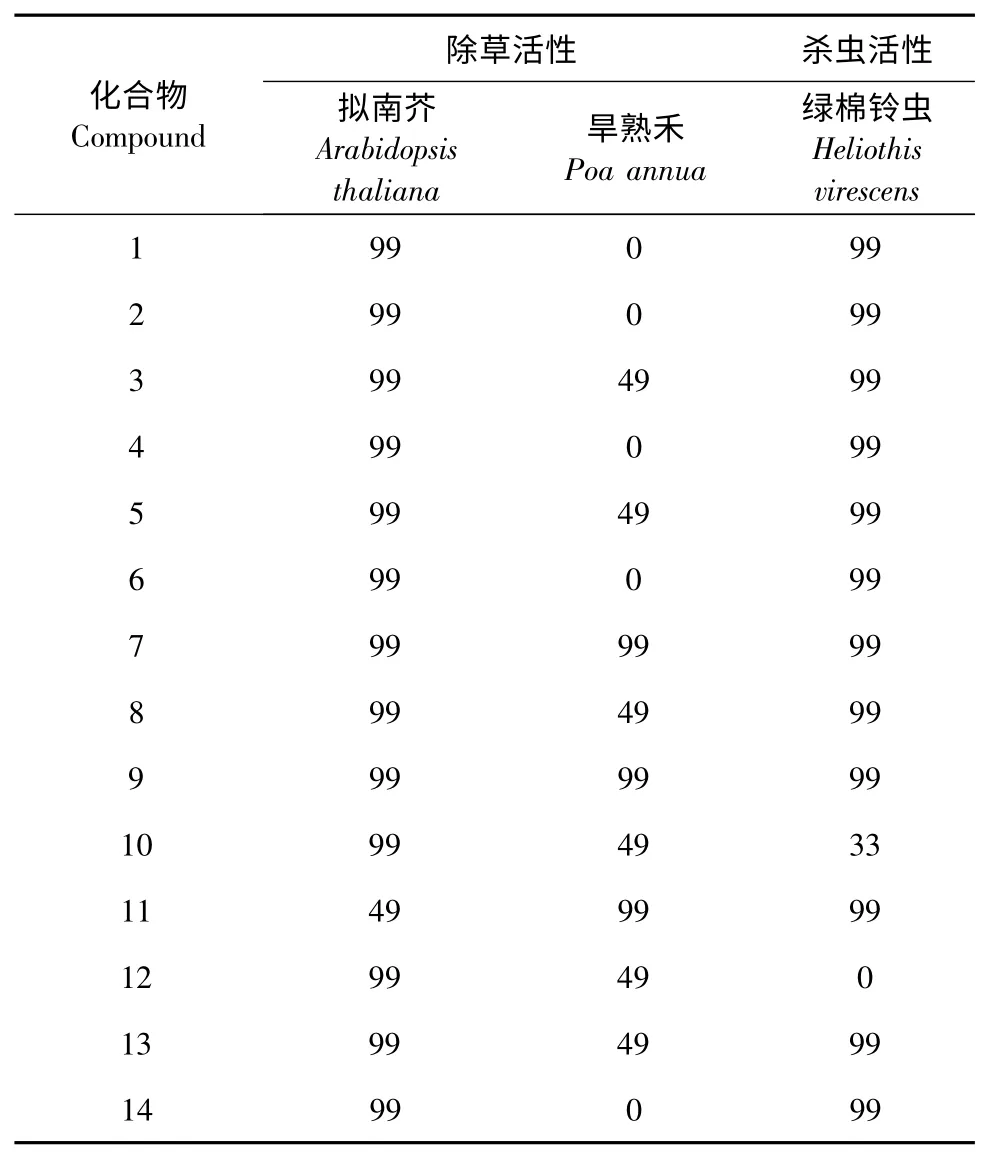
表1 化合物1~14 的除草杀虫活性测试结果Table 1 Herbcidal and pesticidal activities of compounds 1 to 14
4 结果与讨论
一直以来,植物草害和虫害都是导致农作物减产的主要影响因素。而如今由于化学除草剂和杀虫剂的广泛使用,对环境造成了重大的灾害;近年来,为了取代化学农药,开启农业绿色革命,生物防治已成为研究的热点。生物除草剂或杀虫剂相对于化学农药,不仅具有良好的防治效果,而且还具有不污染环境,不影响人类的健康等优点。特别是针对一些特定生物或外侵生物(如水葫芦[21])入侵时,化学防治或人工机械拔出费用太高而达不到有效控制时,采取合适的生物防治能得到有效治理。
本文从羌活内生菌疣孢漆斑菌发酵液的乙酸乙酯部位分离得到14 个单端孢霉烯类化合物,其中化合物2、6、7 为首次从该种真菌中分离得到。我们通过对化合物做了农药活性测试试验发现,10 ppm 的化合物对拟南芥有显著的生长抑制作用,32 ppm 对早熟禾有生长抑制作用,1000 ppm 对绿棉铃虫的致死率大于70%。这些次生代谢产物具有除草,杀虫的作用,尤其对杂草拟南芥和绿棉铃虫的作用效果甚好。为单端孢霉烯的利用提供科学依据,对于将单端孢霉烯类化合物开发为生物除草剂和在棉花的种植中用于杀灭绿棉铃虫都有很好的开发利用前景。
1 Hoagland RE,Weaver MA,Boyette CD.Myrothecium verrucariu fungus:A bioherbicide and strategies to reduce its nontarget risks.Allelopathy J,2007,19:179-192.
2 Bruce BJ,Vivekananda MV,Jacob OM.New Trichoverroids from Myrothecium verrucaria:Verrol and 12,13-Deoxytrichodermadiene.J Med Chem,1983,48:2576-2578.
3 Fernanda FC,Susana J,Betania BC,et al.Antifungal activity of trichothecenes from Fusarium sp.against clinical isolates of Paracoccidioides brasiliensis.Mycoses,2010,54:122-129.
4 Kazuhide K,Akira I,Masafumi K,et al.Cancer preventive potential of trichothecenes from Trichothecium roseum.Bioorg Med Chem,2003,11:2511-2518.
5 Bruce BJ,Jacob OM,Eugene PM.Antileukemic cmpounds derived by chemical modification of macrocyclic Trichothecenes.2.derivatives of Roridins A and H and Verrucarins A and J.J Med Chem,1984,27:239-244.
6 Akira I,Kazuhide K,Hiroki K,et al.Trichothecinols A,B and C,potent anti-tumor promoting sesquiterpenoids from the fungus Trichothecium roseum.Tetrahedr Lett,1996,37:9219-9220.
7 Weaver MA,Boyette CD,Hoagland RE.Bioherbicidal activity from washed spores of Myrothecium verrucaria.World J Microb Biot,2012,28:1941-1946.
8 Zhao HP,Liu YX,Cui ZP,et al.Design,synthesis,and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds.J Agric Food Chem,2011,59:11711-11717.
9 Werner B,Christoph T.13C-NMR-Spectroacopy of the trichothecane derivatives verrucarol,verrucarins A and B and roridins A,D and H.Helv Crim Acta,1975,58:1172-1180.
10 William RR,Timothy AB.Synthesis of Verrucarin B.J Org Chem,1984,49:4332-4339.
11 Melissa M,Wagenaar,Jon C.Two new roridins isolated from Myrothecium sp..J Antibiot,2001,54:517-520.
12 Murakami Y,Okuda T,Shindo K.Roridin L,M and verrucarin M,new macrocyclic trichothecene group antitumor antibiotics,from Myrothecium verrucaria.J Antibiot,2001,54:980-983.
13 Saikawa Y,Okamoto H,Inui T,et al.Toxic principles of a poisonous mushroom Podostroma cornu-damae.Tetrahedron,2001,57:8277-8281.
14 Jarvis BB,Midiwo JO,Desilva T.Verrucarin-L,A new macrocyclic Trichothecene.J Antibiot,1981,34:120-121.
15 Matsumoto M,Minato H,Tori K,et al.Structures of isororidin E,epoxyisororidin E,and epoxy-and diepoxyroridin H,new metabolites isolated from cylindrocarpon species determined by carbon-13 and hydrogen-1 NMR spectroscopy,revision of C-2':C-3' double bond configuration of the roridin group.Tetrahedr Lett,1977,18:4093-4096.
16 Namikoshi M,Akano K,Meguro S,et al.A new macrocyclic Trichothecene,12,13-Deoxyroridin E,produced by the marine-derived fungus Myrothecium roridum collected in Palau.J Nat Prod,2001,64:396-398.
17 Jarvis BB,Stahly GP,Pavanasasivam G,et al.Structure of Roridin-J,a new macrocyclic Trichothecene From Myrothecium-verrucaria.J Antibiot,1980,33:256-258.
18 Wayne ES,Robustelli P,Edens E,et al.Structure and conformational dynamics of Trichothecene mycotoxins.J Nat Prod,2008,71:589-594.
19 Isaka M,Punya J,Lertwerawat Y,et al.Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria.J Nat Prod,1999,62:329-331.
20 Jarvis BB,Wang S,Ammon HL.Trichoverroid stereoisomers.J Nat Prod,1996,59:254-261.
21 Chen ZQ(陈志群).Study on the bio-control research of water hyacinth abroad.Chin J Biol Control(中国生物防治),1996,3:143-145.
