赤芝中一个新的酚性杂萜
2015-01-09周凤娇王心龙王淑美程永现
周凤娇,王心龙,王淑美,程永现*
1广东药学院,广州 510006;2 中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,昆明 650201
灵芝最早记载于《神农本草经》,有仙草之称,被列为上品,又名三秀、茵或芝[1],它是一种坚硬、多孢子和微带苦涩的菌类。灵芝属种类较多,《中华人民共和国药典》仅收载了赤芝(Ganoderma lucidum)或紫芝(Ganoderma sinensis)的干燥子实体作为药材正品来源[2],但实际上灵芝属其它品种市场也流通并药用。灵芝作为名贵中药多年来曾引起众多学者关注,以“Ganoderma”为主题词,通过SciFinder 检索可发现相关研究多达万余篇。研究发现灵芝可调节人体代谢和内分泌功能,对免疫系统、神经系统、心血管系统、呼吸系统等均具有较好的干预效果,同时还可保肝、抗肿瘤、抗氧化、延缓衰老、抗炎等[3]。灵芝临床上或民间被用于治疗多种疾病,如慢性支气管炎、消化不良、神经衰弱、慢性肝炎、高血压、肿瘤、AIDS 等[4,5]。虽然灵芝属的研究已多,但化学成分多局限于三萜、多糖和生物碱[6-8],我们对赤芝的化学成分进行了研究,曾报道lingzhiol 及其肾保护作用[9],对其继续深入研究,从中分离得到9 个化合物,其中包括1 个新的酚性杂萜及2 个首次分离的化合物。该研究将进一步加深和拓宽了人们对灵芝化学的认识。
1 仪器与材料
VGAUTO Spec-3000 及Finnegan MAT 90 质谱仪;Bruker AM-500 及DRX-600 MHz 核磁共振仪(TMS 作为内标,δ 为ppm,J 为Hz)。硅胶(80~100目和200~300 目)(青岛海洋化工厂);40~63 μm RP-18(日本Daiso);45~75 μm MCI gel CHP 20P(日本三菱公司产品);25~100 μm Sephadex LH-20(Pharmacia 公司)。
赤芝于2012年7 月购自中国吉林省北芝菌业科技开发有限公司,样品经中国科学院昆明植物研究所杨祝良研究员鉴定,凭证标本(CHYX-0579)保存于中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室。
2 提取与分离
取赤芝子实体80 kg,粉碎后用95%的乙醇回流提取(2 × 360 L × 2 h),浓缩得总提物。总提物用水适当稀释后用乙酸乙酯萃取3 次,得到乙酸乙酯部分1.1 kg。经硅胶色谱柱进行分离,以氯仿-甲醇(100∶1~1 ∶1)梯度洗脱,得到7 个组分(F1~F7)。F5(31 g)经MCI gel CHP 20P 柱以甲醇-水(10%~90%)梯度洗脱得到3 段组分(F5.1、F5.2、F5.3)。F5.2(22 g)经正相柱硅胶色谱柱,以氯仿-甲醇(50∶1~1∶1)梯度洗脱得到4 段组分(F5.2.1、F5.2.2、F5.2.3、F5.2.4)。F5.2.3(3.5 g)经Sephadex LH-20 柱(MeOH),RP-18 柱,以甲醇-水(50%~70%)洗脱,得到化合物1(1.5 mg)和3(4.2 mg)。F5.2.4(4 g)经MCI gel CHP 20P 柱,以甲醇-水(10%~50%)梯度洗脱,制备薄层硅胶,用氯仿-甲醇(7∶1)展开,再经Sephadex LH-20 柱(MeOH),得化合物2(3.0 mg)。F5.2.1(8 g)经RP-18 柱,以甲醇-水(60%~90%)洗脱,再经Sephadex LH-20柱(MeOH),得化合物5(6.0 mg)、6(17 mg)、7(10 mg)、8(3.0 mg)。F5.2.2(5 g)经Sephadex LH-20(MeOH),制备薄层硅胶,用氯仿-丙酮(3∶1)展开,然后经Sephadex LH-20 柱,得化合物9(3.6 mg)。
3 结构鉴定
化合物1 淡黄色固体,由13C NMR、DEPT 谱及高分辨质谱{m/z:306.1104[M]+(calcd for C16H18O6,306.1103)}确定其分子式为C16H18O6,计算其不饱和度为8。1H NMR 谱中烯区或芳香区有3 个质子(δH7.10,d,J=2.8 Hz,H-3';δH6.88,dd,J=8.9,2.8 Hz,H-5';δH6.67,d,J=8.9 Hz,H-6'),其偶合常数表明结构中芳香区存在一个ABX 系统。中场区的质子信号(δH5.47,s,H-6;δH3.84,s,H-8)分别为双键及与羟基相连碳上的质子。另外,中场区还有两个(δH3.11,s,H-2)质子。1H-1H COSY 谱显示H-5'/H-6'相关,进一步证实了芳环上的取代方式;H-4/H-5/H-6 相关,表明含C-4-C-5-C-6 相连的结构片段。HMBC 谱显示H-4 与C-3,C-8 相关,H-6 与C-7,C-8 相关,H-8 与C-3,C-7 相关,提示结构中存在一个六元环(图1)。H-9 与C-6,C-7,C-8 相关,表明甲基的取代位置,H-2 与C-3(δC50.2),C-4,C-8相关,表明C-2 通过C-3 与环相连,H-3'与H-2 均与C-1(δC206)相关,可以确定苯环与另一环通过C-1-C-2 相连。因为H-2,H-4,H-8 均与C-10(δC183.6)相关,表明羧基是与C-3 相连。至此,化合物1 的平面结构可以得到毫不含糊的确定。ROESY 谱中未发现H-2/H-8 相关,表明H-2 与羧基位于同侧,因此化合物1 的相对构型得以确定。结构中含有2 个手性中心,其比旋光值为-40,手性柱分析只有一个峰,表明化合物1 是一个光学活性结构。该化合物应是一个新杂萜,命名为chizhiol A。–40 (c 0.20,MeOH),ESI-MS:m/z 305[M– H]-,HR-EIMS:m/z 306.1104 [M]+(calcd for C16H18O6,306.1103);1H NMR (600 MHz,CD3OD)δ:3.11(2H,s,H-2),1.99 (2H,m,H-4),1.81 (2H,m,H-5),5.47 (1H,t-like,H-6),3.84 (1H,s,H-8),1.72(3H,s,H-9),7.10 (1H,J=2.8 Hz,H-3'),6.88(1H,dd,J=8.9,2.8 Hz,H-5'),6.67 (1H,d,J=8.9 Hz,H-6');13C NMR (150 MHz,CD3OD)δ:206.2 (C-1),42.1 (C-2),50.2 (C-3),24.1 (C-4),23.9 (C-5),125.4 (C-6),135.9 (C-7),74.3(C-8),27.1 (C-9),183.6 (C-10),156.7 (C-1'),115.7 (C-2'),116.3(C-3'),150.7 (C-4')121.0(C-5'),119.8 (C-6')。
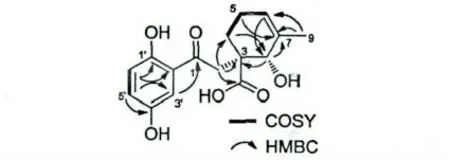
图1 化合物1 的主要HMBC 和COSY 相关Fig.1 Key HMBC and COSY correlations for 1
化合物2 白色粉末,1H NMR (600 MHz,CD3OD)δ:8.11 (1H,s,H-2),8.16 (1H,d,J=8.0 Hz,H-4),7.28 (1H,t-like,J=7.5 Hz,H-5),7.24(1H,t-like,J=7.5 Hz,H-6),7.48 (1H,d,J=8.0 Hz,H-7),9.89 (1H,s,3-CHO);13C NMR (150 MHz,CD3OD)δ:139.8 (C-2),120.1 (C-3),123.6(C-4),122.4 (C-5),125.0 (C-6),113.1 (C-7),125.7 (C-8),138.9 (C-9),187.4 (3-CHO)。以上数据和文献[10]基本一致,故确定化合物2 为吲哚-3-甲醛。
化合物3 淡黄色粉末,1H NMR (600 MHz,CD3OD)δ:7.33 (1H,d,J=2.0 Hz,H-3),7.37(1H,dd,J=8.2,2.0 Hz,H-5),6.79 (1H,d,J=8.2 Hz,H-6),9.55 (1H,s,1-CHO),3.86 (3H,s,2-OCH3);13C NMR (150 MHz,CD3OD)δ:126.9 (C-1),151.3 (C-2),110.2 (C-3),161.9 (C-4),117.6(C-5),130.1 (C-6),192.6 (1-CHO),56.0 (2-OCH3)。以上数据和文献[11]基本一致,故确定化合物3 为2-甲氧基-4-羟基苯甲醛。
化合物4 淡黄色粉末,1H NMR (400 MHz,acetone-d6)δ:7.56 (1H,d,J=1.8 Hz,H-2),7.59(1H,dd,J=8.0,1.8 Hz,H-5),6.91 (1H,d,J=8.0 Hz,H-6),3.89 (3H,s,3-OCH3);13C NMR (100 MHz,acetone-d6)δ:122.7 (C-1),115.4 (C-2),147.9 (C-3),151.9 (C-4),113.3 (C-5),124.8 (C-6),167.6 (1-COOH),56.2 (2-OCH3)。以上数据和文献[12]基本一致,故确定化合物4 为4-羟基-3-甲氧基苯甲酸。
化合物5 针状晶体,1H NMR (400 MHz,CDCl3)δ:3.14 (1H,d,J=8.2 Hz,H-3),4.74(1H,t,J=8.8 Hz,H-7),1.14 (3H,s,H-18),0.81(3H,s,H-19),2.15 (3H,s,H-21),0.96 (3H,s,H-22),0.80 (3H,s,H-23),1.34 (3H,s,H-24);13C NMR (100 MHz,CDCl3)δ:34.7 (C-1),27.6 (C-2),78.2 (C-3),38.8 (C-4),49.0 (C-5),26.5 (C-6),66.7 (C-7),156.3 (C-8),142.7 (C-9),38.6(C-10),196.5 (C-11),49.0 (C-12),44.9 (C-13),58.8 (C-14),216.1 (C-15),35.7 (C-16),54.0 (C-17),18.3 (C-18),19.1 (C-19),205.1 (C-20),31.2 (C-21),28.1 (C-22),15.4 (C-23),24.6 (C-24)。以上数据和文献[13]基本一致,故确定化合物5 为灵芝酮A。
化合物6 淡黄色粉末,1H NMR (400 MHz,CDCl3)δ:3.21 (lH,t,J=9.2 Hz,H-3),4.73 (1H,dt,J=8.4,4.2 Hz,H-7),4.33 (1H,s,H-12),0.74(3H,s,H-18),1.30 (3H,s,H-19),1.93(1H,m,H-20),1.07 (3H,d,J=6.8 Hz,H-21),0.97 (3H,s,H-28),0.84 (3H,s,H-29),1.39 (3H,s,H-30),3.63 (3H,s,OCH3);13C NMR (100 MHz,CDCl3)δ:34.4 (C-1),27.2 (C-2),78.2 (C-3),38.5 (C-4),49.0 (C-5),26.6 (C-6),66.2 (C-7),157.1 (C-8),141.9 (C-9),38.1 (C-10),199.5 (C-11),78.1(C-12),51.8 (C-13),60.1 (C-14),217.4 (C-15),37.3 (C-16),46.4 (C-17),11.8 (C-18),18.7 (C-19),31.4 (C-20),20.5 (C-21),29.6 (C-22),31.9(C-23),174.3 (C-24),27.9 (C-28),15.3 (C-29),23.0 (C-30),51.6 (OCH3)。以上数据和文献[14]基本一致,故确定化合物6 为methyl lucidenate C。
化合物7 淡黄色粉末,1H NMR (400 MHz,CDCl3)δ:4.84 (lH,t,J=7.6 Hz,H-7),1.00 (3H,s,H-18),1.25 (3H,s,H-19),0.96 (3H,d,J=6.8 Hz,H-21),1.12 (3H,s,H-28),1.10 (3H,s,H-29),1.34 (3H,s,H-30);13C NMR (100 MHz,CDCl3)δ:35.6 (C-1),34.2 (C-2),218.0 (C-3),46.2 (C-4),48.8 (C-5),27.6 (C-6),66.2 (C-7),157.9(C-8),141.1 (C-9),38.2 (C-10),197.7 (C-11),50.1 (C-12),44.9 (C-13),59.3 (C-14),216.8 (C-15),41.1 (C-16),46.2 (C-17),17.6 (C-18),18.1(C-19),35.2 (C-20),18.0 (C-21),30.6 (C-22),30.8 (C-23),177.7 (C-24),27.0 (C-28),20.7 (C-29),24.6 (C-30)。以上数据和文献[15]基本一致,故确定化合物7 为赤芝酸A。
化合物8 淡黄色粉末,1H NMR (400 MHz,CDCl3)δ:3.25 (lH,dd,J=11.2,5.2 Hz,H-3),5.61 (1H,s,H-12),0.82 (3H,s,H-18),1.32 (3H,s,H-19),0.98 (3H,d,J=7.6 Hz,H-21),1.01(3H,s,H-28),0.87 (3H,s,H-29),1.72 (3H,s,H-30),3.66 (3H,s,OCH3),2.21 (3H,s,OCOCH3);13C NMR (100 MHz,CDCl3)δ:33.2 (C-1),27.2 (C-2),77.3 (C-3),40.3 (C-4),51.3 (C-5),36.6 (C-6),198.9 (C-7),151.6 (C-8),145.8 (C-9),39.1(C-10),194.1 (C-11),79.3 (C-12),47.8 (C-13),58.4 (C-14),206.2 (C-15),37.6 (C-16),45.3 (C-17),12.0 (C-18),17.9 (C-19),33.2 (C-20),20.1(C-21),30.0 (C-22),31.7 (C-23),173.8 (C-24),27.8 (C-28),15.5 (C-29),21.3 (C-30),51.6(OCH3),20.8 (OCOCH3),170.2 (OCOCH3)。以上数据和文献[14]基本一致,故确定化合物8 为methyl lucideniate E2。
化合物9 淡黄色粉末,1H NMR (500 MHz,DMSO-d6)δ:5.28 (2H,s,H-5),8.69 (1H,s,H-7),8.13 (1H,d,J=8.1 Hz,H-9),7.71 (1H,t-like,J=7.3 Hz,H-10),7.87 (1H,t-like,J=7.3 Hz,H-11),8.17 (1H,d,J=8.1 Hz,H-12),7.35 (1H,s,H-14),5.43 (2H,s,H-17),0.88 (3H,t,J=7.4 Hz,H-18),1.86 (1H,q,H-19);13C NMR (125 MHz,DMSO-d6)δ:152.7 (C-2),145.6 (C-3),50.4(C-5),128.1 (C-6),131.7 (C-7),127.8 (C-8),128.6 (C-9),127.8 (C-10),130.4 (C-11),129.1(C-12),148.0 (C-13),96.9 (C-14),150.1 (C-15),119.2 (C-16),65.3 (C-17),7.9 (C-18),31.4(C-19),72.5 (C-20),172.7 (C-21)。以上数据和文献[16]基本一致,故确定化合物9 为喜树碱。
1 Liu SY(刘思妤),Wang Y(王艳),He RR(何蓉蓉),et al.The chemical constituents of Ganoderma lucidum.J Shenyang Pharm Univ (沈阳药科大学学报),2008,3:183-187.
2 Chinese Pharmacopoeia Commission (国家药典委员会).Pharmacopoeia of China,2000,296.
3 EI-Mekkawy S,Meselhy R,Nakamura N,et al.Anti-HIV-1 and anti-HIV-1-proteasesubs tancesfrom Ganoder malucidum.Phytochemistry,1998,49:1651-1657.
4 Yoshiyuki M,Naoko T,Linda H,et al.Lucidenic acid O and lactone,new terpeneinhibitors of eukaryotic DNA polymerases from abasidiomycete,Ganoderma lucidum.Bioorg Med Chem Lett,1999,7:2047-2052.
5 Chen GL(陈国良),Chen XQ(陈小清).The review of active ingredients research about Ganoderma lucidum.Edible Fungi China(中国食用菌),1995,14(4):7-9.
6 Cheng CR,Yue QX,Wu ZY,et al.Cytotoxic triterpenoids from Ganoderma lucidum.Phytochemistry,2010,73:1579-1585.
7 Ma L(马林),Wu F(吴丰),Chen RY(陈若芸).Analysis of triterpene constituents from Ganoderma lucidum.Acta Pharm Sin (药学学报),2003,38:50.
8 Iwatsuki K,Akihisa T,Tokuda H,et al.Lucidenic acids P and Q,methyl lucidenate P,and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on Epstein-Barr virus activation.J Nat Prod,2003,66:1582-1585.
9 Yan YM,AI J,Zhou LL,et al.Lingzhiols,unprecedented rotary door-shaped meroterpenoids as potent and selective inhibitors of p-Smad 3 from Ganoderma lucidum.Org Lett,2013,15:5488-5491.
10 Wu JY(吴锦玉),Wu YB(吴岩斌),Yi J(易骏).Chemical constituents in leaves of Magnolia officinalis var.biloba.Chin Tradit Herb Drugs(中草药),2013,44:2965-2968.
11 Zhang DW(张德武),Dai SJ(戴胜军),Li GH(李贵海),et al.Chemical constituents in cane of Pueraria lobata.Chin Tradit Herb Drugs(中草药),2011,42:649-651.
12 Zheng J(郑健),Gao HY(高慧媛),Chen GT(陈广通).The active constituents in Mentha spicata L (I).J Shenyang Pharm Univ(沈阳药科大学学报),2006,23:145-147.
13 Hu LL(胡丽丽),Ma QY(马青云),Huang SZ(黄圣卓).Chemical constituents in Ganoderma tropicum.Chin J Med Chem(中国药物化学杂志),2013,23:115-119.
14 Kikuchi T,Kanomi S,Murai Y,et al.Constituents of the fungus Ganoderma lucidum (FR.)KARST.II.:structures of ganoderic acids F,G,and H,lucidenic acids D2 and E2,and related compounds.Chem Pharm Bull,1986,34:4018-4029.
15 Pu QH(普琼惠),Chen H(陈虹),Chen QY(陈若芸).Chemical constituents in Ganoderma tsugae Murr.Chin Tradit Herb Drugs(中草药),2005,36:502-504.
16 Zhou BN,Hoch JM,Johnson RK,et al.Use of compare analysis to discover new natural product drugs:Isolation of camptothecin and 9-methoxycamptothecin from a new source.J Nat Prod,2000,63:1273-1276.
