Advances of stereotactic body radiotherapy in pancreatic cancer
2015-01-08QichunWeiWeiYuLaurenRosatiJosephHerman
Qichun Wei,Wei Yu,Lauren M.Rosati,Joseph M.Herman
1Department of Radiation Oncology,the Second Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou 310009,China;2Department of Radiation Oncology & Molecular Radiation Sciences,Sidney Kimmel Comprehensive Cancer Center,Johns Hopkins University School of Medicine,Baltimore,Maryland,USA
Introduction
Pancreatic cancer (PCA) is the fourth leading cause of cancer-related deaths for both genders in the United States,and it is estimated that 48,960 new PCA cases will be diagnosed and 40,560 will die from the disease in the USA in 2015 (1).In China,PCA is the seventh leading cause of cancer death.According to the National Central Cancer Registry (NCCR) of China,PCA accounted for 3% of all cancer deaths in 2010,with the total number of deaths at 57,735 (2).Overall,despite the advances in surgery,radiotherapy,chemotherapy,immune and targeted therapy,the prognosis of PCA remains to be poor,with a 5-year overall survival (OS) rate of 7% for all stages combined (1).Lack of symptoms at its onset allows PCA to progress to a more advanced stage at the time of diagnosis,with only 20% of cases presenting with a resectable tumor,and about 40% with locally advanced,unresectable disease (3).Surgical resection appears to be the only modality providing a chance of cure (4); however,even resected patients have a poor prognosis,with a 5-year survival of approximately 20% (4,5).The incidence of local recurrence has been reported as 20% to 60% (6-8),and autopsy data reveals even higher rates (9).For those with locally advanced,unresectable disease,the main therapeutic option is a combination of chemotherapy and radiotherapy,with an aim to control the local disease and prevent pain and obstruction,all of which negatively impact the patient’s quality of life.In a report from Johns Hopkins Hospital by Iacobuzio-Donahueet al.,up to 30% of PCA patients died from locally obstructive disease with few or no distant metastases (9).Moreover,advances in systemic chemotherapy and targeted therapy have improved patient outcomes.As patients live longer,the role of local therapy such as radiotherapy becomes even more important.These fi ndings have highlighted the importance of local radiation therapy in the management of PCA.
The role of conventional radiation therapy in the management of PCA
Chemoradiation (CRT) has played a key role in the treatment paradigm for patients with unresectable PCA.Previous clinical trials investigating treatment options for patients with locally advanced pancreatic cancer (LAPC)have demonstrated conflicting results regarding the role of conventional CRT.When compared to chemotherapy alone,an increase in OS with CRT was confirmed in three trials conducted before the 1980s: the Gastrointestinal Tumor Study Group (GITSG) 9283,the Eastern Cooperative Oncology Group (ECOG) 4201,and the Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR)trials.However,a substantial increase in toxicity was also seen in the CRT arms of the first two studies (10,11).In contrast,patients undergoing CRT had decreased OS rates in the Fédération Francophone de Cancérologie Digestive and Société Francophone de Radiothérapie Oncologique(FFCD-SFRO) study (12).
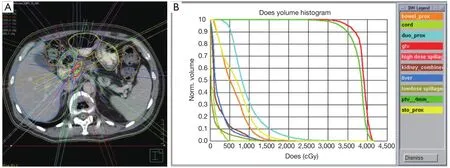
Figure 1 (A) depicts the treatment plan of a patient receiving 33 Gy in 5 fractions to treat a tumor in the head of the pancreas.Note the use of multiple high-dose beams and strict contouring of surrounding organs at risk (OARs).(B) displays the corresponding dose volume histogram (DVH).This demonstrates that dose to the planning target volume (PTV,dark green) and gross tumor volume (GTV,red) is maximized while minimizing dose to the OARs such as the duodenum (light blue),stomach (yellow),bowel (orange),liver (dark blue),kidneys (brown),and spinal cord (light green).
With the aim to settle the controversy regarding the role of standard CRT for LAPC patients,the phase III GERCOR LAP 07 study sought to evaluate the role of CRT following induction chemotherapy (13).After induction chemotherapy with gemcitabine or gemcitabine/erlotinib,LAPC patients were stratified to two additional months of chemotherapy alone or CRT (54 Gy and capecitabine).The investigators reported no significant improvement in OS with the addition of CRT compared to gemcitabine-based chemotherapy alone (13).The study has showed that LAPC patients receiving chemotherapy alone had a slightly higher median OS of 16.5 months compared to patients receiving CRT (15.3 months) (13).However,there was a significant improvement in first local progression in patients who received CRT.It is important to note that the final data analysis of this study has not yet been published.
Nevertheless,CRT currently remains an important component of treatment in patients with unresectable LAPC.Due to inadequate local control (LC) (~50-60%)observed with standard CRT regimens involving threedimensional conformal radiation therapy (3D-CRT),emphasis has been shifted towards improved radiation dose escalation of the primary tumor with intensity-modulated radiation therapy (IMRT) or stereotactic body radiation therapy (SBRT).Ben-Josefet al.reported an impressive median OS of 14.8 months when treating patients with full-dose gemcitabine and IMRT to 50-60 Gy (14).In this study,they incorporated small expansions of the primary tumor and motion management in order to minimize treatment-related toxicity.Similarly,optimizing technologic advancements in radiation dose delivery,image guidance,and motion management,SBRT enables the precise application of multiple high-dose radiation beams to treat the tumor plus a small margin over 1-5 days (Figure 1).
Evolution of SBRT in PCA
The Stanford group reported on the first study to demonstrate the feasibility of a single-fraction SBRT(25 Gy) regimen for LAPC (15).Excellent LC rates were achieved; however,increased rates of late gastrointestinal toxicity were also found in subsequent studies from the same group and Hoyeret al.(16,17).The reasons for higher toxicity rates in these early SBRT studies might have been attributed to the lack of fractionation,inadequate motion management techniques,absence of image guidance using fiducial markers,and lack of specific dose constraints for organs at risk (OARs).
Following these initial reports,SBRT delivered in 3-5 fractions has been investigated thereafter (18-20).Several retrospective studies have revealed similar LC rates and a lower incidence of high-grade toxicity,as compared to those of single-fraction SBRT.This has led to increasing interestin fractionated SBRT.
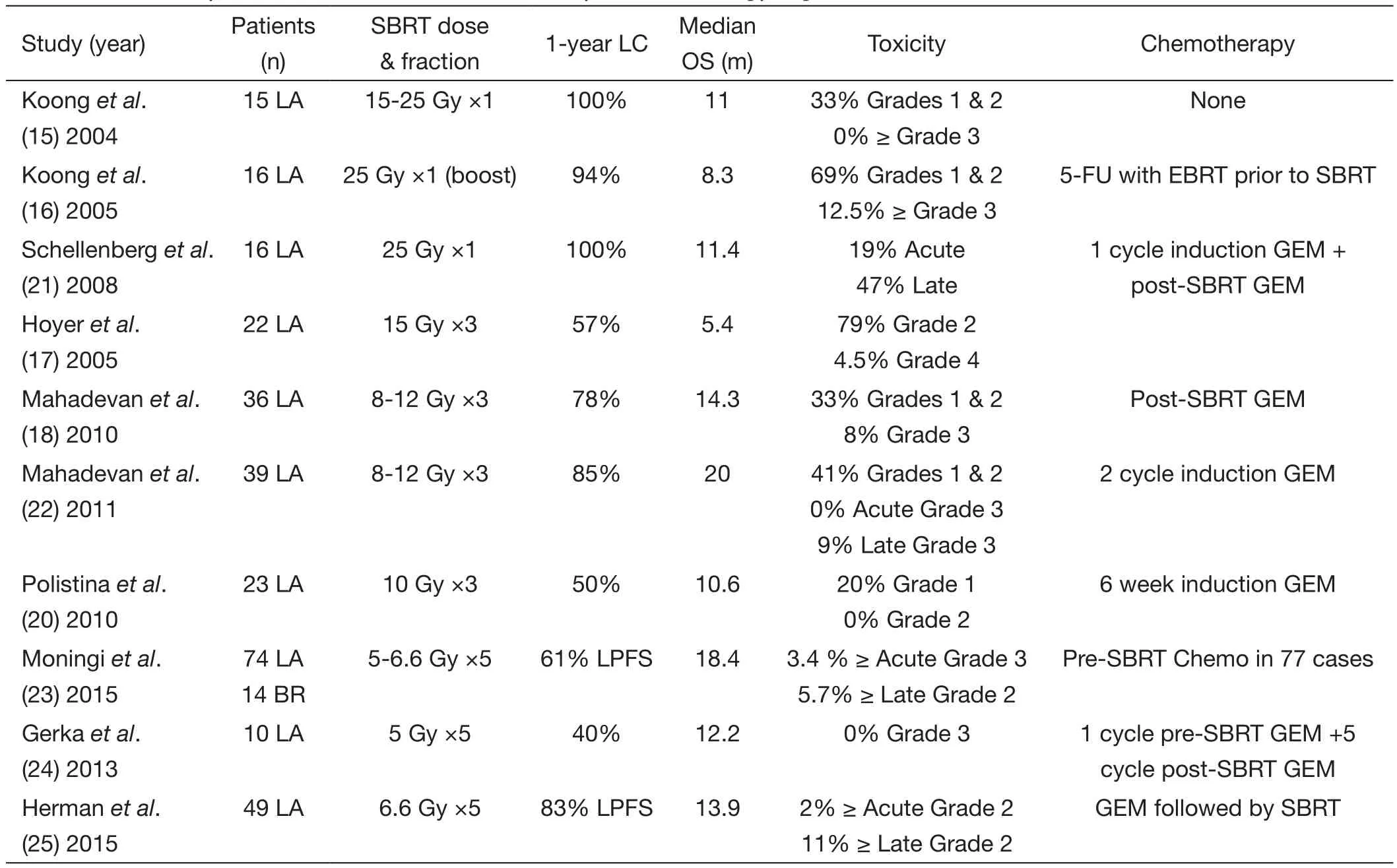
Table 1 A summary of clinical studies of stereotactic body radiation therapy in pancreatic cancer
The application of SBRT provides other advantages.Because SBRT can be completed within a week,the delay to surgery and/or a full-dose chemotherapy course is minimized.Furthermore,a shorter therapeutic course is more convenient for patients.Moreover,the biologically effective dose (BED) delivered with SBRT appears to be higher than conventional fractionation schedules,which may result in improved long-term maintenance of local control.
SBRT in LAPC
Table 1provides an overview of studies that have explored the role of SBRT in the management of LAPC.The initial clinical report on SBRT in the treatment of LAPC was from the Stanford group using CyberKnife.Patients with LAPC were treated to doses of 25 Gy in a single fraction without chemotherapy.Koonget al.reported that the 1-year LC rate was 100%,and the median OS was 11 months.Although none of patients suffered from grade 3 toxicity,33% of patients experienced grade 1-2 toxicity (15).Koonget al.subsequently conducted a phase II study incorporating a SBRT boost of 25 Gy to the pancreatic tumor after a 5-week course of 5-fluorouracil concurrent with external beam radiation therapy.The 1-year LC rate was 94%,69%of patients experienced grade 1-2 toxicity,12.5% of patients suffered from grade 3 toxicity,and the median OS was 8.3 months (16).When combining SBRT with standard CRT,toxicities were higher.Most grade 1 toxicities involved mild nausea,whereas more patients encountered grade 2 and 3 toxicities.Two patients developed duodenal ulcers 4-6 months after therapy.To further explore the effect and toxicity of chemotherapy combined with SBRT,Schellenberget al.conducted a phase II study incorporating one cycle of induction gemcitabine followed by single-fraction SBRT to 25 Gy and maintenance gemcitabine.The 1-year LC rate was 100%,19% of patients experienced acute toxicity,47% of patients suffered from late toxicity,and the median OS was 11.4 months (21).These studies demonstrated excellent LC rates but also showed increased late gastrointestinal toxicity.Lack of fractionation likely contributed to higher toxicity rates.Investigators subsequently shifted to delivering SBRT in 3-5 fractions.
Hypo-fractionated SBRT regimens were adopted as a means to further decrease toxicity while maintaining effective LC.First investigated in a phase II study by Hoyeret al.,a regimen of SBRT to a dose of 45 Gy in three fractions was delivered to 22 LAPC patients.The LC rate was 57%,79% of patients suffered from grade 2 toxicity,4.5% of patients suffered from grade 4 toxicity,and the median OS was 5.4 months (17).Of note,the poor outcomes are likely to have resulted from the lack of accurate positioning and lack of dose constraints to OARs.Mahadevanet al.performed a similar study involving 36 LAPC patients who received three fractions of SBRT to 24 to 36 Gy followed by gemcitabine.At a median follow-up of 24 months (range,12-33 months),the LC rate was 78%with median OS of 14.3 months.The authors also reported low rates of toxicities,with only 25% of patients suffering from grade 2 toxicity and 8% of patients suffering from grade 3 toxicity.Late toxicity occurred in two patients in the form of gastrointestinal bleeding (18).The same group subsequently employed an identical SBRT fractionation scheme following three cycles of induction gemcitabine.The LC rate at 1-year was 85% in 39 LAPC patients with a higher median OS of 20 months,and the rate of late grade 3 toxicities such as bowel obstruction and gastrointestinal bleeding was reported to be 9% (22).An Italian study also evaluated a 3-fraction regimen of 10 Gy SBRT following 6 weeks of pre-SBRT gemcitabine in 23 patients with LAPC (20).The overall LC rate was 82.6% (14 partial response,2 complete response,3 stable disease).Median OS was 10.6 months,which is lower than other similar reports mentioned above.A much lower rate of toxicity was also reported,with no grade 2 or greater acute toxicity in this group of patients (20).However,the definition of LC can vary tremendously between each study,thereby increasing the difficulty of comparison among these reports.
Recently,in a retrospective series at Johns Hopkins Hospital,74 LAPC patients received SBRT to 25-33 Gy in 5 fractions following gemcitabine or FOLFIRINOX-based chemotherapy.The median OS from the date of diagnosis was 18.4 months and 15 (20%) patients underwent successful surgical resection following SBRT (23).Gurkaet al.from the Georgetown group evaluated 10 LAPC patients treated with a multi-fraction SBRT regimen.Patients received one cycle of gemcitabine before SBRT.During week 4 of cycle 1,patients received 25 Gy in 5 fractions,followed by gemcitabine chemotherapy to a maximum of another five cycles (24).The 1-year LC rate was 40% with a median OS of 12.2 months,and no patients suffered from grade 3 acute toxicity (24).
A multi-institutional prospective phase II study involving Johns Hopkins Hospital,Memorial Sloan Kettering Cancer Center,and Stanford University was recently completed (25).In that study,pancreatic fi ducial markers were placed,motion management techniques engaged,and strict dose constraints required.Moreover,all therapeutic plans were centrally reviewed before treatment.A total of 49 LAPC patients received SBRT to a dose of 33 Gy in fi ve fractions followed by gemcitabine.The 1-year freedom from local progression (FFLP) rate was 78%,and the median OS was 13.9 months (25).Only 2% of patients experienced grade 2 or more acute toxicity,and 11% of patients suffered from grade 2 or more late toxicity (25).
The use of fractionated SBRT regimens in patients with LAPC has resulted in promising LC rates that are higher than conventional external beam radiation therapy regimens,with acceptable rates of acute and late gastrointestinal toxicity.
SBRT in BRPC
The literature concerning the application of SBRT in the BRPC is limited.Chuonget al.at Moffitt Cancer Center recently reported on 30 BRPC patients who received neoadjuvant SBRT and concurrent gemcitabine/taxotere/xeloda (GTX) chemotherapy.Twenty-one (70%) patients underwent resection after this regimen.The marginnegative (R0) resection rate was 95% and the node-negative resection rate was 76%.One patient had a near pathologic complete response and two had a partial response.Median OS was 20 months and 1-year progression-free survival(PFS) was 61%.No high-grade (>2) acute toxicity or late grade toxicity was reported (26).Therefore,SBRT in combination with GTX in the neoadjuvant setting was well tolerated with a high conversion rate from borderline resectable to resectable candidates and an increased rate of margin-negative resection (26).
Chuonget al.subsequently performed another retrospective study of 57 BRPC patients who received induction chemotherapy and SBRT.Median doses of 35 Gy were delivered to the region of vessel involvement and 25 Gy to the remainder of the tumor (19).Thirtytwo patients (56.1%) underwent surgery,with 96.9%(31/32) undergoing an R0 resection.Three (9.3%) patients achieved a pathologic complete response and 2 (6.3%)had a near pathologic complete response.Median OS was 16.4 months.No grade 3 or greater acute toxicity was reported whereas 5.3% of patients experienced grade 3 or greater late toxicity (19).Another group from Pittsburgh reported on pathologic response following SBRT for both LAPC (n=5) and BRPC (n=7) patients.Eleven of the 12 (92%) patients received gemcitabine-based or FOLFIRINOX-based chemotherapy before receiving either 24 Gy SBRT in one fraction (n=5) or 36 Gy SBRT in 3 fractions (n=7) (27).Three of the 12 (25%) patients had a pathologic complete response while another two cases(16.7%) demonstrated a near pathologic complete response(<10% viable tumor cells) following tumor resection.Of all resected patients,92% of the cohort achieved a R0 resection.Rates of OS at 1-,2-,and 3-year were 92%,64%,and 51%,respectively (27).
Although the current evidence about SBRT in BRPC is scarce,it appears that BRPC patients may benefit from neoadjuvant SBRT with impressive pathologic response and R0 resection rates.Future research should focus on seeking optimal dose and fractionation regimens in the BRPC setting.
Advances of SBRT as adjuvant th erapy in PCA
The postoperative local recurrence rates in patients with resectable PCA are high,with a range of 20% to 60%(6-8).Therefore,adjuvant therapy is needed with the aim to decrease the risk of local recurrence.The incorporation of SBRT and chemotherapy,which has shown significant potential in the therapy of LAPC,is currently being investigated in the adjuvant setting.Rwigemaet al.reported on 12 patients following a margin-positive resection.The FFLP rate at 1 year was 70.7% and 1-year OS was 81.8%.A median OS of 20.6 months was achieved (28).Rwigemaet al.subsequently conducted a study that 24 resected patients who had close or positive margins received adjuvant SBRT.FFLP at 1 year was 66% and 1-year OS was 80.4%,with a median OS of 26.7 months.No patients suffered from acute grade 3 or greater toxicity (29).Results of this study highlight that adjuvant SBRT in patients with close or positive margins benefited from the treatment.Additional investigation is needed due to the small sample size of the above studies.Future prospective multi-institutional clinical trial is warranted to fully assess the role of SBRT as adjuvant therapy.
Re-irradiation with SBRT after previous conventional CRT
Wildet al.performed a retrospective study from Stanford and Johns Hopkins Hospital on re-irradiation with SBRT for isolated local recurrence or progression of PCA after previous conventionally fractionated CRT.Eighteen locally recurrent or progressive diseases were treated with SBRT to a dose of 20-27 Gy (median,25 Gy) in 5 fractions (30).Rates of FFLP at 6 and 12 months after SBRT were 78% (14/18)and 62% (5/8),respectively,with a median OS of 8.8 months from SBRT.Effective symptom palliation was achieved in 57% of patients.Five patients (28%) experienced grade 2 acute toxicity; none experienced grade 3 or greater acute toxicity.One patient (6%) experienced grade 3 late toxicity in the form of small bowel obstruction (30).Lominskaet al.reported their experience of SBRT for salvage or boost treatment after conventional doses of external beam radiation therapy (31).Twenty-eight patients were evaluated,11 of which were treated with a SBRT boost while the remaining 17 patients underwent salvage SBRT.A dose of 20 to 30 Gy was delivered in 3 to 5 fractions.The rate of FFLP was 86% (12/14),and median OS was 5.9 months (1-27 months) from the date of SBRT treatment.Eleven patients (39%) had 9 months or greater OS.OS at one year was 18%.Patients tolerated the treatment well; only 1 patient had acute grade 2 nausea and vomiting,and two late grade 3 gastrointestinal complications were reported (31).
Although limited treatment options exist for isolated local recurrent PCA after CRT,re-irradiation with SBRT appears to be a safe and reasonable option in well selected cases.
Summary and future directions
While surgical resection appears to be the modality providing an optimal chance of cure,only about 20% of PCA patients present with resectable disease,and 40%present with unresectable,locally advanced disease (3).Even in patients with resectable PCA,the local recurrence rates are high with a range of 20-60% (6-8),and the recurrent lesions are often unresectable.Traditionally,a combination of chemotherapy and radiotherapy are the optimal therapeutic options,with an aim to control the local disease and prevent pain and obstruction which affect the patient’s quality of life.As the role of conventional CRT remains controversial,the dawn of the pancreas SBRT era represents a potential paradigm shift in management of PCA.The advantages of SBRT include the delivery of a higher biological effective dose,the benefit of dose escalation,and a shorter treatment time course.Pancreas SBRT is a therapeutic option to achieve local tumor control; however,whether this translates into improvement in survival remains uncertain.Pancreas SBRT was initially investigated for LAPC and BRPC populations,and has shown promising outcomes in local control for PCA patients as compared to conventional CRT.The acute toxicities have been reported to be mild,with most of them being grade 1 and 2 gastrointestinal side effects,while rates of grade 3 or greater toxicity are less common.The incidence of late complications is also acceptable.Now,SBRT has been expanded to the neoadjuvant setting for resectable disease,adjuvant setting,and recurrent/palliative setting.Exciting data is now accruing such that neoadjuvant SBRT may facilitate margin-negative resection and improve the likelihood of surgical resection among PCA patients who were initially presumed to have unresectable tumors (27,32).
As distant metastases continues to be the most common sites of failure for PCA,there is also a clear need for more effective systemic therapy in these aggressive tumor.The FOLFRINOX regimen has been reported to have superior outcome as compared to gemcitabine for patients with metastatic disease (33-35),thus,investigation of a combination FOLFRINOX or a modified FOLFRINOX followed by SBRT is warranted.Another agent that shows potential is gemcitabine combined with nab-paclitaxel,and exploration of its use in the setting of SBRT in nonmetastatic PCA is necessary (36,37).
Patients may also benefit from individualized therapy by screening out suitable cases for SBRT.It was reported that the genetic status can be used to predict the failure pattern among PCA patients.Those with intact tumor suppressor gene DPC4 had a higher proportion of locally advanced carcinomas with no documented metastatic disease (9).It will be helpful if a local therapy such as SBRT could be reserved for the subset of patients with higher risk of locally destructive disease.
Although there are still many unanswered questions such as dose prescription,fractionation optimization,tumor motion control,dosimetric constraints,and optimal sequence of chemotherapy,it is still hopeful that pancreas SBRT will prove to be an effective emerging technique in the multi-modality treatment of PCA.
Acknowledgements
Funding:Financial support was given from the National Natural Science Foundation of China (No.81071823),and Innovative Multidisciplinary Team for Diagnosis and Treatment of Pancreatic Cancer of Zhejiang Province,China (No.2013TD06).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
1.Siegel RL,Miller KD,Jemal A.Cancer statistics,2015.CA Cancer J Clin 2015;65:5-29.
2.Chen W,Zheng R,Zhang S,et al.Report of cancer incidence and mortality in China,2010.Ann Transl Med 2014;2:61.
3.Willett CG,Czito BG,Bendell JC,et al.Locally advanced pancreatic cancer.J Clin Oncol 2005;23:4538-44.
4.Wagner M,Redaelli C,Lietz M,et al.Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma.Br J Surg 2004;91:586-94.
5.Cameron JL,Riall TS,Coleman J,et al.One thousand consecutive pancreaticoduodenectomies.Ann Surg 2006;244:10-5.
6.Smeenk HG,van Eijck CH,Hop WC,et al.Longterm survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891.Ann Surg 2007;246:734-40.
7.Tepper J,Nardi G,Sutt H.Carcinoma of the pancreas:review of MGH experience from 1963 to 1973.Analysis of surgical failure and implications for radiation therapy.Cancer 1976;37:1519-24.
8.Griffin JF,Smalley SR,Jewell W,et al.Patterns of failure after curative resection of pancreatic carcinoma.Cancer 1990;66:56-61.
9.Iacobuzio-Donahue CA,Fu B,Yachida S,et al.DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer.J Clin Oncol 2009;27:1806-13.
10.Comparative therapeutic trial of radiation with or without chemotherapy in pancreatic carcinoma.Gastrointestinal Tumor Study Group.Int J Radiat Oncol Biol Phys 1979;5:1643-7.
11.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy(chemotherapy plus radiotherapy) to chemotherapy alone.Gastrointestinal Tumor Study Group.J Natl Cancer Inst 1988;80:751-5.
12.Chauffert B,Mornex F,Bonnetain F,et al.Phase III trial comparing intensive induction chemoradiotherapy (60 Gy,infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer.Definitive results of the 2000-01 FFCD/SFRO study.Ann Oncol 2008;19:1592-9.
13.Hammel P,Huguet F,Van Laethem JL,et al.Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC)controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study.J Clin Oncol 2013;31:abstr LBA4003.
14.Ben-Josef E,Schipper M,Francis IR,et al.A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fi xed-dose rate gemcitabine(FDR-G) in patients with unresectable pancreatic cancer.Int J Radiat Oncol Biol Phys 2012;84:1166-71.
15.Koong AC,Le QT,Ho A,et al.Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer.Int J Radiat Oncol Biol Phys 2004;58:1017-21.
16.Koong AC,Christofferson E,Le QT,et al.Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer.Int J Radiat Oncol Biol Phys 2005;63:320-3.
17.Hoyer M,Roed H,Sengelov L,et al.Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma.Radiother Oncol 2005;76:48-53.
18.Mahadevan A,Jain S,Goldstein M,et al.Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer.Int J Radiat Oncol Biol Phys 2010;78:735-42.
19.Chuong MD,Springett GM,Freilich JM,et al.Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated.Int J Radiat Oncol Biol Phys 2013;86:516-22.
20.Polistina F,Costantin G,Casamassima F,et al.Unresectable locally advanced pancreatic cancer:a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration.Ann Surg Oncol 2010;17:2092-101.
21.Schellenberg D,Goodman KA,Lee F,et al.Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer.Int J Radiat Oncol Biol Phys 2008;72:678-86.
22.Mahadevan A,Miksad R,Goldstein M,et al.Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer.Int J Radiat Oncol Biol Phys 2011;81:e615-22.
23.Moningi S,Dholakia AS,Raman SP,et al.The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience.Ann Surg Oncol 2015;22:2352-8.
24.Gurka MK,Collins SP,Slack R,et al.Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety.Radiat Oncol 2013;8:44.
25.Herman JM,Chang DT,Goodman KA,et al.Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma.Cancer 2015;121:1128-37.
26.Chuong MD,Springett GM,Weber J,et al.Induction gemcitabine-based chemotherapy and neoadjuvant stereotactic body radiation therapy achieve high marginnegative resection rates for borderline resectable pancreatic cancer.J Radiat Oncol 2012;1:273-81.
27.Rajagopalan MS,Heron DE,Wegner RE,et al.Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer.Radiat Oncol 2013;8:254.
28.Rwigema JC,Parikh SD,Heron DE,et al.Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas.Am J Clin Oncol 2011;34:63-9.
29.Rwigema JC,Heron DE,Parikh SD,et al.Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins.J Gastrointest Cancer 2012;43:70-6.
30.Wild AT,Hiniker SM,Chang DT,et al.Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: experience from two institutions.J Gastrointest Oncol 2013;4:343-51.
31.Lominska CE,Unger K,Nasr NM,et al.Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas.Radiat Oncol 2012;7:74.
32.Boone BA,Steve J,Krasinskas AM,et al.Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer.J Surg Oncol 2013;108:236-41.
33.Conroy T,Desseigne F,Ychou M,et al.FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer.N Engl J Med 2011;364:1817-25.
34.Marthey L,Sa-Cunha A,Blanc JF,et al.FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort.Ann Surg Oncol 2015;22:295-301.
35.Marsh RW,Talamonti MS,Katz MH,et al.Pancreatic cancer and FOLFIRINOX: a new era and new questions.Cancer Med 2015;4:853-63.
36.Goldstein D,El-Maraghi RH,Hammel P,et al.nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial.J Natl Cancer Inst 2015;107.
37.Von Hoff DD,Ervin T,Arena FP,et al.Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine.N Engl J Med 2013;369:1691-703.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Laparoscopic pancreaticoduodenectomy: a descriptive and comparative review
- Comparison of postoperative complications between internal and external pancreatic duct stenting during pancreaticoduodenectomy: a meta-analysis
- Impact of adjuvant treatment modalities on survival outcomes in curatively resected pancreatic and periampullary adenocarcinoma
- On pancreatic cancer screening by magnetic resonance imaging with the recent evidence by Del Chiaro and colleagues
- Chinese Anti-Cancer Association as a non-governmental organization undertakes systematic cancer prevention work in China
- Prognostic effect analysis of molecular subtype on young breast cancer patients
