海洋酸化没有显著影响成体鹿角杯形珊瑚的钙化作用和光合能力
2015-01-05郑新庆郭富雯刘昕明林荣澄周治东施晓峰
郑新庆 ,郭富雯 ,刘昕明,林荣澄*,周治东,施晓峰
(1. 国家海洋局 第三海洋研究所,福建 厦门 361005;2. 台湾海洋生物博物馆,台湾 屏东 90001;3. 广西壮族自治区海洋研究院,广西 南宁 530022; 4. 福建海洋研究所,福建 厦门 361005)
海洋酸化没有显著影响成体鹿角杯形珊瑚的钙化作用和光合能力
郑新庆1,郭富雯2,刘昕明3,林荣澄1*,周治东4,施晓峰1
(1. 国家海洋局 第三海洋研究所,福建 厦门 361005;2. 台湾海洋生物博物馆,台湾 屏东 90001;3. 广西壮族自治区海洋研究院,广西 南宁 530022; 4. 福建海洋研究所,福建 厦门 361005)
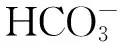
鹿角杯形珊瑚;酸化;珊瑚礁;钙化作用;Fv/Fm
1 引言
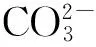
造礁珊瑚被认为是受海水酸化影响最大的类群,因为珊瑚礁体形成过程中需要CaCO3进行相关的物理、生理和化学过程,最后形成珊瑚礁。根据预测,当大气CO2浓度上升到450×10-6时,珊瑚礁的钙化能力将下降到50%左右,这还是没有考虑其他因子(如温度等)协同作用的结果。Langdon等[9]、Barker等[10]发现,当温度处于珊瑚的最适生长温度时,造礁珊瑚的钙化能力与海水文石饱和度(Ωarag)有大致的相关关系。Gattuso等[11]、Langdon和Atkinson[12]指出,当大气CO2浓度上升到450×10-6时,浅水区域造礁珊瑚的主要种类鹿角珊瑚(Acropora)将变得更加脆弱和容易损毁,海水酸度的增加可能会导致大面积珊瑚礁栖息地的退化,珊瑚的造礁过程可能会变缓甚至可能停止或消失。
截至目前,已有大量的实验研究表明pCO2的增加会引起珊瑚钙化率的下降[7,12—19]。不过,来自冷水珊瑚、温带造礁珊瑚以及少数热带造礁珊瑚的数据显示,并不是所有的造礁珊瑚都对海水酸化敏感[14,19—25]。有些造礁珊瑚可以通过上调钙化位点的pH抵消海水酸化的消极影响[26]。而且,造礁珊瑚对酸化的响应似乎还存在地域的特异性,不同区域,它们对酸化的响应可能存在差异[20]。因此,为了评估未来可能的海洋酸化情境对南海北部珊瑚礁的潜在影响,我们必须了解分布于该区域的造礁珊瑚对酸化的生理生态响应及其潜在的结果,并了解其背后的生理及其分子响应机制。然而,与国际上如火如荼的研究相比,国内造礁珊瑚酸化生理的研究还很少[19],目前还未见国内杂志有相关的实验研究报道。
鹿角杯形珊瑚(Pocilloporadamicornis)是印度-太平洋珊瑚礁区常见的造礁珊瑚,被广泛用于造礁珊瑚胁迫生理和生态学、病理学、繁殖生态学等研究的模式生物[27—32],但很少有研究者选择它用于酸化生理的相关研究[14,19—20]。即便是Huang等[19]和Comeau等[14,20]的研究,其结果也存在较大差异。同时,先前的研究也仅局限于珊瑚钙化率的测定,而没有涉及其他的生理参数,例如光合效率、虫黄藻密度等。由于珊瑚的钙化作用与光合作用过程息息相关[33],因此本研究以鹿角杯形珊瑚为研究对象,以珊瑚的钙化率和光合能力为指标,通过气体交换法模拟未来的酸化环境(2100年,pH≈7.8)研究鹿角杯形珊瑚对酸化的生理生态响应。
2 材料与方法
2.1 选择的珊瑚种类及来源
鹿角杯形珊瑚(P.damicornis)是印度-太平洋珊瑚礁区的常见种类,也是我国海域常见的造礁珊瑚种类。本研究从台湾南部垦丁万里桐海域采集直径10 cm的鹿角杯形珊瑚十余棵。
从几个鹿角杯形珊瑚母体剪下珊瑚断枝(fragments),珊瑚的浮力质量(Buoyant weight)约0.4~0.9 g/ind,断枝60棵左右,利用阿隆发胶将珊瑚断枝的剪接位置与不锈钢丝黏在一起,然后扦插在泡沫塑料中(图1),暂养在养殖室内培养系统中1个月,待断枝恢复后,实验开始。

图1 用于实验的鹿角杯形珊瑚断枝Fig. 1 Pocillopora damicornis fragments used for experiments左:刚剪下来的珊瑚断枝;右:与不锈钢丝连接的珊瑚断枝Left:coral fragments removed from large colonies by scissors;right:coral fragments mounted onto stainless steel wire with epoxy resin
2.2 采用的酸化系统
采用图2如下系统模拟海水酸化(图2)。系统通过电磁阀与pH控制器相联,通过监测水体pH的变动,控制气瓶CO2气体的输入。本研究设置酸化处理组和对照组,将酸化处理组的pH控制器的pH值设定为7.8,当水体的pH>7.8时,电磁阀启动,气瓶的CO2输入实验水体,当水体的pH<7.8时,电磁阀关闭。每周校正一次pH控制器的pH探头。
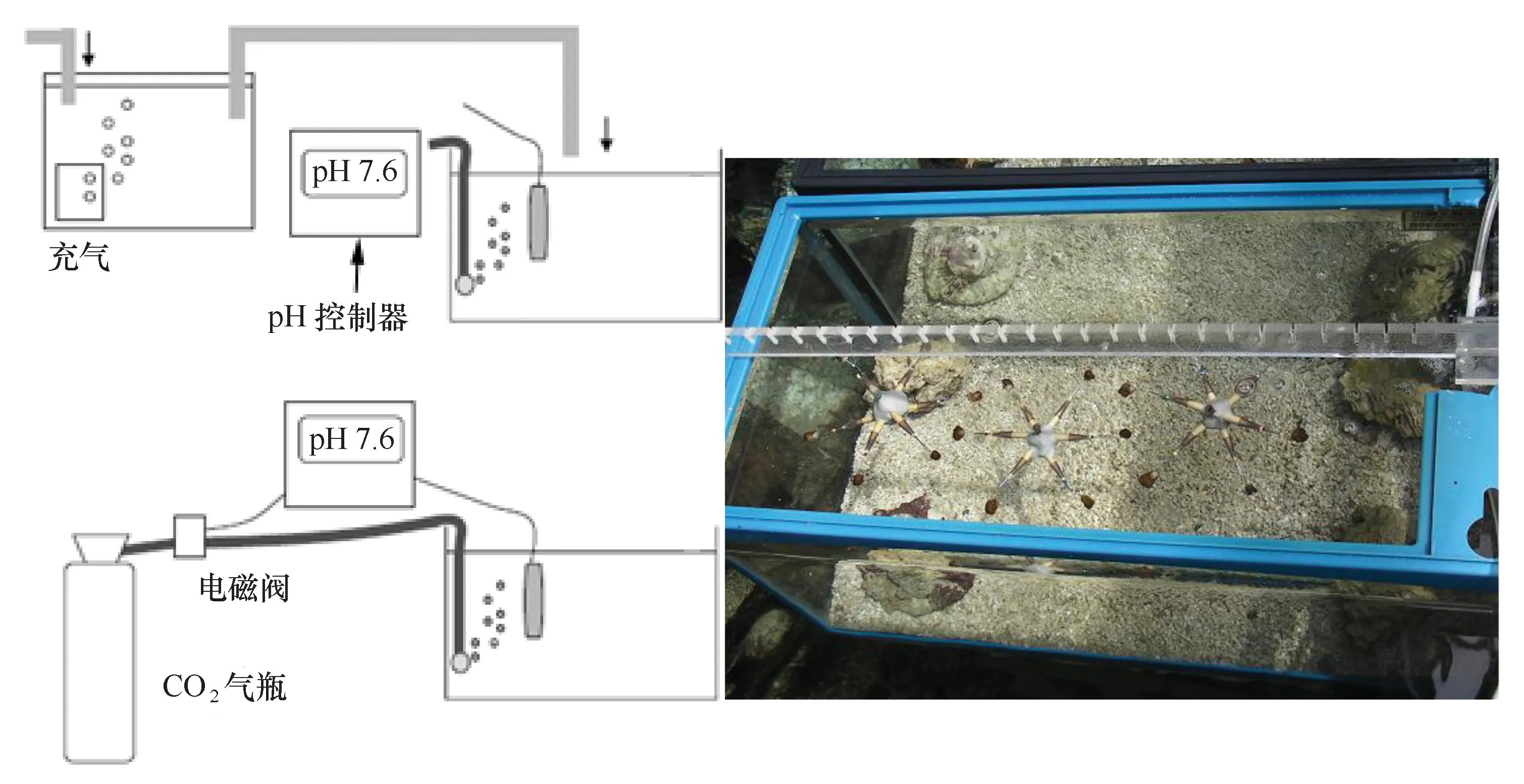
图2 酸化系统的示意图(左)和实验缸的鹿角杯形珊瑚断枝(右)Fig.2 pH-stat systems designed for simulating ocean acidification (left) and Pocillopora damicornis fragments in the experimental tanks (right)
从暂养系统中随机选择断枝用于实验。每个处理组放置18颗断枝,每6颗断枝挂载在六角架上,具体位置如图2所示。实验缸为全透明的玻璃缸,长×宽×高为60 cm×45 cm×45 cm,玻璃缸铺设2~3 cm厚的活沙,沙子的粒径约2~3 mm,两块活石放在缸体的前后侧,实验水体维持在100 L左右。将不同处理的玻璃缸放在水体体积约3 t玻璃纤维缸中水浴,保证不同处理组的水温完全一致,避免温度对实验的干扰。利用2匹的空调制冷机调节玻璃纤维缸的水温,温度变动在25~30℃之间,自然采光。
2.2 实验系统理化参数的测定
(1)利用Hobo温光仪监测实验缸体的温度和光照的变动情况。
(2)实验期间每日的上午(8:00—11:00)、下午(14:00—17:00)和晚上(19:00—22:00)分别记录实验系统的pH值。
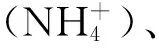
2.3 珊瑚生理参数的测定
选择珊瑚共生虫黄藻叶绿素荧光指数以及珊瑚的钙化率为指标,研究鹿角杯形珊瑚对海水酸化的生理生态响应。每周测量1次,实验4周。
(1)珊瑚的钙化率
利用精度0.01 mg的天平测量所有鹿角杯形珊瑚断枝的浮力质量。根据Spencer Davies (1989)的方法估算鹿角杯形珊瑚的钙化率(G)。
(2)珊瑚的光合能力
利用Diving PAM测量所有鹿角杯形珊瑚断枝的叶绿素荧光指数(Fv/Fm),它反映了珊瑚的光合能力(photosynthesis capacity),当珊瑚受到胁迫时,Fv/Fm会下降。
2.4 数据处理
采用OriginPro 8和Excel 2007作图。采用双因素分差分析(HSD检验)比较酸化处理与否以及不同时间间隔鹿角杯形珊瑚钙化作用的差异;采用成组数据t检验检验每周酸化处理组和对照组鹿角杯形珊瑚光合能力(Fv/Fm)的差异。利用配对数据t检验检验酸化处理组和对照组实验水体pH的差异。显著性水平P<0.05。
3 结果
3.1 环境参数
(1)实验系统温度和光强的变化
从5月20日至6月4日,利用Hobo温光记录仪监测系统温度的变化。结果显示,实验系统的温度介于26.5~29.2℃之间,除24日和25日温度异常升高以外,大部分时间实验系统的温度维持在27~28℃。白天由于日照的关系,温度有所上升(约1℃)。光强介于0~7 000 lx之间,其变化趋势与温度相似,大多数时间光强在2 000~4 000 lx左右。对照组和处理组的温度和光强基本一致(图3)。
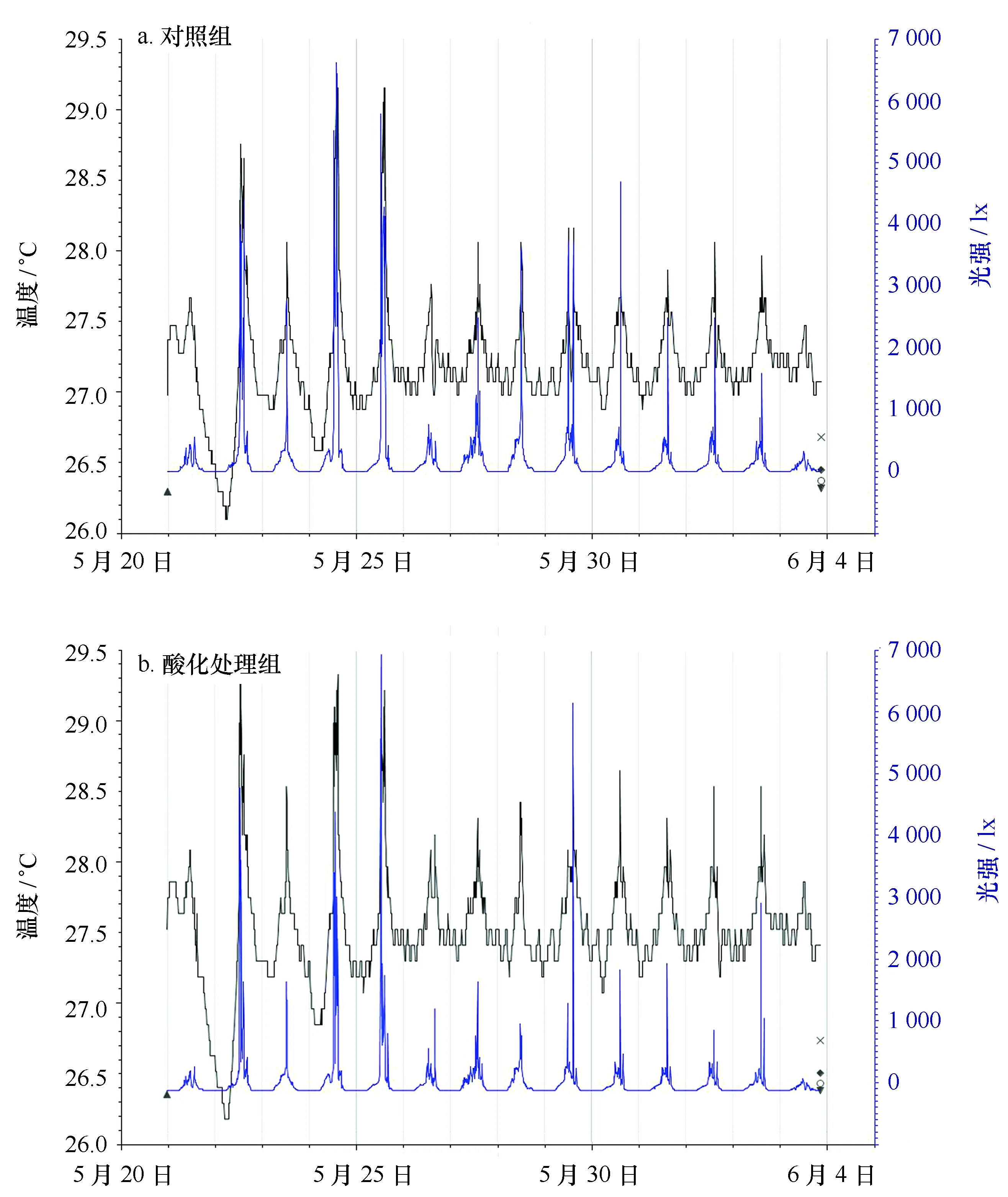
图3 实验水体光强和温度的变化Fig.3 The variation of seawater temperature and light intensity in the experimental seawater
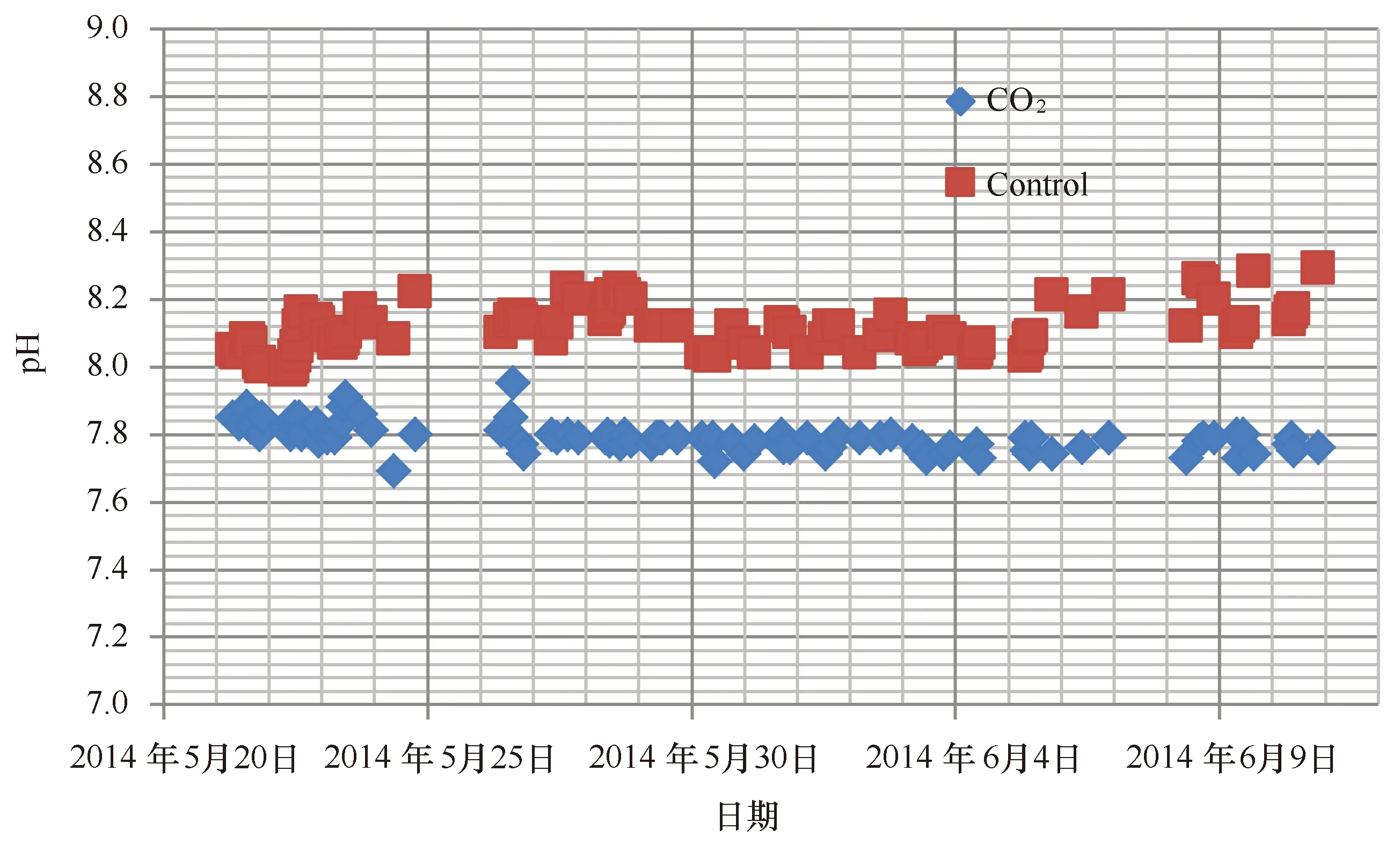
图4 实验水体pH的变化Fig.4 Variation of pH in the experimental seawater
(2)实验系统pH的昼夜变化
实验期间利用酸化系统模拟海洋酸化的实验水体的pH变化如图4所示。结果表明,酸化处理组的pH值介于7.69~7.91(7.78±0.04),而对照组的pH变动较大,介于7.99~8.29(8.11±0.07)。酸化处理组和对照组的pH差异极显著(t=-35.8,df=86,P<0.001)。
(3)实验系统的营养盐水平
实验系统的营养盐水平如表1所示。磷酸盐水平介于0.004~0.017 nmol/L,铵盐介于0.050~0.128 nmol/L,硝酸盐介于0.001~0.017 nmol/L,系统的亚硝酸盐含量低于仪器的检测线,显示实验水体的亚硝酸盐含量极低。总体来说,实验系统的营养盐水平低,落在珊瑚的最适生长范围之内。酸化处理组和对照组的各营养盐指标差异不大。
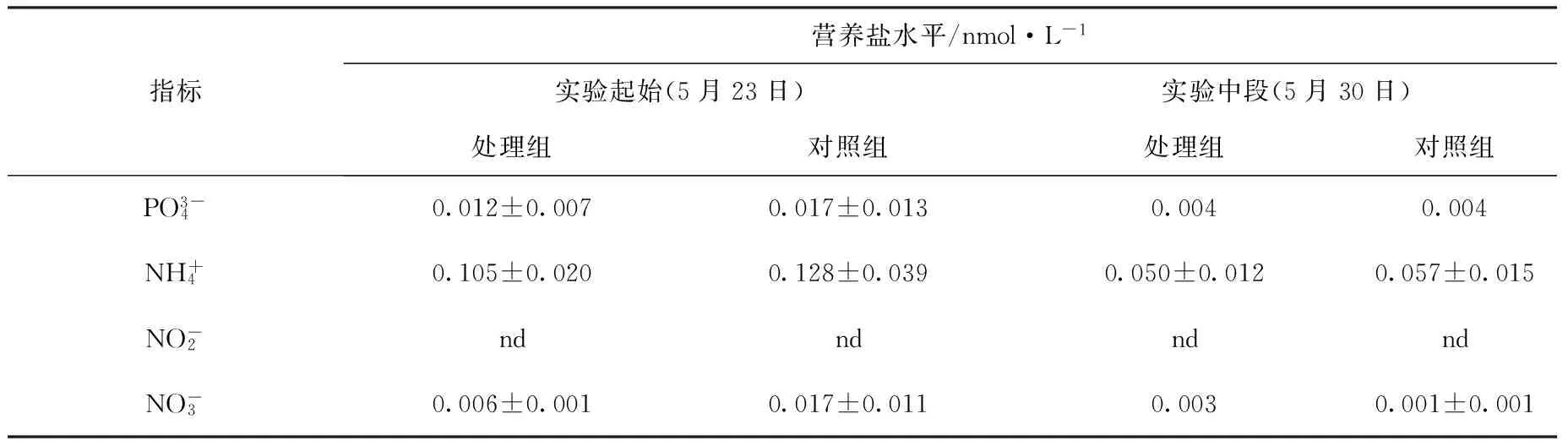
表1 实验系统的营养盐水平Tab.1 Inorganic nutrient in the experimental systems
注:nd表示低于仪器检测线。
3.2 珊瑚的钙化率
鹿角杯形珊瑚的钙化率的变化如图5所示。在实验期间,鹿角杯形珊瑚缓慢生长,酸化处理组鹿角杯形珊瑚平均浮力质量(g/ind)从0.382增加到0.404,对照组则从0.360增加到0.377(见图5A);对照组和酸化处理组鹿角杯形珊瑚的生长率分别介于1.15%~1.92%/周和1.71%~2.09%/周,酸化处理组的平均生长率略高于对照组(见图5B),但二者并没有显著的差异(表2,df=1,F=2.9,P=0.09),不同时段鹿角杯形珊瑚的生长率也没有显著的差异(表2,df=3,F=1.83,P=0.15)。
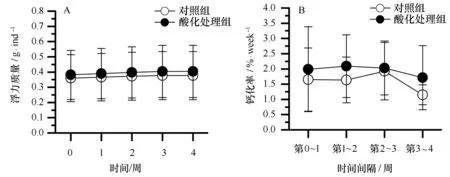
图5 海水酸化对鹿角杯形珊瑚钙化率的影响Fig.5 Effects of ocean acidification to the calcification of Pocillopora damicornisA.珊瑚浮力质量的周变化;B.珊瑚钙化率的周变化A.weekly variation of buoyant weight in P.damicornis; B.weekly variation of the calcification in P.damicomis
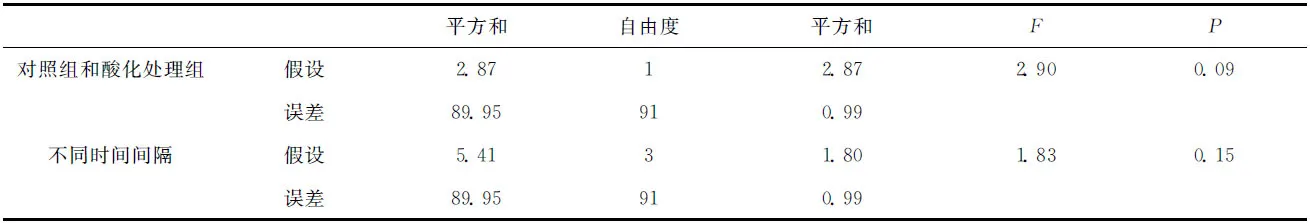
表2 鹿角杯形珊瑚钙化率的统计检验结果Tab.2 Statistic results for the calcification of Pocillopora damicornis
3.3 珊瑚共生虫黄藻的叶绿素荧光指数
从鹿角杯形珊瑚共生虫黄藻叶绿素荧光指数Fv/Fm来看(图6),除第4周略有下降以外,实验期间,Fv/Fm均值在0.7左右,酸化处理组和对照组之间没有显著的差异(P>0.5)。
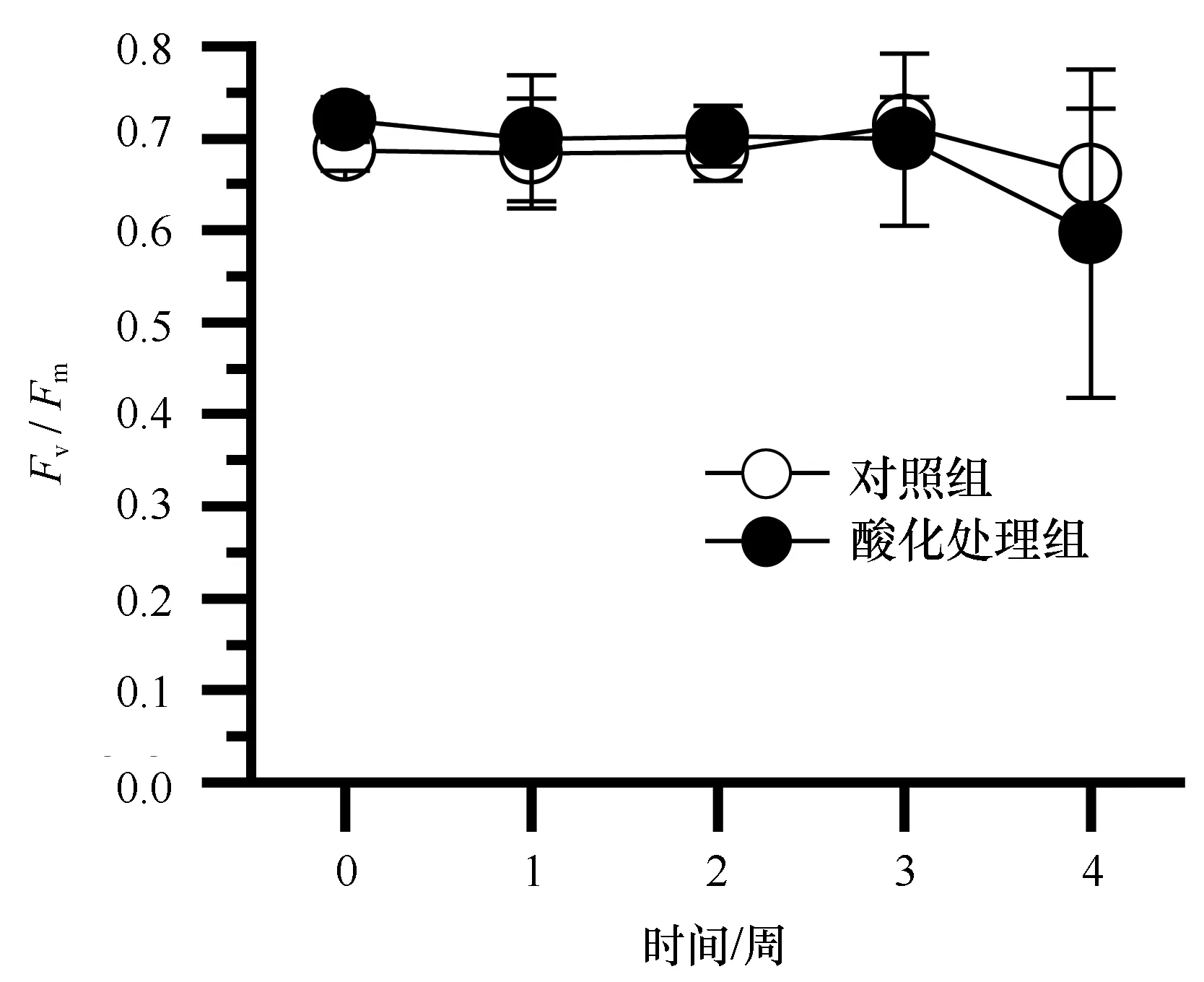
图6 酸化对鹿角杯形珊瑚光合能力(Fv/Fm)的影响Fig.6 Effects of ocean acidification to the photosyn-thesis capacity (Fv/Fm) of Pocillopora damicornis
4 讨论
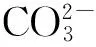
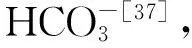
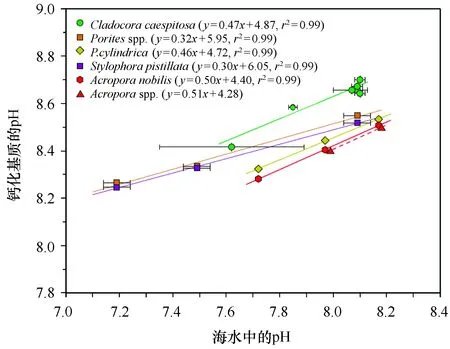
图7 海水中的pH与造礁珊瑚钙化液中的pH(引自McCulloch等[26])Fig.7 Plot of pH in seawater relative to pH at site of calcification of hermatypic corals,modified from McCulloch et al[26]
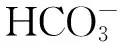
不过,尽管鹿角杯形珊瑚在个体水平上的生理指标(钙化率和光合效率)并没有下降,但是海洋酸化可能会从微观的分子水平上对珊瑚产生影响。例如,Hillhouse和Grammatopoulos发现,酸化对冷水珊瑚Desmophyllumdianthus钙化率和呼吸速率没有显著的影响,但是基因的蛋白表达差异显著,酸化使得与细胞胁迫相关的HSP70和免疫防卫有关的甘露糖结合c型聚合酶表达增加[43]。同时,与珊瑚骨骼合成密切相关的关键蛋白酶——a-碳酸酐酶的合成也增加了[44]。这表明在酸化条件下珊瑚可能会重建其钙化能力,即使其最终表现出的钙化率指标并没有显著的差异。因此,理解造礁珊瑚对酸化生理响应过程背后的分子机制也是非常重要,这需要今后研究进一步的深入。
[1] Sabine C L,Feely R A,Gruber N,et al. The oceanic sink for anthropogenic CO2[J]. Science,2004,305(5682): 367-371.
[2] Raven J,Caldeira K,Elderfield H,et al. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide[M]. London: The Royal Society,2005.
[3] Houghton J T,Ding Y,Griggs D J,et al. Climate change 2001: the scientific basis[R]//Contribution of Working Group I to the Third Assessment Report of the Intergoverment Panel on Climate Change. Cambridge: Cambridge University Press,2001: 881.
[4] Brewer P G. Ocean chemistry of the fossil fuel CO2signal: the haline signal of “business as usual”[J]. Geophysical Research Letters,1997,24(11): 1367-1369.
[5] Feely R A,Sabine C L,Lee K,et al. Impact of anthropogenic CO2on the CaCO3system in the oceans[J]. Science,2004,305(5682): 362-366.
[6] Kleypas J A,Langdon C. Coral reefs and changing seawater chemistry[M]//Phinney J T,Hoegh-Guldberg O,Kleypas J,eds. Coral Reefs and Climate Change: Science and Management. Washington,DC: American Geophysical Union,2006: 73-110.
[7] Anthony K R N,Kline D I,Diaz-Pulido G,et al. Ocean acidification causes bleaching and productivity loss in coral reef builders[J]. Proceedings of the National Academy of Sciences of the United States of America,2008,105(45): 17442-17446.
[8] Fabry V J,Seibel B A,Feely R A,et al. Impacts of ocean acidification on marine fauna and ecosystem processes[J]. ICES Journal of Marine Science,2008,65(3): 414-432.
[9] Langdon C,Takahashi T,Sweeney C,et al. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef[J]. Global Biogeochemical Cycles,2000,14(2): 639-654.
[10] Baker A C,Glynn P W,Riegl B. Climate change and coral reef bleaching: an ecological assessment of long-term impacts,recovery trends and future outlook[J]. Estuarine,Coastal and Shelf Science,2008,80(4): 435-471.
[11] Gattuso J P,Allemand D,Frankignoulle M. Photosynthesis and calcification at cellular,organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry[J]. American Zoologist,1999,39(1): 160-183.
[12] Langdon C,Atkinson M J. Effect of elevatedpCO2on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment[J]. Journal of Geophysical Research: Oceans,2005,110(C9): C09S07.
[13] Albright R,Langdon C. Ocean acidification impacts multiple early life history processes of the Caribbean coralPoritesastreoides[J]. Global Change Biology,2011,17(7): 2478-2487.
[14] Comeau S,Edmunds P J,Spindel N B,et al. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations[J]. Limnology and Oceanography,2014,59(3): 1081-1091.
[15] Chauvin A,Denis V,Cuet P. Is the response of coral calcification to seawater acidification related to nutrient loading?[J]. Coral Reefs,2011,30(4): 911-923.
[16] Dufault A M,Ninokawa A,Bramanti L,et al. The role of light in mediating the effects of ocean acidification on coral calcification[J]. The Journal of Experimental Biology,2013,216: 1570-1577.
[17] Jokiel P L,Rodgers K S,Kuffner I B,et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation[J]. Coral Reefs,2008,27(3): 473-483.
[18] Ries J B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification[J]. Geochimica et Cosmochimica Acta,2011,75(14): 4053-4064.
[19] Huang Hui,Yuan Xiangcheng,Cai Weijun,et al. Positive and negative responses of coral calcification to elevatedpCO2: case studies of two coral species and the implications of their responses[J]. Marine Ecology Progress Series,2014,502: 145-156.
[20] Comeau S,Carpenter R C,Nojiri Y,et al. Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification[J]. Proceedings of the Royal Society of London B: Biological Sciences,2014,281(1790): 20141339.
[21] Comeau S,Edmunds P J,Spindel N B,et al. The responses of eight coral reef calcifiers to increasing partial pressure of CO2do not exhibit a tipping point[J]. Limnology and Oceanography,2013,58(1): 388-398.
[22] Edmunds P J. Zooplanktivory ameliorates the effects of ocean acidification on the reef coralPoritesspp[J]. Limnology and Oceanography,2011,56(6): 2402-2410.
[23] Rodolfo-Metalpa R,Martin S,Ferrier-Pagès C,et al. Response of the temperate coralCladocoracaespitosato mid-and long-term exposure topCO2and temperature levels projected for the year 2100 AD[J]. Biogeosciences,2010,7: 289-300.
[24] Takahashi A,Kurihara H. Ocean acidification does not affect the physiology of the tropical coralAcroporadigitiferaduring a 5-week experiment[J]. Coral Reefs,2013,32(1): 305-314.
[25] Movilla J,Orejas C,Calvo E,et al. Differential response of two Mediterranean cold-water coral species to ocean acidification[J]. Coral Reefs,2014,33(3): 675-686.
[26] McCulloch M,Falter J,Trotter J,et al. Coral resilience to ocean acidification and global warming through pH up-regulation[J]. Nature Climate Change,2012,2(8): 623-627.
[27] Ben-Haim Y,Zicherman-Keren M,Rosenberg E. Temperature-regulated bleaching and lysis of the coralPocilloporadamicornisby the novel pathogenVibriocoralliilyticus[J]. Applied and Environmental Microbiology,2003,69(7): 4236-4242.
[28] Ben-Haim Y,Thompson F L,Thompson C C,et al.Vibriocoralliilyticussp. nov.,a temperature-dependent pathogen of the coralPocilloporadamicornis[J]. International Journal of Systematic and Evolutionary Microbiology,2003,53(1): 309-315.
[29] Bourne D G,Munn C B. Diversity of bacteria associated with the coralPocilloporadamicornisfrom the Great Barrier Reef[J]. Environmental Microbiology,2005,7(8): 1162-1174.
[30] Lesser M P,Weis V M,Patterson M R,et al. Effects of morphology and water motion on carbon delivery and productivity in the reef coral,Pocilloporadamicornis(Linnaeus): diffusion barriers,inorganic carbon limitation,and biochemical plasticity[J]. Journal of Experimental Marine Biology and Ecology,1994,178(2): 153-179.
[31] Richmond R H,Jokiel P L. Lunar periodicity in larva release in the reef coralPocilloporadamicornisat Enewetak and Hawaii[J]. Bulletin of Marine Science,1984,34(2): 280-287.
[32] Clausen C D,Roth A A. Effect of temperature and temperature adaptation on calcification rate in the hermatypic coralPocilloporadamicornis[J]. Marine Biology,1975,33(2): 93-100.
[33] Erez J,Reynaud S,Silverman J,et al. Coral calcification under ocean acidification and global change[M]//Dubinsky Z,Stambler N,eds. Coral Reefs: An Ecosystem in Transition. Berlin: Springer,2011: 151-176.
[34] Wicks L C,Roberts J M. Benthic invertebrates in a high-CO2world[M]//Gibson R N,Atkinson R J A,Gordon J,eds. Oceanography and Marine Biology: An Annual Review,2012,50: 127-188.
[35] Kleypas J A,Yates K K. Coral reefs and ocean acidification[J]. Oceanography,2009,22(4): 108-117.
[36] Ries J B,Cohen A L,McCorkle D C. A nonlinear calcification response to CO2-induced ocean acidification by the coralOculinaarbuscula[J]. Coral Reefs,2010,29(3): 661-674.
[37] Comeau S,Carpenter R C,Edmunds P J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate[J]. Proceedings of the Royal Society B: Biological Sciences,2015,280(1753): 20122374.
[38] Jury C P,Whitehead R F,Szmant A M. Effects of variations in carbonate chemistry on the calcification rates ofMadracisauretenra(=Madracismirabilissensu Wells,1973): bicarbonate concentrations best predict calcification rates[J]. Global Change Biology,2010,16(5): 1632-1644.
[39] Jokiel P L,Bahr K D,Rodgers K S. Low-cost,high-flow mesocosm system for simulating ocean acidification with CO2gas[J]. Limnology and Oceanography: Methods,2014,12(5): 313-322.
[40] Burris J E,Porter J W,Laing W A. Effects of carbon dioxide concentration on coral photosynthesis[J]. Marine Biology,1983,75(2/3): 113-116.
[41] Goiran C,Al-Moghrabi S,Allemand D,et al. Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellate association I. Photosynthetic performances of symbionts and dependence on sea water bicarbonate[J]. Journal of Experimental Marine Biology and Ecology,1996,199(2): 207-225.
[42] Iguchi A,Ozaki S,Nakamura T,et al. Effects of acidified seawater on coral calcification and symbiotic algae on the massive coralPoritesaustraliensis[J]. Marine Environmental Research,2012,73: 32-36.
[43] Hillhouse E W,Grammatopoulos D K. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology[J]. Endocrine Reviews,2006,27(3): 260-286.
[44] Carreiro-Silva M,Cerqueira T,Godinho A,et al. Molecular mechanisms underlying the physiological responses of the cold-water coralDesmophyllumdianthusto ocean acidification[J]. Coral Reefs,2014,33(2): 465-476.
Ocean acidification does not significantly affect the calcification and photosynthesis capacity of hermatypic coral Pocillopora damicornis
Zheng Xinqing1,Kuo Fuwen2,Liu Xinming3,Lin Rongcheng1*,Zhou Zhidong4,Shi Xiaofeng1
(1.TheThirdInstituteofOceanography,StateOceanicAdministration,Xiamen361005,China; 2.NationalMuseumofMarineBiologyandAquarium,Pingtung90001,China; 3.GuanxiAcademyofOceanography,Nanning530022,China; 4.FujianInstituteofOceanography,Xiamen361005,China)
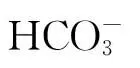
Pocilloporadamicornis; ocean acidification; coral; calcification;Fv/Fm
2015-03-25;
2015-06-15。
南方海洋中心海洋产业核心和关键技术攻关项目(14CZY037HJ11);福建省重点科技项目(2012Y0071);福建省自然科学基金资助项目(2015J05083,2014J01127);海洋公益性行业科研专项(201105012);广西科技兴海专项项目(GXZC2015-G3-3696-JZ);厦门海洋开发研究院项目(K140301)。
郑新庆(1983—),男,福建省泉州市人,博士,助理研究员,从事珊瑚礁生态学研究。E-mail:zhengxinqing@tio.org.cn
*通信作者:林荣澄,男,研究员,从事海洋生物学研究。E-mail:linrongcheng@tio.org.cn
10.3969/j.issn.0253-4193.2015.10.006
Q145.2
A
0253-4193(2015)10-0059-10
郑新庆,郭富雯,刘昕明,等. 海洋酸化没有显著影响成体鹿角杯形珊瑚的钙化作用和光合能力[J].海洋学报,2015,37(10):59—68,
Zheng Xinqing,Kuo Fuwen,Liu Xinming,et al. Ocean acidification does not significantly affect the calcification and photosynthesis capacity of hermatypic coralPocilloporadamicornis[J]. Haiyang Xuebao,2015,37(10):59—68,doi:10.3969/j.issn.0253-4193.2015.10.006
