自组装型Pt/γ-Al2O3催化剂用于低温去除挥发性有机物
2015-01-04李加衡李小青许响生徐潇潇严新焕浙江工业大学绿色化学合成技术国家重点实验室培育基地杭州3004浙江大学能源清洁利用国家重点实验室杭州3004
李加衡 敖 平 李小青 许响生 徐潇潇 高 翔 严新焕,*(浙江工业大学绿色化学合成技术国家重点实验室培育基地,杭州3004;浙江大学能源清洁利用国家重点实验室,杭州3004)
自组装型Pt/γ-Al2O3催化剂用于低温去除挥发性有机物
李加衡1敖 平1李小青1许响生1徐潇潇1高 翔2严新焕1,*
(1浙江工业大学绿色化学合成技术国家重点实验室培育基地,杭州310014;2浙江大学能源清洁利用国家重点实验室,杭州310014)
分别通过自组装法(AS)和浸渍法(WI)制备得到纳米催化剂Pt/γ-Al2O3-AS和Pt/γ-Al2O3-WI,并用于评价甲苯、异丙醇、丙酮、乙酸乙酯等易挥发性有机物(VOCs)的氧化性能.通过各种表征手段探究了催化剂形态、结构及表面性质与催化剂氧化活性的关系.结果表明,Pt/γ-Al2O3-AS在低温下即可实现VOCs的完全氧化.在气体浓度(体积分数)为1000×10-6,空速为18000 mL·g-1·h-1的条件下,甲苯、异丙醇、丙酮、乙酸乙酯被Pt/γ-Al2O3-AS催化剂完全氧化的温度分别为130、135、145、215°C,展现出了优异的氧化性能,且具有很好的稳定性.该催化剂较高的比表面积、较小的Pt纳米粒径、较好的Pt纳米颗粒分散度、更好的低温还原效果及丰富的表面羟基是具有较高催化活性的重要因素.
Pt/γ-Al2O3;易挥发性有机物;催化燃烧;组合型催化剂
©Editorial office ofActa Physico-Chimica Sinica
1 Introduction
In recent years,environmental legislations have imposed increasingly stringent targets for permitted levels of atmospheric emissions.Because of their toxic,malodorous,mutagenic,and carcinogenic nature,volatile organic compounds(VOCs)are considered as atmospheric pollutants.1VOCs are also important precursors for the formation of ozone,particulate matter(PM), and smog.Diverse sources,such as automobile exhausts,petrochemical industries,manufacturing plants,and solid and liquid waste treatment facilities,release VOCs into the atmosphere.In several countries,including USA,EU,Japan,and China,stringent legislationshave been passedtominimize theemission ofVOCs.2,3
The main approaches in use for the removal of VOCs include adsorption,thermal incineration,and catalytic oxidation.Among these methods,catalytic oxidation,i.e.,complete oxidation of VOCs to CO2and H2O,is recognized as one of the most promising processes;4it is an efficient,inexpensive,and a green chemical degradation process with a faster reaction rate and lower operating temperature.Moreover,catalytic oxidations conducted at relatively low temperatures and under controlled conditions do not emit undesirable by-products,such as dioxins and NOx.5,6
The catalysts currently being used for minimizing VOC emissions can be divided into two groups:noble metal-based7-13and metal oxide-based14-18catalysts.The noble metal-based catalysts,because of their high specific activity and recyclability,are frequently used for the oxidation of various VOCs.They are widely used despite their low resistance to poisons and relatively higher costs(when compared with metal oxides).To the best of our knowledge,the catalytic oxidation of VOCs over precious metal catalysts is typically carried out between 160 and 300°C.19-21Besides,catalytic oxidation of VOCs is greatly influenced by the nature of pollutants.Approximately 60%-80%of various VOCs are emitted in the cold-start period.Among the noble metal-based catalysts,Pt-based catalysts have been widely used in oxidations for minimizing the emission of hydrocarbons,CO,and the soluble organic fraction of PM.In these processes,the efficient oxidation of VOCs requires relatively high temperatures.To realize the total oxidation of VOCs at lower temperature,it is important to develop more efficient catalysts.
In general,the catalytic activity of a platinum-based catalyst depends on the method of its preparation and the nature of support.22The method of catalyst preparation can influence properties such as crystallite size,surface area,dispersion,oxidation state, and oxygen defect concentration;these features can influence the activity of catalysts.Alarge body of work related to the complete oxidation of VOCs over supported Pt-based catalysts prepared by various methods,such as wet impregnation(WI),11,23,24precipitation-deposition,25-27and ion exchange,28-30has been recorded. Among these,WI is the most widely used method for catalyst preparation.Here,Pt nanoparticles were synthesized by the reduction of precursors at high temperatures or under H2atmosphere;this can sometimes lead to poor distribution of the metal on the surface and weak interactions between Pt and the support.31Therefore,optimization of the preparation method is critical for the generation of catalysts with higher efficiencies and longer lifetimes.Additionally,properties of the support also play an important role in the efficiency of the catalyst.The inexpensive and stable alumina(Al2O3)is the most commonly used catalyst support in a variety of applications.
In this study,a catalytic Pt/γ-Al2O3nanomaterial(nanocatalyst) was successfully prepared by self-assembly and is effective at minimizing the emission of VOCs at low temperatures.The key factors controlling the activity of the nanocatalyst,i.e.,Pt dispersion,Pt size,and reducibility of Pt sites,are discussed in the context of the preparation method.
2 Experimental
2.1 Material
K2PtCl4(AR)was purchase from Sinopharm Chemical Reagent Co.,Ltd.China.Propylene carbonate(AR)was purchase from Shandong Shenjie Chemical Co.,Ltd.China.γ-Al2O3powder was purchase from Hangzhou Wangjing New Material Co.Ltd.,China, surface area SBET=180 m2·g-1.
2.2 Preparation of nanocatalyst
Pt2(dba)3(dba=dibenzylideneacetone)was prepared following a protocol described in the literature,32with a few modifications. Asolution of K2PtCl4(1.40 g,AR)in distilled water(12 mL)was added to a solution of sodium acetate(2.8 g,AR)and bis-dba (2.36 g)in ethanol(60 mL,AR)at 50°C.The initial pale yellow suspension dissolved when the mixture was refluxed at 90°C. After refluxing for 1 h,a dark violet precipitate formed.The mixture was allowed to settle overnight.The liquid was discarded after filtration,while the solid was washed three times with water (20 mL),dried overnight under vacuum,further washed three times with n-pentane(20 mL,AR),and finally dried under vacuum overnight.
We prepared Pt/γ-Al2O3-AS according to a procedure reported previously by us,33but using a different support and with modifications in the post-treatment:a certain amount of Pt2(dba)3was added into propylene carbonate(AR)solution in a steel autoclave pre-equilibrated with H2(to exclude the inside air),followed by pressurizing to 3 MPa with H2.The solution was vigorously stirred for 2 h at room temperature(RT)to afford a brown colloid of Pt. To deposit the sol on a support,γ-Al2O3powder was added to the above solution,and the stirring continued for 24 h.The solid catalyst was separated by filtration and dried at 80°C.Only trace levels of Pt were observed in the clear filtrate,indicating a complete adsorption of the Pt on the carrier.Thus,the supported catalyst,Pt nanoparticles(1.0%Pt loading)on γ-Al2O3,was prepared by the assembled method(Pt/γ-Al2O3-AS).
For comparison,a reference catalyst was prepared by impregnation in excess solvent as follows:γ-Al2O3(1.0 g)was added to an aqueous solution of K2PtCl4(50 mL,concentration of K2PtCl4was adjusted to give 1%(w,mass fraction)of Pt in the final solid). The suspension was stirred at RT for 2 h and then heated overnight at 70°C in a vacuum oven.Finally,the sample was reducedby H2at 300°C in order to obtain the Pt nanoparticles.
2.3 Characterization
N2adsorption-desorption isotherms were measured at liquid nitrogen temperature using a Micromeritics ASAP 2010 equipment.Specific surface areas were calculated according to the Brunauer-Emmett-Teller(BET)method.Micropore volume was estimated by the t-plot method.Total pore volume was determined by nitrogen adsorption at a relative pressure of 0.98 and pore size distributions from the adsorption isotherms by the Barrett-Joyner-Halen(BJH)method.
The structures of the supports and catalysts were analyzed by X-ray diffraction(XRD)on a Rigaku D/Max-2500/propylene carbonate(PC)powder diffraction system using Cu Kαradiation (40 kV and 100 mA)over the angular range 20°-50°,2θ,with a 0.05°step size and a counting time of 20 s per step.The assignment of the various crystalline phases was based on the JPDS powder diffraction file cards.
Transmission electron microscopy(TEM)images were recorded using a JEOL JEM-1200EX microscope with an accelerating voltage of 60 kV.One drop of the sample solution was placed on the copper grid coated by a polymer or carbon film. Average sizes of Pt particles were calculated from the size distributions obtained by measuring the diameter of over 100 Pt particles in bright-field TEM.
For temperature-programmed reduction of H2(H2-TPR),the catalysts(50 mg)were subjected to flow of 10%(volume fraction, the same below)H2/Ar(total flow rate=30 mL·min-1)at RT for 1 h to stabilize the thermal conductivity detector(TCD)signal. After that,the temperature was increased from 30 to 800°C at a rate of 10°C·min-1.The temperature dependent change in the concentration of H2was recorded on an on-line TCD.
Fourier transform infrared(FTIR)experiments were carried out using a Nicolet 740 FTIR spectrometer equipped with a diffuse reflectance(DRIFT)cell(Spectra Tech),an mercury cadmium telluride(MCT)detector,and a KBr beam splitter.In a typical experiment,the catalyst powder in the DRIFT cell was pretreated with a flow of He at 450°C for 10 min and then cooled down to RT.At different temperatures during the cooling stage,background spectra were collected.The flow was then switched to 1% CO in He at room temperature for 30 min.
2.4 Catalytic activity measurements
Catalytic activity tests were carried out in a conventional fixedbed reactor at atmospheric pressure and with reaction temperatures in the range 60-300°C.The fixed-bed reactor was placed inside a temperature-regulated electrical furnace,and the temperature in the reaction zone was measured by a thermocouple placed in the middle of the catalyst bed.To ensure the measurement of steady-state data,the reactor was maintained at each temperature for 20 min.All the runs were performed using 500 mg of catalyst. The concentration(volume fraction)of VOCs in the incoming feed stream and gas hourly space velocity(GHSV)were maintained at~1000×10-6-3000×10-6and~18000-54000 mL·g-1·h-1, respectively.The analysis of the outgoing stream(raw reactant or product)was performed using a gas chromatograph(Fuli GC-9790)equipped with flame ionization detector(FID)and TCD. The conversion(X)of VOCs was calculated by using the following equation:
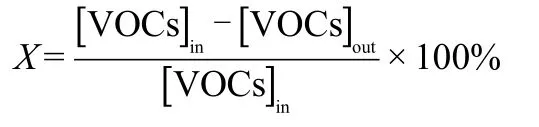
where[VOCs]inis the inlet VOC concentration,and[VOCs]outis the outlet VOC concentration.The catalytic activity was evaluated by its T100value,i.e.,the temperature for 100%conversion of VOCs.
3 Results and discussion
3.1 Catalytic performance of Pt/γ-Al2O3catalysts toward the total oxidation of VOCs
3.1.1 Effect of preparation method
The activity of 1.0%Pt/γ-Al2O3-AS in the total catalytic oxidation of four representative VOCs(toluene,isopropanol,acetone, and ethyl acetate)was evaluated by analyzing the conversiontemperature plots(light-off curves)(Fig.1).For comparison,the activity of the pure support(γ-Al2O3only)was also measured.The support γ-Al2O3is active only at temperatures above 330°C. Moreover,the detected products(carbon dioxide and water)indicate the occurrence of combustion during the reaction with pure γ-Al2O3.The complete oxidation of toluene,isopropanol,acetone, and ethyl acetate over Pt/γ-Al2O3-AS occurs at~130,~135,~145, and~215°C,respectively,while with Pt/γ-Al2O3-WI(catalyst prepared by WI),complete oxidations occur at~170,~160,~165, and~250°C,respectively.Consistent with previous observations, the oxidation activity of the nanocatalyst is highly dependent on the preparation method.Besides,the T100values for the VOCs with the catalyst Pt/γ-Al2O3-AS are much lower than those reported for other catalysts.10,12,13,19,20,34-37
3.1.2 Effect of concentration of VOCs
It is crucial to evaluate the performance of Pt/γ-Al2O3-AS nanocatalyst at different concentrations of VOCs in the incoming feed,because various real sources emit varying amounts of VOCs. An increase in the concentration of a pollutant is accompanied by a decrease in the nanocatalyst activity,a phenomenon typical of any catalytic oxidation process.Nevertheless,complete removal of toluene,isopropanol,acetone,and ethyl acetate at highest evaluated concentration(3000×10-6)was achieved at relatively low temperatures of 140,145,150,and 230°C,respectively. Thus,even at high concentrations of VOCs,Pt/γ-Al2O3-AS has sufficient ability at relatively low temperatures to minimize the release of pollutants.
3.1.3 Effect of GHSV
The catalytic performance of Pt/γ-Al2O3-AS nanocatalyst in the conversions of VOCs(maintained at 1000×10-6)at different temperatures and flow rates were measured.The GHSVs studied were 18000,36000,and 54000 mL·g-1·h-1accordingly.With the increase of GHSV,the temperature of completely conversion was also increased.This is probably because the quantity of VOCs inthe outlet is increased,when elevating GHSV values,the contact time between VOCs and catalyst is reduced.When the GHSV is increased to 36000 mL·g-1·h-1,the T100values for toluene,isopropanol,acetone,and ethyl acetate are 135,140,150,and 220°C, respectively;the corresponding T100values are 140,150,155,and 230°C when the value of GHSV is 54000 mL·g-1·h-1.The data indicate that GHSV negatively affected different kinds of VOCs, and these results are much lower than those reported in literature.7,38Taken together,these studies indicate that the Pt/γ-Al2O3-AS nanocatalyst can be used for the combustion of VOCs in a wide range of GHSVs.

Fig.1 Performance of Pt/γ-Al2O3catalysts with different preparation methods and VOCs
3.1.4 Time-on-stream behavior of the nanocatalyst
In commercial applications,besides the catalytic activity,stability,and durability are two very desirable aspects in a catalyst. In industrial-scale operations,the operation temperature of a reaction can be increased in order to improve the catalytic activity or selectivity;such a change in temperature can affect the stability of a nanocatalyst.Therefore,studies to evaluate the stability of Pt/ γ-Al2O3-AS nanocatalyst in the conversions of toluene and ethyl acetate(because toluene and ethyl acetate have the lowest and highest T100values,respectively)were conducted.Fig.2 shows the results of stability test for the Pt/γ-Al2O3-AS nanocatalyst.There was no decline in the conversions(maintained at 100%)of toluene (at 130°C)and ethyl acetate(at 215°C)by the nanocatalyst after 200 h on-stream.In other words,the nanocatalyst was catalytically durable for the complete removal of various VOCs.
3.2 Structural and textural properties

Fig.2 Time-on-stream behavior of total oxidation of VOCs on Pt/γ-Al2O3-AS nanocatalyst
The nitrogen adsorption-desorption isotherms of support(γ-Al2O3only)and γ-Al2O3-supportedplatinum catalysts are shown in Fig.3,and the textural properties derived from these isotherms are listed in Table 1.The isotherms of both Pt/γ-Al2O3samples(AS and WI)display similar patterns.These can be classified as type IV isotherms.10Interestingly,the conventional catalyst prepared by WI method exhibits a lower surface area and smaller pore volume. This indicates that the γ-Al2O3pores were significantly occupied/ blocked by the Pt species during the impregnation process. However,the self-assembly method of preparation partially prevents such blocking of pores,which can be attributed to the use of PC solution as a dispersant in the preparation process.Most ofthe Pt nanoparticles are directly adsorbed on the surface of the support and do not occupy the pore-channels of γ-Al2O3because of the surface tension of PC.The above results indicate that the textural properties of the supported Pt nanocatalysts are influenced by the preparation method.The invariance of surface area, pore volume,and pore diameter of Pt/γ-Al2O3-AS catalyst particularly enhances the catalytic activity,by improving the dispersion of platinum.Adsorption is one of the important steps for the progress of a heterogeneous catalytic reaction.The high surface area of the supported catalyst can provide more active sites for the adsorption of large amounts of VOCs and oxygen molecules,thus acting as“VOC reservoirs”which feed the Pt particles,where the catalytic oxidation takes place.Therefore,the likely reason for the excellent performance of Pt/γ-Al2O3-AS in the catalytic combustion of VOCs is provided by the unique surface and Pt dispersion in AS nanocatalyst that leads to a higher contacting efficiency,an enhanced mass/heat transfer as well as a shorter diffusion path.37
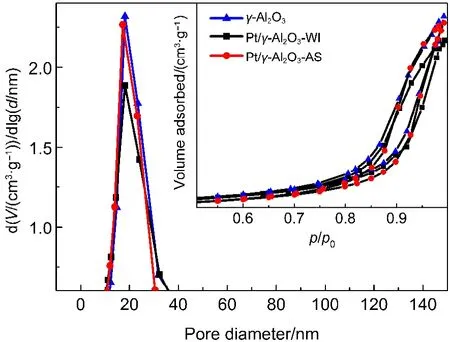
Fig.3 N2adsorption-desorption isotherms of support(γ-Al2O3only)and γ-Al2O3-supportedplatinum catalysts(inset)and distribution curves of catalyst pore sizes
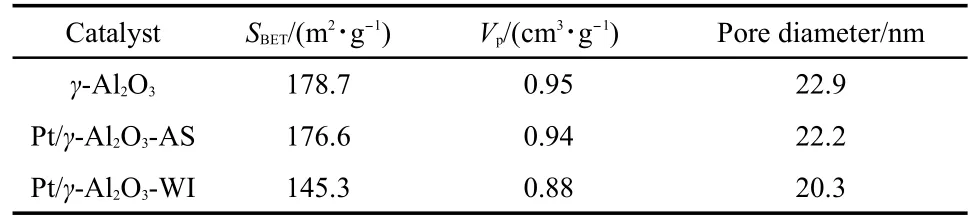
Table 1 Textural properties of γ-Al2O3,Pt/γ-Al2O3-AS,and Pt/γ-Al2O3-WI catalysts
Fig.4 shows the XRD patterns of pure γ-Al2O3and synthesized Pt/γ-Al2O3nanocatalysts.The diffraction peaks of alumina-supported Pt catalysts are similar to that of pure γ-Al2O3(JCPDS 75-0921),indicating that the incorporation of Pt into the structure of γ-Al2O3caused no significant changes in the structure.However, in the present case,the diffraction peaks of Pt at 40.1°(110),46.4° (200),and 67.9°(220)mask those of γ-Al2O3phases at 39.9°(222), 46.5°(400),and 67.0°(440),respectively.39,40Furthermore,no sharp peak for Pt was observed,indicating that a low loading can denote a small size in the obtained nanoparticles.

Fig.4 Wide-angle XRD patterns of γ-Al2O3(a),Pt/γ-Al2O3-AS(b), and Pt/γ-Al2O3-WI(c)
Fig.5 shows the TEM patterns of 1.0%Pt/γ-Al2O3-WI and 1.0% Pt/γ-Al2O3-AS samples as well as the Pt particle size distribution. When compared with the catalyst prepared by WI,the Pt nanoparticles in the AS synthesized nanocatalyst are evenly loaded on the γ-Al2O3,and a large number of these spherical nanoparticles are isolated from each other.Additionally,a more homogeneous distribution of Pt nanoparticles was observed in the AS synthesized catalyst.41Moreover,particle size distributions of Pt nanoparticles of AS(average 2.5 nm)and WI(average 5.0 nm) samples are significantly different.These results can be rationalized as follows:reduction by H2at high temperature promotes the formation of Pt clusters in case of the Pt/γ-Al2O3-WI nanocatalyst.In contrast,the presence of PC in the preparation of Pt/γ-Al2O3-AS nanocatalyst hinders the aggregation of Pt nanoparticles.The oxidation of VOCs by platinum group metal catalysts has sometimes been speculatively classified as structure-sensitive reactions.Finer dispersion and smaller size of the active phase significantly promoted the Pt-based activity towards the complete oxidation of VOCs.7,21,42,43
3.3 Redox and surface properties of the Pt catalystsH2-TPR is a powerful tool to study the reduction behavior of the catalysts.Fig.6 shows the hydrogen uptakes as a function of temperature for 1%Pt/γ-Al2O3-WI,1%Pt/γ-Al2O3-AS,and γ-Al2O3. No reduction of γ-Al2O3was observed between 30 and 800°C.The weak peaks at~98°C observed in both the Pt-loaded catalysts were attributed to the reduction of the surface PtOxspecies to metallic Pt.Notably,the reduction peak of Pt/γ-Al2O3-AS(470°C) is at a lower temperature when compared with that of Pt/γ-Al2O3-WI(550°C).Furthermore,the intensity of the peak in the AS-synthesized catalyst was nearly three-fold higher than that in the WI-synthesized catalyst.On the one hand,the strong interaction between Pt nanoparticles and γ-Al2O3as well as surface oxygen species facilitate the reduction of Pt/γ-Al2O3-AS at low temperature,which is beneficial for the enhancement in the catalytic performance because the oxidation of VOCs has been reported to proceed via a Mars-van Krevelen(redox)mechanism,11,44,45while on the other hand,the oxygenated compounds on the surface of the catalyst synthesized byAS are more easily reduced than those on the surface of the catalyst synthesized by WI.In other words, the active oxygen species formed on the surface of theAS sample are more reactive.It has previously been suggested that this canbe due to the activation of the surfaceAl―OH bond to form Pt―OH bond with hydrogen bonding on the surface of γ-Al2O3caused by the presence of Pt,9which was responsible for the excellent performance of low-temperature VOC total oxidation.

Fig.5 TEM images(a,b)and Pt particle size distributions of(c,d)γ-Al2O3supported Pt samples

Fig.6 H2-TPR profiles of γ-Al2O3(a),1%Pt/γ-Al2O3-WI(b), 1%Pt/γ-Al2O3-AS(c)
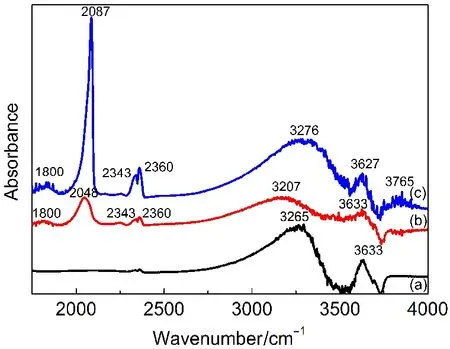
Fig.7 FTIR spectra of γ-Al2O3(a),Pt/γ-Al2O3-WI(b),and Pt/γ-Al2O3-AS catalysts(c)
The surface properties of catalysts synthesized by AS and WI were investigated by FTIR.The corresponding spectra are shown in Fig.7.In the 3800-3200 cm-1region,all the samples show bands corresponding to the O―H stretching of surface hydroxyl groups.46The absorption at higher wavenumber(3765 cm-1)is typically attributed to the vibrations of isolated O―H species, while the peaks at 3633 and 3627 cm-1are attributed to bridging OHs,and those at 3207-3276 cm-1are attributed to hydrogenbonded hydroxyls.47A careful comparison of the spectra of catalysts synthesized by AS and WI methods shows that the absorption peaks are broader and more intense in the AS sample, indicating the presence of more amounts of hydroxyl groups. More Pt―OH bonds are formed on γ-Al2O3because of hydrogen bonding on the catalyst surface,which can be confirmed by the theory of H2-TPR.Moreover,the OH vibrational bands shift to higher wavenumbers,indicating that the hydroxyl groups become more basic compared to the WI sample.In the region 1800-2500 cm-1,both the samples exhibit bands at 2087 and 2048 cm-1, which are attributed to the CO linearly adsorbed on the top and step sites on the zerovalent Pt crystallites,and the band at 1800cm is characteristic of bridge-bonded CO on Pt sites.The bands appeared at 2343 and 2360 cm-1are attributed to the absorption peak of Ptδ+.48,49Notably,the peak at 2087 cm-1of AS was more intensive and appeared at higher wavenumbers than that at 2048 cm-1of the WI sample.These data indicate that more small Pt nanoparticles are prepared by theAS method on the surface of γ-Al2O3with fine dispersion,while most of the Pt nanoparticles prepared by the WI method poorly dispersed on γ-Al2O3with large size.The results are in accordance with the TEM information. Moreover,the intensity of the peak of Ptδ+for theAS sample was much higher than that for the WI sample,probably because of the electron transfer from Pt to γ-Al2O3due to the interaction between Pt and support.49The results could be proved by the H2-TPR information.
4 Conclusions
γ-Al2O3-supported Pt nanocatalysts were successfully prepared by the self-assembly method.The as-prepared Pt/γ-Al2O3-AS catalyst shows superior activity in the complete conversion of each of the four representative VOCs,toluene,isopropanol,acetone,and ethyl acetate,when present in the incoming feed (GHSV of 18000 mL·g-1·h-1)at a concentration of 1000×10-6at temperatures below 130,135,145,and 215°C,respectively.The excellent catalytic activity can be attributed to the novel preparation process,self-assembly,which leads to a higher surface area, smaller size,and finer dispersion of Pt nanoparticles in the nanocatalyst.The self-assembly process of preparation also provides the supported catalyst with better low-temperature reducibility and a higher amount of hydroxyls groups.The excellent catalytic performance of this Pt-based nanocatalyst may find potential applications in the catalytic combustion of VOCs.
(1) Ao,P.;Xu,X.S.;Xu,X.X.;Li,J.H.;Yan,X.H.Acta Phys.-Chim.Sin.2014,30,950.[敖 平,许响生,徐潇潇,李加衡,严新焕.物理化学学报,2014,30,950.]doi:10.3866/ PKU.WHXB201403111
(2) Masui,T.;Imadzu,H.;Matsuyama,N.;Imanaka,N.J.Hazard. Mater.2010,176,1106.
(3) Jones,A.P.Atmos.Environ.1999,33,4535.
(4) Li,W.B.;Gong,H.Acta Phys.-Chim.Sin.2010,26,885. [黎维彬,龚 浩.物理化学学报,2010,26,885.]doi:10.3866/ PKU.WHXB20100436
(5) Hosseini,S.A.;Sadeghi-Sorkhani,M.T.;Kafi-Ahmadi,L.; Alemi,A.;Niaei,A.;Salari,D.Chin.J.Catal.2011,32, 1465.[Hosseini,S.A.,Sadeghi-Sorkhani,M.T.,Kafi-Ahmadi,L.,Alemi,A.,Niaei,A.,Salari,D.催化学报,2011,32,1465.]doi:10.1016/S1872-2067(10)60257-4
(6) Vandenbroucke,A.M.;Morent,R.;De,G.N.;Leys,C.J.Hazard.Mater.2011,195,30.doi:10.1016/j. jhazmat.2011.08.060
(7) Morales-Torres,S.;Maldonado-Hodar,F.J.;Perez-Cadenas,A. F.;Carrasco-Marin,F.J.Hazard.Mater.2010,183,814.doi: 10.1016/j.jhazmat.2010.07.100
(8) Ye,Q.;Huo,F.F.;Yan,L.N.;Wang,J.;Cheng,S.Y.;Kang,T. F.Acta Phys.-Chim.Sin.2011,27,2872.[叶 青,霍飞飞,闫力娜,王 娟,程水源,康天放.物理化学学报,2011,27, 2872.]doi:10.3866/PKU.WHXB20112872
(9) Chen,B.B.;Shi,C.A.;Crocker,M.;Wang,Y.;Zhu,A.M.Appl.Catal.B2013,132,245.
(10) Rahmani,F.;Haghighi,M.;Estifaee,P.Microporous Mesoporous Mat.2014,185,213.doi:10.1016/j. micromeso.2013.11.019
(11) Liu,Y.X.;Dai,H.X.;Deng,J.G.;Xie,S.H.;Yang,H.G.;Tan, W.;Han,W.;Jiang,Y.;Guo,G.S.J.Catal.2014,309,408.doi: 10.1016/j.jcat.2013.10.019
(12) Liu,Z.S.;Chen,J.Y.;Peng,Y.H.J.Hazard.Mater.2013,256, 49.
(13) Wu,X.Q.;Zong,R.L.;Zhu,Y.F.Acta Phys.-Chim.Sin.2012,28,437.[吴小琴,宗瑞隆,朱永法.物理化学学报,2012,28,437.]doi:10.3866/PKU.WHXB201112082
(14) Tsoncheva,T.;Issa,G.;Nieto,J.M.L.;Blasco,T.;Concepcion, P.;Dimitrov,M.;Atanasova,G.;Kovacheva,K.Microporous Mesoporous Mat.2013,180,156.doi:10.1016/j. micromeso.2013.06.017
(15) Jin,L.Y.;Lu,J.Q.;Luo,M.F.;Xie,G.Q.;He,M.Acta Phys.-Chim.Sin.2007,23,1691. [金凌云,鲁继青,罗孟飞,谢冠群,何 迈.物理化学学报,2007,23,1691.]doi:10.1016/ S1872-1508(07)60083-7
(16) Konsolakis,M.;Carabineiro,S.A.;Tavares,P.B.;Figueiredo,J. L.J.Hazard.Mater.2013,261,512.doi:10.1016/j. jhazmat.2013.08.016
(17) Meng,Z.H.;Yang,P.;Zhou,R.X.Acta Phys.-Chim.Sin.2013,29,391.[孟中华,杨 鹏,周仁贤.物理化学学报,2013,29, 391.]doi:10.3866/PKU.WHXB201212072
(18) Shang,J.;Zhu,Y.F.;Xu,Z.L.;Jing,L.Q.;Du,Y.G.Chin.J. Catal.2003,24,369.[尚 静,朱永法,徐自力,井立强,杜尧国.催化学报,2003,24,369.]
(19) Takeguchi,T.;Aoyama,S.;Ueda,J.;Kikuchi,R.;Eguchi,K.Top.Catal.2003,23,159.doi:10.1023/A:1024888724146
(20) Liotta,L.F.Appl.Catal.B2010,100,403.doi:10.1016/j. apcatb.2010.08.023
(21) Papaefthimiou,P.;Ioannides,T.;Verykios,X.E.Appl.Catal.B1997,13,175.doi:10.1016/S0926-3373(96)00103-8
(22) Ivanova,A.S.;Slavinskaya,E.M.;Gulyaev,R.V.;Zaikovskii, V.I.;Stonkus,O.A.;Danilova,I.G.;Plyasova,L.M.; Polukhina,I.A.;Boronin,A.I.Appl.Catal.B2010,97,57.doi: 10.1016/j.apcatb.2010.03.024
(23) Zangeneh,F.T.;Mehrazma,S.;Sahebdelfar,S.Fuel Process. Technol.2013,109,118.doi:10.1016/j.fuproc.2012.09.046
(24) Zou,J.J.;Liu,C.J.;Yu,K.L.;Cheng,D.G.;Zhang,Y.P.;He, F.;Du,H.Y.;Cui,L.Chem.Phys.Lett.2004,400,520.doi: 10.1016/j.cplett.2004.11.003
(25) Chytil,S.;Glomm,W.R.;Blekkan,E.A.Catal.Today2009,147,217.doi:10.1016/j.cattod.2008.09.003
(26) Kong,W.Z.;Tian,B.Z.;Zhang,J.L.;He,D.N.;Anpo,M.Res. Chem.Intermediat.2013,39,1701.doi:10.1007/s11164-012-0903-4
(27) Salim,V.M.M.;Cesar,D.V.;Schmal,M.;Duarte,M.A.I.; Frety,R.Preparation of Catalysts VI1995,91,1017.
(28) Ryoo,R.;Ko,C.H.;Kim,J.M.;Howe,R.Catal.Lett.1996,37, 29.doi:10.1007/BF00813515
(29) Tao,B.;Fletcher,A.J.J.Hazard.Mater.2013,244,240.
(30) Zhu,S.;Wang,S.L.;Jiang,L.H.;Xia,Z.X.;Sun,H.;Sun,G. Q.Int.J.Hydrog.Energy2012,37,14543.doi:10.1016/j. ijhydene.2012.07.043
(31) Basile,F.;Fornasari,G.;Gazzano,M.;Vaccari,A.J.Mater. Chem.2002,12,3296.doi:10.1039/b205146j
(32) Huang,B.S.;Su,E.C.;Wey,M.Y.Chem.Eng.J.2013,223, 854.doi:10.1016/j.cej.2013.03.076
(33) Xu,X.S.;Li,X.Q.;Gu,H.Z.;Huang.Z.B.;Yan,X.H.Appl. Catal.A2012,429,17.
(34) Sedjame,H.J.;Fontaine,C.;Lafaye,G.;Barbier,J.,Jr.Appl. Catal.B2014,144,233.doi:10.1016/j.apcatb.2013.07.022
(35)Takamitsu,Y.;Yoshida,S.;Kobayashi,W.;Ogawa,H.;Sano,T.J.Environ.Sci.Heal.A2013,48,667.doi:10.1080/ 10934529.2013.744563
(36) Diehl,F.;Barbier,J.;Duprez,D.;Guibard,I.;Mabilon,G.App. Catal.B2010,95,3.
(37) Chen,H.;Zhang,H.;Yan,Y.Ind.Eng.Chem.Res.2013,52, 12819.
(38) Chen,C.;Zhu,J.;Chen,F.;Meng,X.;Zheng,X.;Gao,X.; Xiao,F.S.Appl.Catal.B2013,140-141,199.
(39) Matam,S.K.;Kondratenko,E.V.;Aguirre,M.H.;Hug,P.; Rentsch,D.;Winkler,A.;Weidenkaff,A.;Ferri,D.Appl.Catal. B2013,129,214.doi:10.1016/j.apcatb.2012.09.018
(40) Kumar,M.S.;Hammer,N.;Ronning,M.;Holmen,A.;Chen, D.;Walmsley,J.C.;Oye,G.J.Catal.2009,261,116.doi: 10.1016/j.jcat.2008.11.014
(41) Medina-Mendoza,A.K.;Cortes-Jacome,M.A.;Toledo-Antonio,J.A.;Angeles-Chavez,C.;Lopez-Salinas,E.; Cuauhtemoc-Lopez,I.;Barrera,M.C.;Escobar,J.;Navarrete, J.;Hernandez,I.App.Catal.B2011,106,14.
(42) Mistry,H.;Behafarid,F.;Zhou,E.;Ono,L.K.;Zhang,L.; Roldan,C.B.ACS Catal.2014,4,109.doi:10.1021/cs400888n
(43) Garetto,T.F.;Apesteguia,C.R.Appl.Catal.B2001,32,83. doi:10.1016/S0926-3373(01)00128-X
(44) Liu,B.C.;Liu,Y.;Li,C.Y.;Hu,W.T.;Jing,P.;Wang,Q.; Zhang,J.Appl.Catal.B2012,127,47.doi:10.1016/j. apcatb.2012.08.005
(45) Djeddi,A.;Fechete,I.;Garin,F.Appl.Catal.A2012,413,340.
(46) Montanari,T.;Matarrese,R.;Artioli,N.;Busca,G.Appl.Catal. B2011,105,15.doi:10.1016/j.apcatb.2011.03.021
(47) Li,Y.;Wei,Z.H.;Sun,J.M.;Gao,F.;Peden,C.H.F.;Wang,Y.J.Phys.Chem.C2013,117,5722.doi:10.1021/jp310512m
(48) Albertazzi,S.;Busca,G.;Finocchio,E.;Glockler,R.;Vaccari, A.J.Catal.2004,223,372.doi:10.1016/j.jcat.2004.01.024
(49) Panagiotopoulou,P.;Kondarides,D.I.J.Catal.2008,260, 141.doi:10.1016/j.jcat.2008.09.014
Removal of Volatile Organic Compounds at Low Temperature by a Self-Assembled Pt/γ-Al2O3Catalyst
LI Jia-Heng1AO Ping1LI Xiao-Qing1XU Xiang-Sheng1XU Xiao-Xiao1GAO Xiang2YAN Xin-Huan1,*
(1State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology,Zhejiang University of Technology, Hangzhou 310014,P.R.China;2State Key Laboratory of Clean Energy Utilization, Zhejiang University,Hangzhou 310014,P.R.China)
Pt/γ-Al2O3catalyst nanoparticles were prepared by self-assembly(AS)and wetness impregnation (WI)methods,and evaluated for the oxidation of volatile organic compounds including toluene,isopropanol, acetone,and ethyl acetate.The morphology,structure,and surface properties of the catalyst particles were correlated to their oxidation activity.Toluene,isopropanol,acetone,and ethyl acetate(1000×10-6,volume fraction)in the feed stream(gas hourly space velocity of 18000 mL·g-1·h-1)were completely oxidized and removed by Pt/γ-Al2O3-AS at below 130,135,145,and 215°C,respectively.Pt/γ-Al2O3-AS exhibited outstanding activity and stability at high concentrations and space velocities.The high catalytic activity of Pt/γ-Al2O3-AS was attributed to its high surface area,small size,finely dispersed Pt nanoparticles,better reproducible activity at low temperature,and a higher number of hydroxyl groups.
Pt/γ-Al2O3;Volatile organic compound;Catalytic combustion;Assembled catalyst
O643
10.3866/PKU.WHXB201411131www.whxb.pku.edu.cn
Received:July 11,2014;Revised:November 13,2014;Published on Web:November 13,2014.
∗Corresponding author.Email:xinhuanyan139@hotmail.com;Tel:+86-571-88320791.
The project was supported by the Key Innovation Team of Science&Technology in Zhejiang Province,China(2011R50017)and National High Technology Research and Development Program of China(863)(2013AA065005).
浙江省重点创新团队(2011R50017)和国家高技术研究发展计划项目(863)(2013AA065005)资助
