抗米勒管激素用于年轻乳腺癌患者卵巢功能抑制个体化治疗的评价
2015-01-04李华萍殷志强邓克红张保华徐文怡
李华萍,郭 祯,殷志强,邓克红,张保华,徐文怡
1.上海市浦南医院妇产科,上海 200125;
2.江西省南昌市第三医院乳腺科,江西 南昌 330009;
3.上海交通大学附属仁济医院乳腺科,上海 200001;
4.郑州大学第二附属医院妇产科,河南 郑州 450014
抗米勒管激素用于年轻乳腺癌患者卵巢功能抑制个体化治疗的评价
李华萍1,郭 祯2,殷志强3,邓克红4,张保华1,徐文怡1
1.上海市浦南医院妇产科,上海 200125;
2.江西省南昌市第三医院乳腺科,江西 南昌 330009;
3.上海交通大学附属仁济医院乳腺科,上海 200001;
4.郑州大学第二附属医院妇产科,河南 郑州 450014
背景与目的:年轻乳腺癌患者应用促黄体生成素释放激素类似物戈舍瑞林治疗没有个体化用药方案,并缺乏临床可用的指导依据。该研究皆在探讨抗米勒管激素(anti-Müllerian hormone,AMH)在年轻乳腺癌患者卵巢功能抑制个体化治疗的评价作用。方法:选取2012年5月—2014年1月在上海交通大学附属仁济医院因雌激素受体(estrogen receptor,ER)和孕激素受体(progesterone receptor,PR)阳性的乳腺癌41例患者,术前随机分为戈舍瑞林6个疗程+化疗组(简称戈舍瑞林组)20例,化疗组21例,30例同年龄组健康妇女为正常对照组,随访(17.4±6.2)个月。观察两组治疗后的停经时间与复潮时间,于术前1个月、戈舍瑞林组或化疗组术后月经复潮后3、6个月检测AMH、促卵泡激素(follicle-stimulating hormone,FSH)和E2水平。正常对照组在相应时间段内检测AMH、FSH和E2水平。结果:3组患者的术前临床资料及术前FSH、E2水平差异均无统计学意义(P>0.05),乳腺癌患者术前AMH水平较正常对照组降低,差异有统计学意义(P=0.04);戈舍瑞林组较化疗组停经时间更短,差异有统计学意义(P=0.00);戈舍瑞林组较化疗组复潮时间更短,差异有统计学意义(P=0.00);与正常对照组及术前比较,戈舍瑞林组及化疗组FSH、E2水平在5个测定时间的差异均无统计学意义(P>0.05);戈舍瑞林组及化疗组在5个测定时间的AMH水平均显著降低,差异均有统计学意义(P=0.00),两组在复潮后3、6个月AMH水平逐渐上升,差异均有统计学意义(P<0.05);与化疗组相比,戈舍瑞林组的AMH水平下降明显,戈舍瑞林组的AMH水平在复潮6个月后比化疗组升高,差异均有统计学意义(P<0.05)。结论:年轻乳腺患者在卵巢功能抑制治疗+化疗过程中,AMH较其他评价卵巢储备的指标明显下降,在其他指标恢复术前水平后仍提示卵巢受损,提示AMH可以作为评价年轻乳腺癌患者卵巢功能的指标,亦有可能成为戈舍瑞林个体化治疗的评价指标。
抗米勒管激素;戈舍瑞林;年轻乳腺癌患者;卵巢功能抑制
根据美国癌症研究所1975—2006年的流行病学统计,约2%的乳腺癌患者发生在20~34岁,约11%的患者发生在35~44岁[1],研究表明化疗可导致年轻乳腺癌患者卵巢储备下降及卵巢早衰[2-6]。年轻乳腺癌患者推荐年卵巢功能抑制治疗(ovarian function suppression,OFS)已达成国际共识,即应用一种促黄体激素释放激素类似物戈舍瑞林治疗,可有效保护年轻患者的卵巢功能,不影响化疗效果[7],戈舍瑞林治疗建立在个体化治疗上[8]。但目前对年轻乳腺癌患者戈舍瑞林治疗没有个体化用药方案,并缺乏临床可用的指导依据。血清抗米勒管激素(anti-Müllerian hormone,AMH)水平与卵巢卵泡数具有相关性,近年来成为优于年龄、促卵泡激素(follicle-stimulating hormone,FSH)和抑制素B等反映卵巢储备的良好指标[9-11]。本文研究雌激素受体(estrogen receptor,ER)和孕激素受体(progesterone receptor,PR)阳性的年轻乳腺癌患者术后应用戈舍瑞林+化疗或化疗,探讨AMH在年轻乳腺癌戈舍瑞林个体化用药方案的评价作用。
1 资料和方法
1.1 资料
1.1.1 患者分组
选取2012年5月—2014年1月在上海交通大学附属仁济医院因ER和PR阳性的乳腺癌将行手术治疗的58例患者,随机分为戈舍瑞林6个疗程+化疗组(以下简称戈舍瑞林组)、化疗组,随访(17.4±6.2)个月,因失访剔除17例。选取同年龄组健康妇女为正常对照组。患者入选标准:① 年龄30~40岁;② 无内分泌疾病、卵巢囊肿病史,无子宫卵巢手术史;③ 无激素服用史,无内外科合并症;④ 经病理或细胞学诊断为乳腺癌,ER和(或)PR阳性;⑤已行乳腺癌手术切除;⑥ 既往未行化疗,Karnofsky评分≥80,目前病情需要;⑦ 无化疗禁忌证,化疗方案均为TAC(多西他赛75 mg/m2+多柔比星350 mg/m2+环磷酰胺500 mg/m2,第1天,每3周1次,共6次);⑧ 患者知情同意,主观上有强烈的维持正常月经或生育要求;⑨ 化疗结束后继续口服他莫昔芬治疗。正常对照组入选标准:①年龄30~40岁;②无内分泌疾病、卵巢囊肿病史、无子宫卵巢手术史;③无激素服用史,无内外科合并症。具体情况见表1。患者均签署知情同意书,经上海市浦南医院伦理委员会批准。

表1 戈舍瑞林组、化疗组与正常对照组的临床情况Tab. 1 The clinical situation of goserelin group, chemotherapy group and normal control group
1.1.2 实验材料
AMH ELISA试剂盒购自德国贝-克曼二代公司,FSH、E2化学发光试剂盒及酶标仪购自德国西门子公司。所有激素测定均由上海达安公司完成。
1.2 方法
1.2.1 术前观察指标
正常对照组月经第2~5天上午抽肘静脉血,患者术前1个月月经第2~5天上午抽肘静脉血,采用化学发光法检测FSH和E2水平,ELISA法检测AMH水平。
1.2.2 手术和用药方法
戈舍瑞林组20例和化疗组21例,手术均由同一组医师完成。戈舍瑞林组:化疗前应用1次戈舍瑞林3.6 mg,化疗中每28 d应用1次戈舍瑞林3.6 mg,一共应用6次,化疗方案为TAC,化疗结束后停用戈舍瑞林。化疗组:常规化疗,化疗方案同上。
1.2.3 术后观察指标及随访
患者均完成手术,术后戈舍瑞林组在注射戈舍瑞林3、6针后,如月经规律在月经第2~5天上午抽血查AMH、FSH和E2,如停经在戈舍瑞林注射后2天内上午抽血查AMH、FSH和E2;停戈舍瑞林月经复潮后3、6个月随诊1次,在月经第2天上午抽血查AMH、FSH和E2;化疗组术后在化疗3、6次后,如月经规律在月经第2天上午抽血查AMH、FSH和E2,如停经在化疗结束后2天内上午抽血查AMH、FSH和E2;正常对照组在同时期内月经第2~5天上午抽血查AMH、FSH和E2。
1.3 统计学处理
采用SPSS 10.0软件进行统计学分析,计量资料采用χ±s表示,假设检验均为双侧检验。各组比较采用重复测量数据方差分析,各组间比较采用单因素方差分析、秩和检验;计数资料比较采用χ2检验。P<0.05为差异有统计学意义。
2 结 果
2.1 两组治疗后闭经情况及月经恢复时间
戈舍瑞林组使用戈舍瑞林后的闭经时间显著低于化疗组,疗程结束后月经规律率高于化疗组,复潮时间显著低于化疗组。戈舍瑞林组在应用戈舍瑞林治疗(2.20±0.62)个疗程后闭经,化疗组在应用化疗(4.14±0.91)个疗程后闭经,差异有统计学意义(U=12.50,P=0.00)。两组月经复潮率均为100%,戈舍瑞林组在结束治疗后(68.05±13.18)个月复潮,化疗组在结束治疗后(91.43±21.45)个月复潮,差异有统计学意义(U=68.00,P=0.00);戈舍瑞林组月经不规律5例,化疗组月经不规律7例,差异无统计学意义(χ2=0.51,P=0.29,表2)。
2.2 激素水平
戈舍瑞林组及化疗组术前AMH水平均低于正常对照组,戈舍瑞林组在应用戈舍瑞林+化疗6次后AMH水平显著低于化疗组及正常对照组,戈舍瑞林组在停用戈舍瑞林+化疗月经复潮3个月后AMH水平逐渐恢复,6个月后AMH水平显著高于化疗组。
3组术前E2和FSH水平差异均无统计学意义(F=2.29和1.94,P=0.11和0.13);两组在应用戈舍瑞林+化疗或化疗6次、月经复潮6个月后不同的测量时间差异均有统计学意义(P均=0.00),戈舍瑞林组与化疗组在两个测量时间的E2和FSH水平上差异均无统计学意义(P=0.34和0.08)。戈舍瑞林组和化疗组术前AMH水平低于正常对照组,差异有统计学意义(F=4.32,P=0.04)。3组的AMH测量时间和分组差异均有统计学意义(F=52.89和24.76,P均=0.00),两两比较,5个测定时间差异均有统计学意义(P均=0.00);戈舍瑞林组及化疗组在3、6个疗程后AMH水平低于正常对照组及术前,差异均有统计学意义(P均=0.00),两两比较,戈舍瑞林组与化疗组在6个疗程后AMH水平的差异有统计学意义(P=0.03),两组在复潮6个月后AMH水平的差异均有统计学意义(P=0.01,表3)。

表2 戈舍瑞林组和化疗组患者的闭经情况及恢复月经时间Tab. 2 The amenorrhea and menstrual recovery time of goserelin group and chemotherapy group
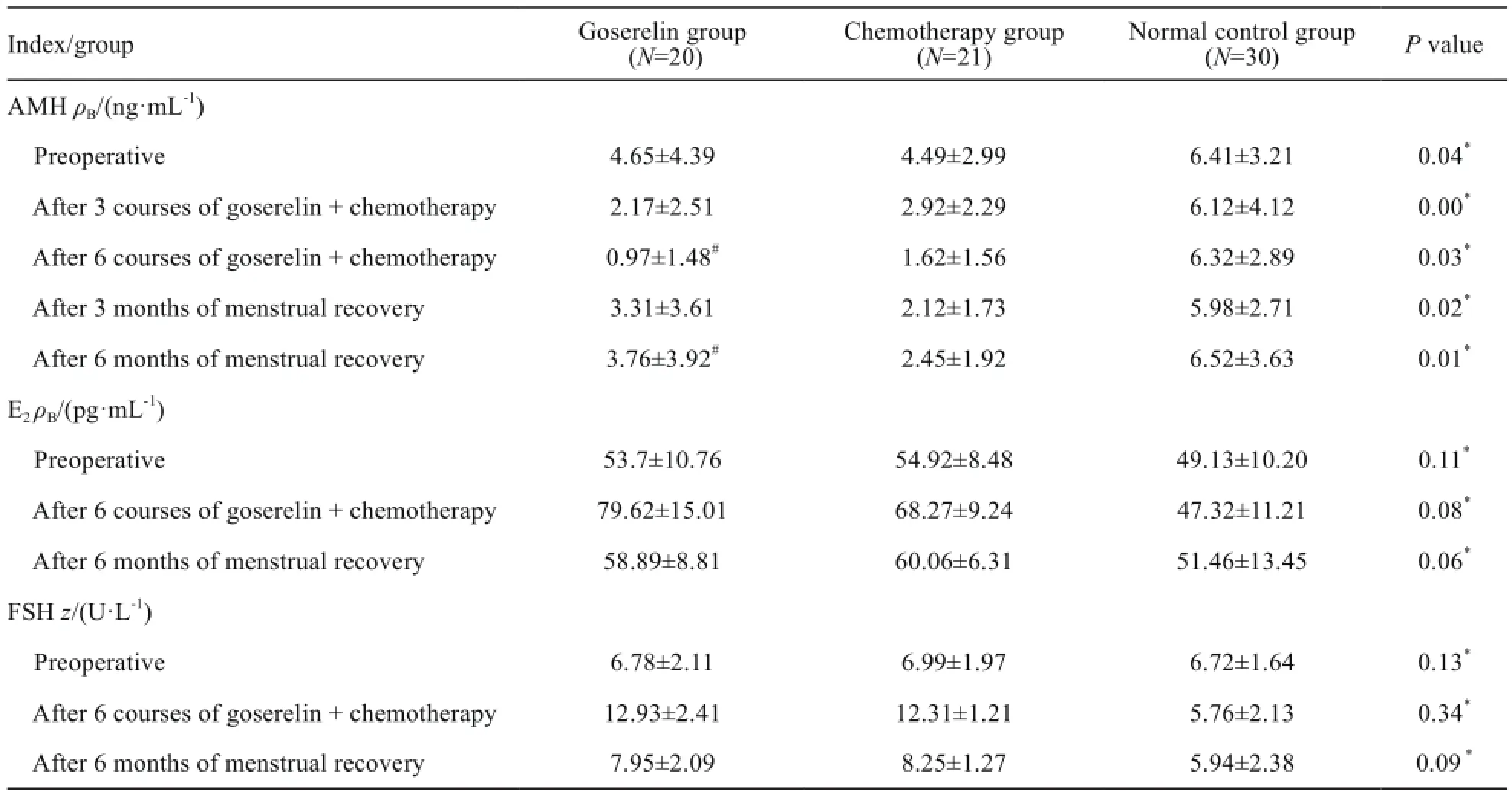
表3 戈舍瑞林组、化疗组与正常对照组患者的激素水平Tab. 3 The hormone levels of goserelin group, chemotherapy group and normal control group(χ±s)
3 讨 论
越来越多的年轻乳腺癌患者在考虑癌症治疗、保留生育能力及生存质量的问题,针对年轻乳腺癌患者戈舍瑞林治疗的个体化在临床工作中显得尤为重要。去年国际乳腺癌联盟建议年轻乳腺癌患者卵巢功能抑制治疗5年[8]。一项回顾性的戈舍瑞林用于绝经前乳腺癌患者治疗的成本效益分析表明,接受戈舍瑞林治疗较长之间的患者生存期较长[(122.5±6.3)个月 vs (112.2±6.7)个月],比化疗获得更好生存率及生存质量,并且有更好的成本效益[12]。关于放化疗后保留生育功能的癌症患者在辅助生殖技术结局中的研究表明,激素受体阳性的乳腺癌患者,接受卵细胞体外成熟后体外受精-胚胎移植获卵数及AMH水平均较低,差异有统计学意义(P=0.000 7)[9]。
生育期女性的AMH主要由次级卵泡、窦前和窦状卵泡颗粒细胞产生[13],卵泡发育到4~6 mm后分泌AMH,到窦前及小窦卵泡期达到高峰,随着卵泡发育其表达水平逐渐降低[14],AMH水平与卵巢卵泡数目具有相关性。AMH在正常周期女性血中波动很小,不受周期影响[15-16]。近年AMH成为优于年龄、FSH和抑制素B等反映卵巢储备的良好指标。有学者对147例生育期的晚期乳腺癌患者临床研究表明,AMH、FSH和抑制素B能够预测卵巢储备功能的下降,AMH能更早、更准确地反映卵巢储备功能的下降,不受外源激素的影响[4],并且成为评价乳腺癌患者卵巢储备的指标[17-18]。Su等[19]研究表明,在均衡了年龄、身体质量指数、妊娠、种族、月经模式和吸烟等因素,年轻乳腺癌患者的平均血清AMH水平和健康对照组差异无统计学意义(0.85 ng/mL vs 0.76 ng/mL,P=0.06)。本研究比较了年轻乳腺癌患者接受戈舍瑞林+化疗或化疗及正常生育期妇女的5个不同时间段AMH、E2和FSH的血清水平,以及戈舍瑞林+化疗或化疗后闭经情况及月经恢复时间。结果表明,两组年轻乳腺癌患者术前AMH水平均低于正常对照组(P均<0.05),与Su等[19]的研究结果不同,可能与样本量较小有关。戈舍瑞林组在应用戈舍瑞林+化疗6次后AMH水平显著低于化疗组及正常对照组(P<0.05),戈舍瑞林组在停用戈舍瑞林+化疗月经复潮3个月后AMH水平逐渐恢复(P<0.05),6个月后AMH水平显著高于化疗组(P<0.05)。两组应用戈舍瑞林+化疗或化疗6个疗程、月经复潮6个月后戈舍瑞林组与化疗组在2个测量时间FSH、E2水平上差异有统计学意义(P<0.05)。戈舍瑞林组使用戈舍瑞林后的闭经时间显著低于化疗组,疗程结束后月经规律率高于化疗组,复潮时间显著低于化疗组。本研究结果表明,戈舍瑞林可以保护年青乳腺腺癌患者的卵巢功能,AMH可以作为评价年轻卵巢癌患者的卵巢功能的指标,亦有可能成为OFS个体化治疗的评价指标。
年轻乳腺癌患者在考虑保留生育能力和提高生活质量方面的目的不同,未生育年轻乳腺癌患者在完成乳腺癌常规治疗后,应用戈舍瑞林和内分泌治疗多久可以妊娠,已生育的年轻乳腺癌患者如何能在生存期内有较高的生存质量,目前在临床工作中缺乏可用的评价指标,而且个体化治疗已迫在眉睫。本研究将继续扩大样本量,加长随访时间,将会得到一个更科学的结论。
[1] PETRU E. Fertility preservation and infertility treatment in breast cancer patients [J]. Wien Med Wochenschr, 2010, 160(19-20): 487-492.
[2] WARNE G L, FAIRLEY K F, HOBBS J B, et al. Cyclophosphamide-induced ovarian failure[J]. N Engl J Med, 1973, 289(22): 1159-1162.
[3] ANDERSON R A, ThEMMEN A P, AIQAHTANI A, et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer[J]. Hum Reprod, 2006, 21(10): 2583-2592.
[4] SU H I, SAMMEL M D, GREEN J, et al. Anti-Müllerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors[J]. Cancer, 2010, 116(3): 592-599.
[5] PARTRIDGE A H, GELBER S, PEPPERCORN J, et al. Fertility and menopausal outcomes in young breast cancer survivors[J]. Clin Breast Cancer, 2008, 8(1): 65-69.
[6] SCHOVER L R. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility[J]. J Clin Oncol, 2008, 26(5): 753-758.
[7] DELMASTRO L, CATZEDDU T, BONI L, et al. Prevention of chemotherapy-induced meno-pause by temporary ovarian suppression with goserelin in young,early breast cancer patients[J]. Ann Oncol, 2006, 17(1): 74-78.
[8] MOORE H C, UNGER J M, PHILLIPS K A, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy[J]. N EngI J Med, 2015, 372(10): 923-932.
[9] HWU Y M, WU F S, LI S H, et al. The impact of endometriomas and laparoscopic cystectomy on serum anti-Müllerian hormone levels[J]. Reprod Biol Endocrinol, 2011, 9(9): 80.
[10] BUSACCA M, RIPARINI J, SOMIGLIANA E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas[J]. Am J Obstet Gynecol, 2006, 195(2): 421-425.
[11] SUN W, STEGMANN B J, HENNE M, et al. A new approach to ovarian reserve testing[J]. Fertil Steril, 2008, 90(6): 2196-2202.
[12] CHENG T F, WANG J D, UEN W C. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer[J]. BMC Cancer, 2012, 12: 33
[13] ERCAN C M, SAKINCI M, DURU N K, et al. Anti-Müllerian hormone levels after laparoscopic endometrioma stripping surgery[J]. Gynecol Endocrinol, 2010, 26(6): 468-472.
[14] DUMESIC D A, LESNICK T G, STASSART J P, et al. Intrafollicular anti-Müllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenicovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer[J]. Fertil Steril, 2009, 92(2): 217-221.
[15] VANDISSELDORP J, FADDY M J, THEMMEN A P, et al. Relationship of serum anti-Müllerian hormone concentration to age at menopause[J]. J Clin Endocrinol Metab, 2008, 93(6): 2129-2134.
[16] SEIFER D B, MACLAUGHLIN D T. Müllerian inhibiting substance is an ovarian growth factor of emerging clinical significance[J]. Fertil Steril, 2007, 88(3): 539-546
[17] GERBER B, VONMINCKWITZ G, STEHLE H,et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study[J]. J Clin Oncol, 2011, 29(17): 2334-2341.
[18] LUTCHMANSINGH K, MUTTUKRISHNA S, et al. Predictors of ovarian reserve in young women with breast cancer[ J]. Br J Cancer, 2007, 96(12): 1808-1816.
[19] SU H I,FlATT S W, NATARAJAN L, et al. Impact of breast cancer on anti-Müllerian hormone levels in young women[J]. 2013, 137(2): 571-577.
Anti-Müllerian hormone as a new marker of the individualized ovarian function suppression treatment in the young breast cancer patients
LI Huaping1, GUO Zhen2, YIN Zhiqiang3, DENG Kehong4, ZHANG Baohua1, XU Wenyi1(1.Department of Obstetrics and Gynecology, the Punan Hospital of Shanghai, Shanghai 200315, China; 2.Department of Breast, the Nanchang Third Hospital of Jiangxi Province, Nanchang 330009, Jiangxi Province, China; 3.Department of Breast, the Renji Hospital Affiliated to Shanghai Jiao Tong University, Shanghai 200001, China; 4.Department of Gynecology and Obstetrics, the Second Affiliated Hospital of Zhengzhou University, Zhengzhou 450014, Henan Province, China)
YIN Zhiqiang E-mail: zdlhp@126.com
Background and purpose:The young breast cancer patients were treated with goserelin without individualized regimen, and lack of available clinical marker. The aim of this study was to investigate the role of anti-Müllerian hormone (AMH) in evaluation of individualized treatment of ovarian function suppression in the young breastcancer patients.Methods:Forty-one young patients with estrogen receptor (ER) and progesterone receptor (PR) positive breast cancer from May 2012 to Jan. 2014 were randomly divided into 2 groups to undergo radical resection of breast cancer. According to postoperative treatment, one group was treated with goserelin + chemotherapy (n=20), and the other group received chemotherapy alone (n=21). Thirty female patients in the same age group were selected as normal control group. The time of menopause and menstrual recovery after the goserelin + chemotherapy or chemotherapy alone were observed in 2 groups. In early follicular phase (day 3-5) of the cycle preceding the operation and 3, 6 courses after the goserelin + chemotherapy treatment or chemotherapy treatment, serum levels of AMH, FSH and E2were measured in 2 groups. Accordingly, serum levels of AMH, FSH and E2were evaluated as well in normal control group.Results:There were no significant differences in preoperative general conditions and preoperative serum FSH and E2levels among the 3 groups (P>0.05). Compared with normal control group, the preoperative serum AMH levels of young breast cancer patients were decreased significantly (P=0.04). The menopause time and menstrual recovery time in 2 chemotherapy groups were significantly shorter than that in normal control group (P=0.00). Compared with normal control group and preoperative measurement, the differences in serum FSH and E2levels were not statistically significant in goserelin + chemotherapy group or chemotherapy alone group (P<0.05). The serum AMH levels measured at different time points of the goserelin + chemotherapy group and chemotherapy alone group were decreased significantly (P<0.05). Compared with the chemotherapy group, the serum AMH levels of the goserelin + chemotherapy group after 6 courses were significantly decreased, and then significantly increased 6 months after menstrual recovery (P<0.05).Conclusion:This study demonstrated that the serum AMH levels were obviously decreased after the ovarian function suppression treatment and increased after the menstrual recovery compared with evaluation of other ovarian reserve index. The serum AMH level could suggest ovarian reserve damage even after ovarian function has recovered to the noticeable level. Thus, AMH could be used clinically to evaluate the ovarian reserve of breast cancer patients as a potential marker for the individualized ovarian function suppression treatment in young breast cancer patients.
Anti-Müllerian hormone; Goserelin; Young breast cancer patients; Ovarian function suppression
10.3969/j.issn.1007-3969.2015.12.011
R737.9
A
1007-3639(2015)12-0983-06
2015-05-11
2015-06-29)
上海市浦东新区青年医学人才项目(PWRq2012-31);上海市卫计委课题(201440468)。
殷志强 E-mail:zdlhp@126.com
