不同还原温度制备RGO/MnO2复合材料对电容增效的影响
2015-01-01王令云章海霞王晓敏
王令云,王 勇,章海霞,王晓敏
(太原理工大学 材料科学与工程学院,山西 太原 030024)
1 Introduction
MnO2is a promising candidate to replace the noble-metal oxides as supercapacitor electrode material,owing to its high theoretical pseudocapacitance,low cost and environmental friendliness[1,2].MnO2has been synthesized via various methods,such as reduction,hydrothermal,and co-precipitation methods[3-5].The values of specific capacitance reported for manganese oxide are mostly between 100 and 250 F·g-1,which is far from the theoretical value of 1 000 F·g-1,which is attributed to its intrinsic poor conductivity,easy aggregation,various crystal structures and morphology of MnO2[3,6].To improve its performance,carbon materials have been applied to composite with MnO2to achieve excellent conductivity and large specific surface area[7-9].
Graphene is a two-dimensional new carbon material with the advantages of the large specific surface area and outstanding electrical conducting properties,which make it a good carrier material for MnO2.Positive effects have been already attained as a support of MnO2[10-12].As an intermediate product of graphene prepared by the oxidation-reduction method,graphene oxide (GO)has a large specific surface area as well as a large number of oxygen-containing functional groups,providing both double layer capacitance and pseudocapacitance[13].However,for the poor electrical conductivity of GO,composite of MnO2with GO can’t improve significantly the capacitance of MnO2[14,15].
In this paper,we synthesized composite materials of MnO2with reduced graphene oxide(RGO)as supercapacitor electrode materials.The relationships between specific capacitance and thermal reduction temperature of GO,microstructure,electrical conductivity of the composite materials were analyzed,suggesting a balanced use of RGO’s electrical conductivity and pseudocapacitance in supercapacitor electrode materials.
2 Experimental
2.1 Preparation of RGO/MnO2composite materials
GO was synthesized by the modified Hummers method,and then was dissolved into deionized water to prepare GO by ultrasonic treatment[16,17].Then GO powder was heated at different temperatures from 200 to 800 ℃to obtain RGO in Ar atmosphere.Then the RGO was sonicated in 25% alcoholic solution,to which KMnO4was added under stirring.KMnO4was reduced to MnO2by alcohol and then MnO2grew on the RGO sheets.RGO/MnO2composites were obtained after washing and drying.GO samples after thermal reduction were marked by X-RGO and the composites by X-RGO/MnO2,where X standed for the heating temperature of GO.The mass fraction of RGO in the composites was all controlled to be 20%.
2.2 Characterization
Scanning electron microscopy (SEM)was employed to characterize the morphology.X-ray diffraction (XRD,Y-2000X)was used to characterize the size and the structure of all samples.Fourier Translation Infrared Spectroscopy (FT-IR,FTS165)was performed to measure the characteristic functional groups.Four probe method is used to measure the electrical conductivity of the RGO.Electrochemical workstation (CHI660D)was used to evaluate the electrochemical performance of samples,the cyclic voltammetry (CV)for the samples was tested in a standard three-electrode test system.The counter electrode was a piece of Pt foil,the reference electrode was Hg/Hg2SO4electrode and the working electrode was assembled from the RGO/MnO2composites as follows.Electrode material was made of the composites (77%,~16-20 mg),acetylene black (13%)and polytetrafluoroethylene (PTFE,10%).The mixture was painted on Ni foil current collectors and pressed under 25 MPa to make the electrode.The applied potential range for CV measurement was-0.8~0.0 V(vs.Hg/Hg2SO4),the test was conducted in 6 mol·L-1KOH electrolyte.Calculation method of specific capacitance was based on the formula:
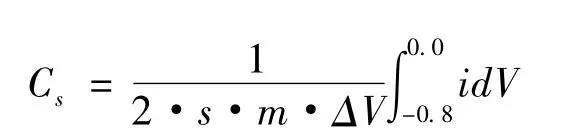
Where Csis the specific capacitance of the composites,s stands for the scan rate in the CV test,m is the weight of the composite,ΔV stands for scanning voltage range,i and V represent for electric current and voltage,respectively.
3 Results and discussion
3.1 Morphology and structures
The obtained materials were analyzed by SEM.Fig.1a shows a low magnification SEM image of the as-prepared MnO2sample without the addition of RGO,in which the particles were aggregated into bulks with a particle size greater than 5 μm.The high magnification SEM image of MnO2shown in Fig.1b revealed a typical sheet-like structure of δ-MnO2,which was a potential material for the study in supercapacitors.Smaller RGO/MnO2particles are shown in Fig.1c,while MnO2was dispersed by GO and the agglomeration was not obvious.In the high magnification SEM image of RGO/MnO2shown in Fig.1d,loose and small pieces of particles can be seen clearly,which were different from the bulks in Fig.1b,indicating that the aggregation was alliviate and dispersion was improved by RGO addition[18-20].Fig.1e is the low magnification SEM image of 600-RGO/MnO2.Compared with Fig.1c,there was no obvious change in the particle size,but in the high magnification SEM image of the sample in Fig.1f,looser and smaller fragments were presented,indicating that thermal reduction broke RGO into much smaller pieces and finally made the MnO2particles more dispersedly grown on RGO and thus increased the specific surface area of the composites.

Fig.1 SEM images of (a,b)pure MnO2,(c,d)GO/MnO2and (e,f)600-RGO/MnO2.
Fig.2 shows the XRD patterns of the GO/MnO2,200-RGO/MnO2,400-RGO/MnO2,600-RGO/MnO2and 800-RGO/MnO2.In each pattern,characteristic diffraction peaks of MnO2were all located at the 2θ values of~12°,37°,67°,corresponding to δ-MnO2(JCPDS file 80-1098).The crystal type of the MnO2was not changed by the addition of RGO,in accordance with the analysis of SEM images.The δ-MnO2was also considered to have a relatively high capacitance as well[21].The structure destruction of RGO was caused by the thermal reduction.So the characteristic peak of the (002)plane was broadened,which agreed well with the result in SEM image,which suggested that the edges of RGO after heat treatment appeared to be loose and blurry[22].Besides,with the increase of the heating temperature,the 2θ value of the (001)plane peak of GO gradually increased,indicating that oxygen-containing functional groups located between the layers of GO were reduced during heat treatment,and the interlayer spacing was decreased[23,24].However,the (001)plane peak of RGO sample after thermal reduction at 800 ℃ didn’t drift to the location of characteristic peak of the (002)planes (2θ=26°)completely,which indicated that some of the oxygen-containing functional groups were still left in the RGO.The patterns of sample d and e with higher heating temperatures obviously showed that the peaks of the (001)and (002)planes coincided and broadened,which indicated the structure of GO was destructed to some extent during the thermal reduction.The destruction was presented as the loosen edges of RGO,leading to an increase of its specific surface area and making it easier to disperse MnO2.
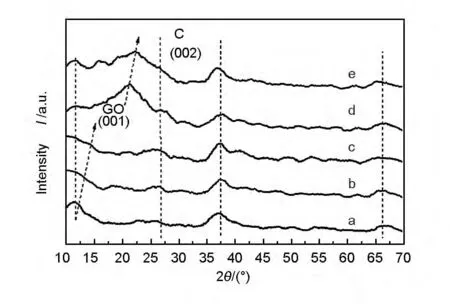
Fig.2 XRD patterns of the samples:(a)GO/MnO2,(b)200-RGO/MnO2,(c)400-RGO/MnO2,(d)600-RGO/MnO2and (e)800-RGO/MnO2.
The FT-IR spectra of GO,200-RGO,400-RGO,600-RGO and 800-RGO are shown in Fig.3.The characteristic peaks at 3480,1750 and 1408cm-1could be attributed to the stretching vibration of—H—O—H from interlayer water molecules,C=O from aromatic aldehydes,and C—O from carboxy group,respectively[25].The intensity of the peak at 3480 cm-1weakened with the increasing temperature,which indicated that heat treatment decreased the interlayer water content[26].The intensity of the peaks at 1750cm-1and 1408cm-1weakened when the heating temperature was increased,demonstrating that the thermal reducing ability was enhanced with the rising temperature.All of the weakened peaks indicated that the reduction of oxygen-containing functional groups would lead to the decrease of the interlayer spacing,which was proved by the XRD spectra.The existence of oxygen-containing functional groups also caused a high electrical resistance of GO,so their removal would improve the electrical conductivity of RGO[27,28].
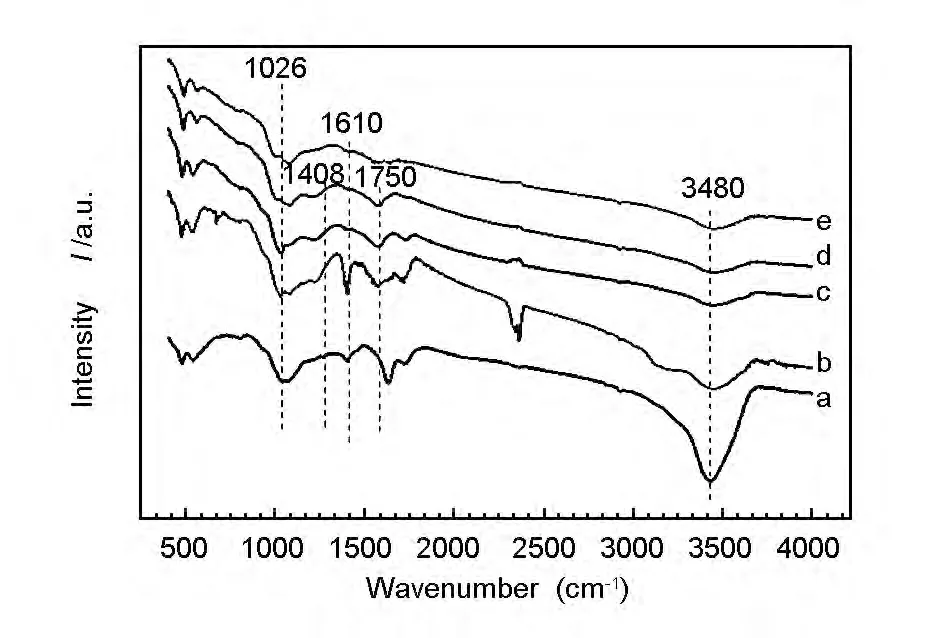
Fig.3 FT-IR spectra of (a)GO,(b)200-RGO,(c)400-RGO,(d)600-RGO and (e)800-RGO.
The electrical resistances of the prepared RGO are listed in Table 1.These data confirmed our prediction and was in accordance with the analysis of the FT-IR spectra.

Table 1 Electrical conductivity of different RGO samples.
3.2 Electrochemical characterization
Fig.4 shows the cyclic voltammograms of different RGO samples.Fig.5 shows the cyclic voltammograms of different RGO/MnO2composite samples.Table 2 and Table 3 list the specific capacitance of all samples obtained from Fig.4 and Fig.5,respectively.Fig.6 indicates the capacitance retention rate of the 600-RGO/MnO2composite.
The redox reaction would take place and electric energy would be stored due to the existence of oxygen-containing functional groups of GO and RGO.The reaction would be reflected in the curves by the redox peaks[29],just like the obvious peaks in cyclic voltammetry curve of GO and 200-RGO.For other samples,the peaks changed to be weaker as the thermal reduction temperature got higher,this was because that the functional groups were partly removed and the reaction was thus weakened accordingly.On the other hand,the impurities in the materials and electrolyte might also have redox reactions and bring out some peaks.
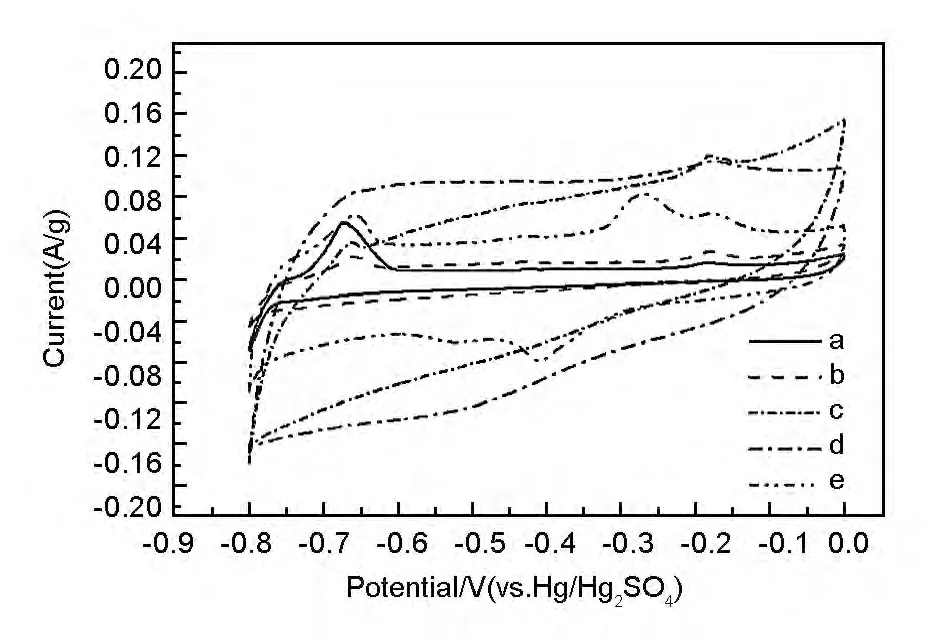
Fig.4 Cyclic voltammetry curves of (a)GO,(b)200-RGO,(c)400-RGO,(d)600-RGO and (e)800-RGO.

Fig.5 Cyclic voltammetry curves of (a)GO/MnO2,(b)200-RGO/MnO2,(c)400-RGO/MnO2,(d)600-RGO/MnO2and (e)800-RGO/MnO2.
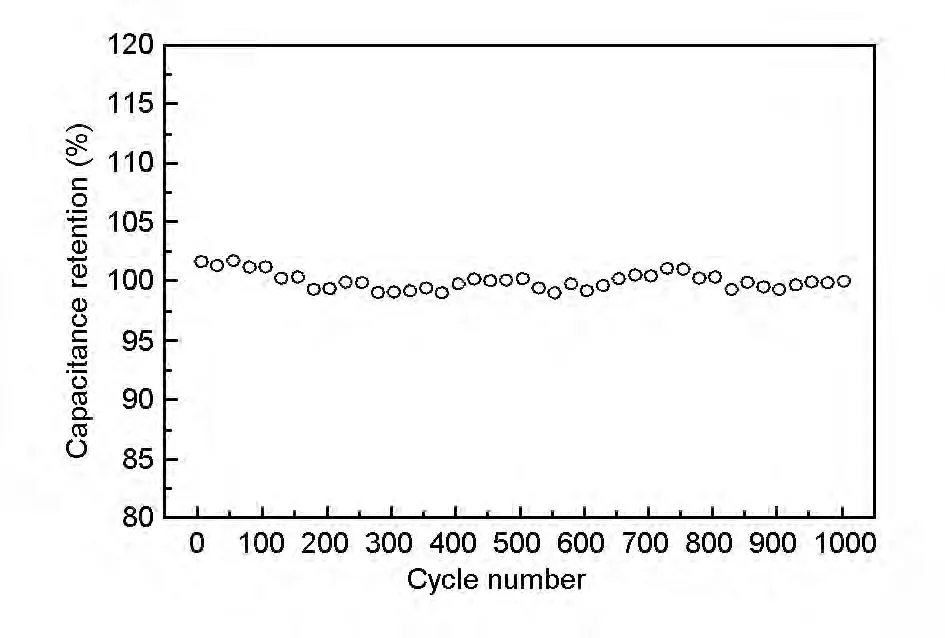
Fig.6 Relationship between capacitance retention and cycle number for the 600-RGO/MnO2composite.
The results showed that the specific capacitance of the composites increased with increasing heating temperatures of GO,and reached a maximum of 321 F·g-1at 600 ℃for 600-RGO/MnO2,which was increased by 87% compared with that of pure MnO2(171 F·g-1)and by 50% compared with GO/MnO2(214 F·g-1).This was a compromising result contributed by two countering factor of electrical conductivity and pseudocapacitance.In one hand,as the electrical conductivity of the RGO improved with the increasing heating temperature,the specific capacitance of the composite also went up.On the other hand,the remaining oxygen-containing functional groups of RGO could provide considerable pseudocapacitance at the same time,which decreased with increasing heating temperature[30].The trend of specific capacitance of RGO samples (Table 2)was consistent with the composite samples (Table 3),which in turn supported the analysis above.On the other hand,the capacitance retention of 600-RGO/MnO2didn’t change too much even cycled for 1 000 times,indicating that the sample had a good stability (Fig.6).

Table 2 Specific capacitances of different RGO samples.

Table 3 Specific capacitances of different RGO/MnO2samples.
4 Conclusions
The RGO/MnO2composites were prepared after the thermal reduction of GO in the composites at different temperatures.RGO has a large specific surface area,disperses MnO2well and enhances the efficiency of MnO2.With increasing the temperature,the amounts of oxygen-containing functional groups decreased,the electrical conductivity of RGO was increased,which favored an increase of the capacitance.However,the pseudocapacitance provided by the remaining oxygen-containing functional groups decreased with the temperature,leading to a specific capacitance decrease.When GO was reduced at 600 ℃,the 600-RGO/MnO2composite achieved the highest specific capacitance of 321 F·g-1,which is 87% higher than that of the pure MnO2.Also,the 600-RGO/MnO2composite had a good cycling stability.
[1]Yu Gui-hua,Hu Liang-bing,Liu Nian,et al.Enhancing the supercapacitor performance of graphene MnO2nanostructured electrodes by conductive wrapping[J].Nano Lett,2011,11(10):4438-4442.
[2]Wei Wei-feng,Cui Xin-wei,Chen Wei-xing,et al.Manganese oxide-based materials as electrochemical supercapacitor electrodes[J].Chem Soc Rev,2011,40(3):1697-1721.
[3]S Devaraj,N Munichandraiah.Effect of crystallographic structure of MnO2on its electrochemical capacitance properties[J].Journal of Physical Chemistry C,2008,112(11):4406-4417.
[4]Yang Yu-juan,Huang Cheng-de.Effect of synthetical conditions,morphology,and crystallographic structure of MnO2on its electrochemical behavior[J].J Solid State Electr,2010,14(7):1293-1301.
[5]V Subramanian,Zhu Hong-wei,Bingqing Wei.Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudocapacitance properties in neutral electrolyte[J].CPL,2008,453(4):242-249.
[6]Daniel Bélanger,L Brousse,Jeffrey W Long.Manganese oxides:battery materials make the leap to electrochemical capacitors[J].The Electrochemical Society Interface,2008,17(1):49-52.
[7]Wang Yu-qin,Yuan An-bao,Wang Xiu-ling.Pseudocapacitive behaviors of nanostructured manganese dioxidecarbon nanotubes composite electrodes in mild aqueous electrolytes:effects of electrolytes and current collectors[J].J Solid State Electr,2008,12(9):1101-1107.
[8]Wang Jian-gan,Yang Ying,Huang Zheng-hong,et al.Incorporation of nanostructured manganese dioxide into carbon nanofibers and its electrochemical performance[J].Mat L,2012,72(4):18-21.
[9]Fan Zhuang-jun,Yan Jun,Wei Tong,et al.Asymmetric supercapacitors based on graphene MnO2and activated carbon nanofiber electrodes with high power and energy density[J].Adv Funct Mater,2011,21(12):2366-2375.
[10]Deng Ling-juan,Zhu Guang,Wang Jian-fang,et al.Graphene-MnO2and graphene asymmetrical electrochemical capacitor with a high energy density in aqueous electrolyte[J].J Power Sources,2011,196(24):10782-10787.
[11]A.K.Geim,Graphene:status and prospects[J].Science,2009,324(5934):1530-4.
[12]JIN Yu,CHEN Hong-hai,CHEN Ming-hai,et al.Carbon nanotube polyaniline graphene composite paper and its electrochemical capacitance behaviors[J].Acta Phys.-Chim.Sin,2009,28,(03):609-614.(靳 瑜,陈宏源,陈名海.碳纳米管聚苯胺石墨烯复合纳米碳纸及其电化学电容行为[J].物理化学学报,2012,28(03):609-614.)
[13]Xu Bin,Yue Shu-fang,Sui Zhu-yin,et al.What is the choice for supercapacitors:graphene or graphene oxide[J].Energy &Environmental Science,2011,4(8):2826-2830.
[14]Chen Sheng,Zhu Jun-wu,Wu Xiao-dong,et al.Graphene oxide MnO2nanocomposites for supercapacitors[J].Acs Nano,2010,4(5):2822-2830.
[15]Dmitriy A Dikin,Sasha Stankovich,Eric J Zimney,et al.Preparation and characterization of graphene oxide paper[J].Nature,2007,448(7152):457-460.
[16]WANG Yong-zhen,WANG Yan,HAN Fei,et al.The effect of heat treatment on the electrical conductivity of highly conducting graphene films[J].New Carbon Materials,2012,27(4):266-270.(王永祯,王 艳,韩 非.还原热处理对石墨烯薄膜导电性的影响[J].新型炭材料,2012,27(4):266-270.)
[17]Goki Eda,Giovanni Fanchini,Manish Chhowalla.Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material[J].Nature nanotechnology,2008,3(5):270-274.
[18]Myeongjin Kim,Yongseon Hwang,Kyungchan Min,et al.Introduction of MnO2nanoneedles to activated carbon to fabricate high-performance electrodes as electrochemical supercapacitors[J].Electrochim Acta,2013,113(0):322-331.
[19]Ha Fei,Wang Xiao-min,Lian Jie,et al.The effect of Sn content on the electrocatalytic properties of Pt-Sn nanoparticles dispersed on graphene nanosheets for the methanol oxidation reaction[J].Carbon,2012,50(15):5498-5504.
[20]Jiang Rong-rong,Huang Tao,Tang Yang,et al.Factors influencing MnO2multi-walled carbon nanotubes composite's electrochemical performance as supercapacitor electrode[J].Electrochim Acta,2009,54(27):7173-7179.
[21]Ragupathy P,Park D H,Campet G,et al.Remarkable capacity retention of nanostructured manganese oxide upon cycling as an electrode material for supercapacitor[J].The Journal of Physical Chemistry C,2009,113(15):6303-6309.
[22]LIU Yan-zhen,LI Yong-feng,YANG Yong-gang,et al.The effect of thermal treatment at low temperatures on graphene oxide films[J].New Carbon Materials,2011,26(1):41-45.(刘燕珍,李永锋,杨永岗.低温热处理对氧化石墨烯薄膜的影响[J].新型炭材料,2011,26(1):41-45.)
[23]Wang Da-wei,Li Feng,Wu Zhong-shuai,et al.Electrochemical interfacial capacitance in multilayer graphene sheets:Dependence on number of stacking layers[J].Electrochem Commun,2009,11(9):1729-1732.
[24]Zhao Bing,Liu Peng,Jiang Yong,et al.Supercapacitor performances of thermally reduced graphene oxide[J].J Power Sources,2012,198(0):423-427.
[25]Geng Jian-xin,Liu Lei-jing,Yang Seung-bo,et al.A simple approach for preparing transparent conductive graphene films using the controlled chemical reduction of exfoliated graphene oxide in an aqueous suspension[J].Journal of Physical Chemistry C,2010,114(34):14433-14440.
[26]James G Radich,Prashant V Kamat.Making graphene holey.Gold nanoparticle mediated hydroxyl radical attack on reduced graphene oxide[J].ACS Nano,2013,7(6):5546-5557.
[27]Sung Mook Choi,Min Ho Seo,Hyung Ju Kim,et al.Synthesis of surface-functionalized graphene nanosheets with high Ptloadings and their applications to methanol electrooxidation[J].Carbon,2011,49(3):904-909.
[28]ZHANG Li-li,ZHAO Xin,Meryl D.Stoller,et al.Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors[J].Nano Lett,2012,12(4):1806-1812.
[29]Frackowiak E,Beguin F.Carbon materials for the electrochemical storage of energy in capacitors[J].Carbon,2001,39(6):937-950.
[30]Chen Cheng-meng,Zhang Qiang,Yang Mang-guo,et al.Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors[J].Carbon,2012,50(10):3572-3584.
