Synthesis, Structure and Electrochemical Property of a New 3D Ag(I) Coordination Polymer Constructed by Biphenyl-2,2΄,4,4΄-tetracarboxylate①
2014-12-15WANGJiJiangTANGLongHOUXiangYangGAOLouJunFUFengZHANGMeiLi
WANG Ji-Jiang TANG Long HOU Xiang-Yang GAO Lou-Jun FU Feng ZHANG Mei-Li
Synthesis, Structure and Electrochemical Property of a New 3D Ag(I) Coordination Polymer Constructed by Biphenyl-2,2΄,4,4΄-tetracarboxylate①
WANG Ji-Jiang②TANG Long HOU Xiang-Yang GAO Lou-Jun FU Feng ZHANG Mei-Li
(716000)
biphenyl-2, 2΄,4,4΄-tetracarboxylic acid, Ag(I), electrochemical property
1 INTRODUCTION
The construction of new coordination polymers (CPs) based on metal ions and multidentate organic ligands is of great interest in recent years, not only due to their structural diversity and intriguing topo- logies but also to their significant potential applica- tions as functionalized materials[1-6]. In the con- struction of new CPs, organic aromatic polycar- boxylate ligands have been proven to be excellent structural constructors because their different coordination modes lead to diverse multidimen- sional architecturesand interesting properties[7-11]. In our strategy, H4btc was chosen as the organic ligand based on the following considering: i) its four carboxylic groups can help to construct novel and various coordination polymers; ii)their two phenyl rings can freely rotate around the C–C single bond according to the small change in the coor- dination environment in order to minimize the steric hindrance.
To extend our previous research work[12, 13]and fully understand the coordination chemistry of H4btc ligand, we selected H4btc as an organic ligand and Ag (I) as the metal center in the presence of bpp ligand to construct new CPs. Herein, we report the syntheses, crystal structures, and characterizations of a new Ag (I) CP, namely, [Ag8(btc)2(bpp)2](1). Furthermore, thermal sta- bility and electrochemical property of 1 are also investigated in detail.
2 EXPERIMENTAL
2. 1 Materials and methods
All reagents and solvents employed were commercially available and used without further purification. The C, H and N microanalyses were carried out with a Vario EL elemental analyzer. The IR spectrum was recorded with a Shimadzu Prestige-21 spectrometer using the KBr pellet technique. Thermogravimetric analysis was per- formed on a NETZSCH STA 449F3 analyzer.A LK98BII Electrochemical Workstation was used to control the electrochemical measurements and for data collection.
2. 2 Synthesis of complex 1
A mixture of AgAc(0.2 mmol), H4btc (0.05 mmol), bpp (0.05 mmol), NaOH (0.2 mmol), and 10 mL H2O was stirred for 30 min. The mixture was then placed in a 25 mL Teflon-lined stainless steel vessel and kept at 140 ℃ for 3 d. Colorless block crystals were obtained when the mixture was cooled to room temperature.Yield: ca. 46% based on Ag.Calcd. for C58H40Ag8N4O16(%): C, 36.44; H, 2.11; N, 2.93. Found (%): C, 36.46; H, 2.14; N, 2.90. IR (KBr pellet, cm-1): 3446 s, 1577 m, 1464 m,1370 s, 1251 m, 1011 w, 751 w, 678 w, 512 w.
2. 3 Crystal structure determination
Diffraction intensities for complex 1 were collected at 296(2) K on a Bruker Smart APEX II CCD diffractometer equipped with a graphite- monochromated Mo-radiation (= 0.71073Å) using an-scanmode. A semi-empirical ab- sorption correction was applied using the SAD- ABS program[14]. The structure was solved by direct methods and refined by full-matrix least- squares on2using the SHELXS-97 and SHE- LXL-97 programs, respectively[15, 16]. Non- hy- drogen atoms were refined anisotropically and hydrogen atoms were placed in the geometrically calculated positions. A total of 13444 reflections of complex 1 were collected in the range of 2.31<25.25 (–16≤≤16, –16≤≤15, –19≤≤16) and 9490 were independent withint= 0.0257, of which 6794 with> 2() (refine- ment on2) were observed and used in the succeeding structure calculation. The final= 0.0481,= 0.0955 (= 1/[2(F2) +(0.0327)2+ 11.7369], where= (F2+ 2F2)/3), (Δ)max= 2.775 and (Δ)min= –2.139 e/Å3. The selected bond lengths and bond angles are listed in Table 1.
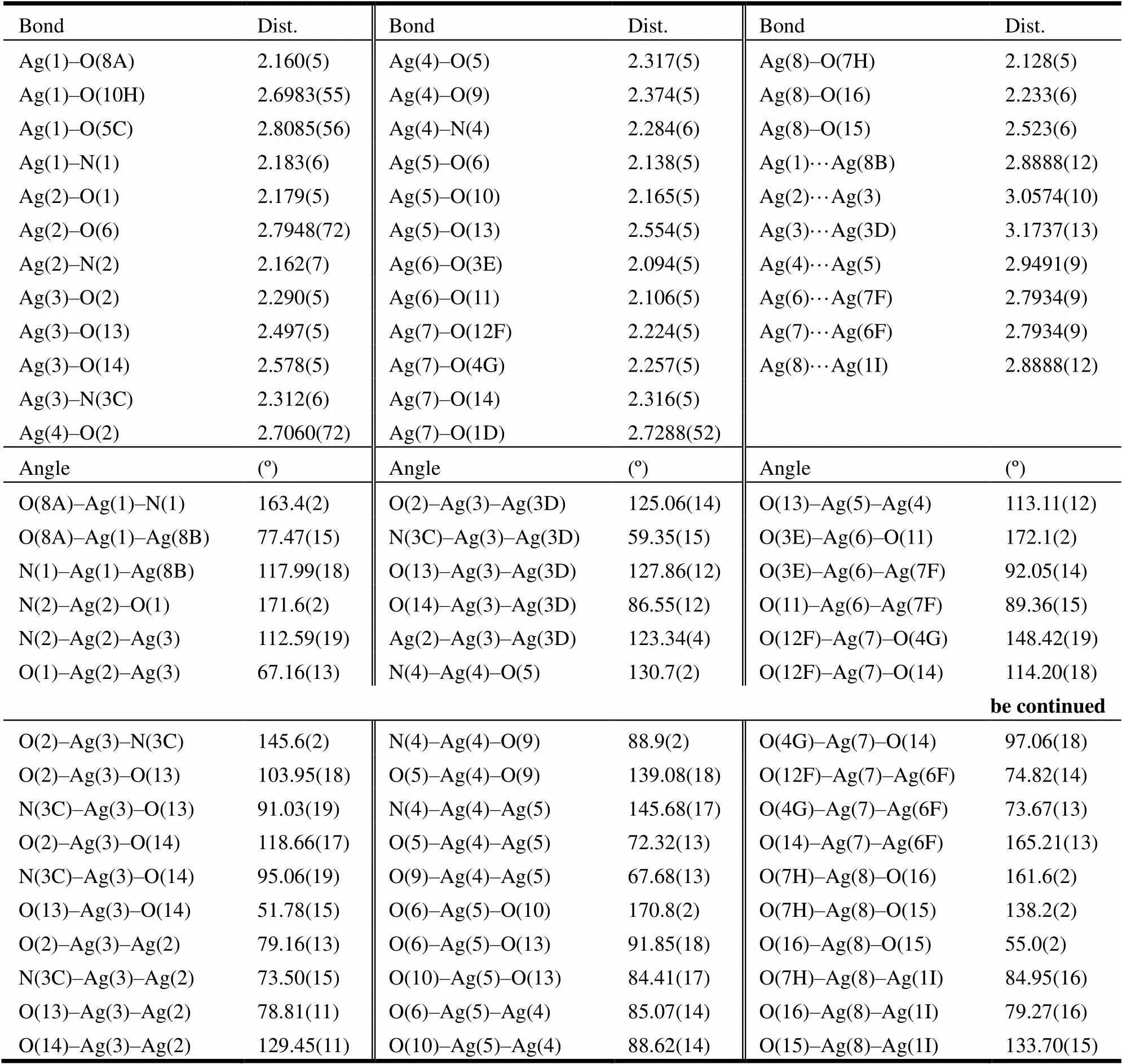
Table 1. Selected Bond Lengths (Å) and Bond Angles (º) for Complex 1
Symmetry operations: A: –, ––1, –; B:–1,,; C:–1,–1,; D: –+2, –, –+1;
E: –+3, –+1, –+1; F: –+3, –, –+1; G:,–1,; H: –+1, ––1, –; I:+1,,
3 RESULTS AND DISCUSSION
3. 1 Crystal structure
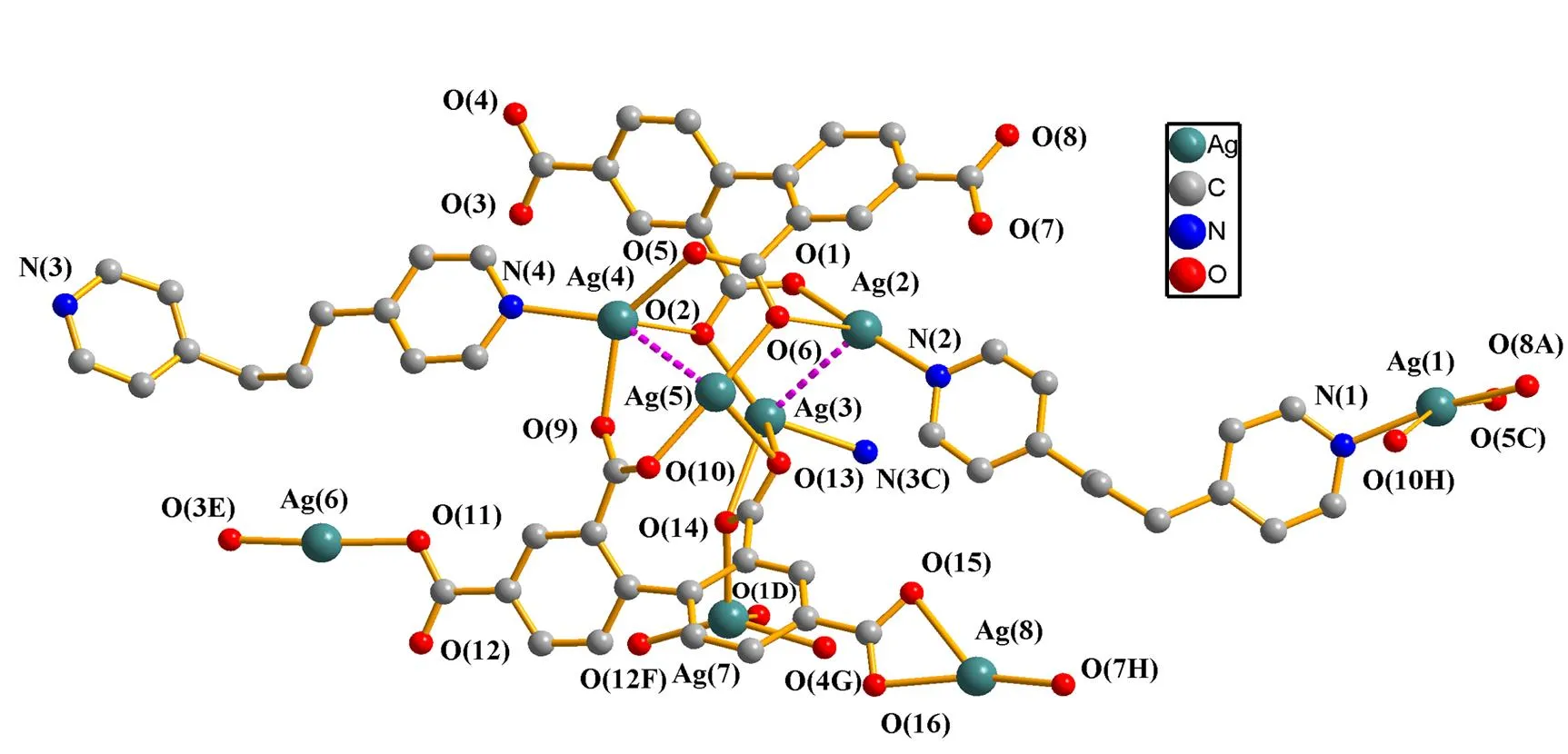
Fig. 1. Coordination environment of Ag(I)in complex 1
In complex 1, the (btc)4-anions display two different coordination modes. In the first one, four carboxylate groups show bis(bidentate) and bis(bridging bidentate) (Scheme 1a), whereas they adopt chelating-bidentate, bidentate, brid- ging bidentate and chelating/bridging bidentate modes in the second one (Scheme 1b). On these connection modes, the Ag(I) ions are linked by (btc)4-ligands to form a 1D chain containing [Ag16(COO)16] as a secondary building block. Notably, the strong Ag···Ag interactions (Ag···Ag = 2.7934(9)~3.1737(13) Å, Table 1) are obser- ved in this secondary building block(Fig. 2a). The Ag···Ag distances are significantly shorter than the van der Waals contact distance for Ag···Ag 3.40 Å[20], which provide supporting evidence for the significance of argentophilicity. The chains are further interconnected with each other to form a 3D metal-organic framework in theplane by (btc)4-ligands between adjacent chains. Furthermore, the 3D framework is further consolidated by bpp ligands (Fig. 2b).

Scheme 1. Coordination modes of (btc)4-incomplex 1
Fig. 2. (a) View of the 1D chain containing [Ag16(COO)16] as a secondary building block in 1 (The dotted lines indicate Ag···Ag interactions). (b) 3D framework by linking the 1D chains with (btc)4-and bpp (All hydrogen atoms have been omitted for clarity)
3. 2 Thermal analysis
The stability of complex 1 was investigated by thermogravimetric analysis (Fig. 3). The rapid weight loss is observed from 175 to 650 ℃, which is attributed to the removal of bpp and (btc)4-ligands (The DSC curve shows two exo- thermic peaks at about 265 and 466 ℃, respec- tively), with a weight loss of 54.67% (calcd. 54.85%). Above 650 ℃, no weight loss is obser- ved, indicating the complete decomposition of 1.
3. 3 Electrochemical property
In the CV measurement, a tri-electrode system was used with glass/C as the working electrode, Pt as the auxiliary electrode and SCE as the reference electrode. The solvent is the mixture of methanol and water with complex condensation of 2.0 × 10-5mol·L-1. KCl was used as the supporting electrolyte. As depicted in Fig. 4, one reduction peak (–0.822 V) corresponds to the Ag(I) → Ag(0) single-electron reduction, and the other oxidation peak (0.260 V) corresponds to the Ag(0) → Ag(I) single-electron oxidation.Obviously, the transfer in the electrode reaction is irreversible.
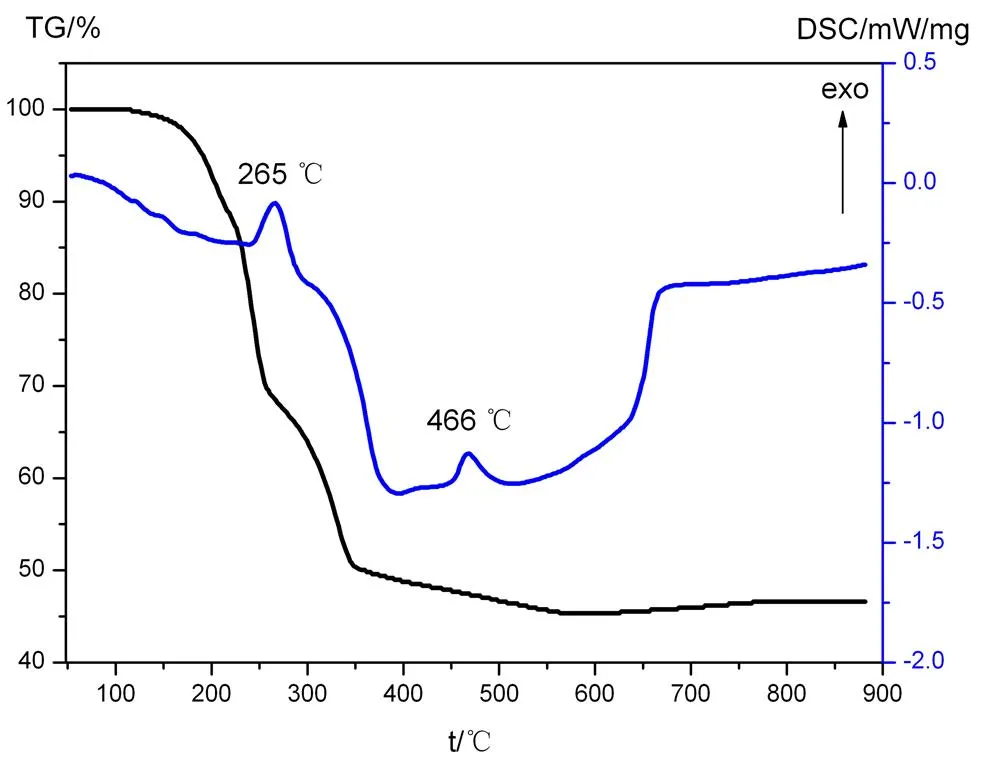
Fig. 3. TG-DSC curves of complex 1
Fig. 4. Cyclic voltammograms of complex 1
4 CONCLUSION
In summary, we have prepared a3D frame- work formed by Ag(I)-carboxylate chains containing the [Ag16(COO)16] secondary building block. The thermal stability and electrochemical property of 1 were also discussed. This result will not only provide a new coordination polymer but also promote the further development of Ag(I)- containing CPs electric functional materials.
(1) Yang, Y.; Du, P.; Liu, Y. Y.; Ma, J. F.A series of coordination polymers constructed by flexible 4-substituted bis(1,2,4-triazole) ligands and polycarboxylate anions: syntheses, structures, and photoluminescent properties.. 2013, 13, 4781–4795.
(2) Makal, T. A.; Wang, X.; Zhou, H. C. Tuning the moisture and thermal stability of metal-organic frameworks through incorporation of pendant hydrophobic groups.. 2013, 13, 4760–4768.
(3) Chen, S. M.; Chen, Y. F.; Lin, R.; Lei, X. P.; Zhang, J. Organic templates promoted photocatalytic and photoluminescent properties between two coordination polymers.. 2013,15, 10423–10426.
(4) Wang, Y. F.; Li, Z.; Sun, Y. C.; Zhao, J. S.; Wang, L. Y. Structural modulation and properties of four cadmium(II) coordination architectures based on 3-(pyridin-4-yl)-5-(pyrazin-2-yl)-1H-1,2,4-triazole and aromatic polycarboxylate ligands.. 2013,15, 9980–9987.
(5) Liu, C. B.; Li, Q.; Wang, X.; Che, G. B.; Zhang, X. J. A series of lanthanide(III) coordination polymers derived via in situ hydrothermal decarboxylation of quinoline-2, 3-dicarboxylic acid.2014, 39, 56–60.
(6) Wang, Y. Q.; Liu, Z. L.; Gao, E. Q. Structure and magnetic properties of a manganese complex with 1-carboxymethylpyridinium-3-carboxylate.2013, 32, 1761–1766.
(7) Hao, H. J.; Sun, D.; Li, Y. H.; Liu, F. J.; Huang, R. B.; Zheng, L. S. Effect of different carboxylates on a series of Ag(I) coordination compounds with benzoguanamine ligand.. 2011, 11, 3564–3578.
(8) Cheng, L.; Gou, S. H.; Wang, J. Q. Three new 3D coordination polymers constructed by biphenyl-2,2΄,4,4΄-tetracarboxylic acid: effect of metal ions and the second ligands.. 2011,991, 149–157.
(9) Lin, X.; Jia, J. H.; Zhao, X. B.; Thomas, K. M.; Blake, A. J.; Walker, G. S.; Champness, N. R.; Hubberstey, P.; Schröder, M. High H2adsorption by coordination-framework materials.2006, 45, 7358–7364.
(10) Li, D. S.; Ke, X. J.; Zhao, J.; Du, M.; Zou, K.; He, Q. F.; Li, C. Unusual 3D ZnIIcoordination networks with mixed tetrahedral and square-planar building units: from 2-fold interpenetrating bbf architecture to self-penetrating 86topological frameworks.
. 2011,13, 3355–3359.
(11) Li, C.; Li, D. S.; Zhao, J.; Mou, Y. Q.; Zou, K.; Xiao, S. Z.; Du, M. Structural diversity and fluorescent properties of Zn(II)/Cd(II) coordination polymers with a versatile tecton 2-(carboxymethoxy)benzoic acid and N-donor co-ligands.. 2011,6601–6609.
(12) Wang, J. J.; Gao, L. J.; Cao, P. X.; Wu, Y. P.; Fu, F.; Zhang, M. L.; Ren, Y. X.; Hou, X. Y.Synthesis, characterization, and crystal structures of two Ag(I) coordination polymers based on biphenyl-2,2΄,4,4΄-tetracarboxylate.2012,65, 3614–3622.
(13) Wang, J. J.; Wang, T. T.; Tang, L.; Hou, X. Y.; Gao, L. J.; Fu, F.; Zhang, M. L.Syntheses, crystal structures, and photoluminescent properties of two new Cd(II) coordination polymers based on biphenyl-2,2΄,4,4΄-tetracarboxylate and dipyridyl-containing ligands.
2013,66, 3979–3988.
(14) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(15) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(16) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(17) Cheng, X.; Liu, T.; Duan, X. Y.; Wang, F. G.; Meng, Q. J.; Lu, C. S. A novel 2D coordination polymer with unprecedented [Ag]7chains and two 3D silver-organic frameworks constructed by methylenediisophthalic acid (H4MDIP) with strong Ag–Ag interactions.
. 2011,13, 1314–1321.
(18) Hao, H. J.; Sun, D.; Li, Y. H.; Liu, F. J.; Huang, R. B.; Zheng, L. S. Effect of different carboxylates on a series of Ag(I) coordination compounds with benzoguanamine ligand.2011, 11, 3564–3578.
(19) Yang, G. P.; Wang, Y. Y.; Liu, P.; Fu, A. Y.; Zhang, Y. N.; Jin, J. C.; Shi, Q. Z.Formation of three new silver(I) coordination polymers involving 1,2-phenylenediacetic acid via the modulation of dipyridyl-containing ligands.2010, 10, 443–1450.
(20) Jansen, M. Homoatomic10-10interactions: their effects on structure and chemical and physical Properties.1987, 26, 1098–1110.
20 January 2014;
20 March 2014 (CCDC 982193)
① This project was supported by the National Natural Science Foundation of China (No. 21373178) and the Natural Scientific Research Foundation of Shaanxi Provincial Education Office of China (No. 13JS124)
. Male, born in 1973, associate professor, majoring in functional coordination chemistry. E-mail: yadxwjj@126.com
杂志排行
结构化学的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid
