海洋链霉菌L211次生代谢产物分离和结构鉴定
2014-12-02郭书举李宪璀史大永朱校斌
郭书举 , 苏 华, 李宪璀 史大永 朱校斌
(1. 中国科学院 海洋研究所, 山东 青岛 266071; 2. 中国科学院 海洋研究所 海洋科学与技术研究与发展中心, 江苏 南通 226006; 3. 中国科学院 青岛生物能源与过程研究所, 山东 青岛 266101)
海洋放线菌是一类重要的海洋微生物, 可以产生多种多样的代谢产物, 如含氮杂环类、多肽类、萜类、糖苷类及大环内酯类化合物[1-2], 链霉菌作为放线菌的重要组成, 也是新结构化合物的重要来源。本研究以海洋链霉菌Streptomyces sp. L211为研究对象,对其发酵液的乙酸乙酯相提取物进行了分离, 先期分离了15个化合物, 并通过1H-NMR、13C-NMR等方法对其中的 10个进行了结构鉴定, 分别为:Kalamycin (1), Medermycin (2), Menoxymycin-B (3),spatozoate (4), 邻氨基苯甲酸(5), 3-吲哚乙醇(6), 1-乙酰基咔啉(7), 对羟基苯基乙醇(8), 吲哚-3-乙酸(9),吲哚-3-甲酸(10)。
1 材料与方法
1.1 仪器与材料
BRUKER AVANCE-500型核磁共振仪; 三氯甲烷、二氯甲烷、四氯化碳、石油醚、乙酸乙酯、冰乙酸、三氟乙酸酐、溴素、香草醛、N-溴代丁二酰亚胺、乙醇钠、乙醇均为分析纯; 柱色谱硅胶(160~200 目)和薄层色谱硅胶 GF254(60型)为青岛海洋化工厂产品。
1.2 实验方法
1.2.1 菌株培养
取适量菌种接种到固体培养基(麦芽提取物10 g/L,酵母提取物4 g/L, 葡萄糖4 g/L, 琼脂粉15 g/L, 海水, pH 7.8)的平板上, 28℃培养48 h后, 作为种子接种到液体培养基(麦芽提取物10 g/L, 酵母提取物4 g/L,葡萄糖4 g/L, 海水, pH 7.8)中, 共接种20 L, 28℃,110 r/min于摇床培养4 d。
1.2.2 提取分离
发酵物离心将菌体和发酵液分离, 发酵液用同体积的乙酸乙酯萃取3次后, 减压蒸干; 菌体在丙酮中超声破碎提取3次后, 减压蒸干; 将两部分粗提物TLC对比, 发现成分相似, 合并后得到7.50g浸膏。浸膏经硅胶柱色谱分离(石油醚: 乙酸乙酯, 氯仿:甲醇梯度洗脱), 得到8个组分, 各组再经过凝胶柱、硅胶柱及制备薄层色谱分离后, 得到 15个化合物,利用核磁共振波谱和质谱, 鉴定了其中的10个化合物, 见图1。
2 波谱数据与结果鉴定
化合物(1), 黄色固体, C16H12O6。1H-NMR(CDCl3,300MHz) δ: 11.8 (s, 9-OH), 7.30 (dd, 7.5, 2.1 Hz, H-8),7.73-7.65 (overlapping, H-6, 7), 5.27 (d, 3.0 Hz, H-4),5.09 (q, 6.9 Hz, H-1), 4.70 (dd, 5.1, 3.0 Hz, H-5), 2.97(dd, 17.7, 5.2 Hz, H-13β), 2.71 (d, 17.7, H-13α), 1.57(d, 6.9 Hz, 1-CH3)。 以上数据与Kalamycin波谱数据一致[3]。
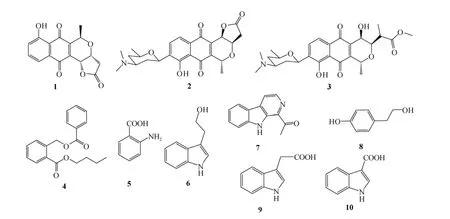
图1 化合物1-10的结构Fig.1 Structure of the Compounds 1-10
化合物(2), 黄色固体, C24H27NO8。1H-NMR(CDCl3, 300MHz) δ: 7.91(1H, d, J=7.8), 7.71(1H, d,J=7.8), 5.25(1H, d, J=2.9), 5.08(1H, q, J=7.0),4.87(1H, dd, J=10.9, 2.0), 4.69(1H, dd, J=5.1, 2.9),3.53(1H, dp, J=8.9, 6.2), 3.2(1H, dd, J=9.5, 8.9),2.97(1H, dd, J=17.6, 5.1), 2.78(1H, ddd, J=12.5, 9.5,3.8), 2.69(1H, d, J=17.6), 2.34(6H, s), 2.26(1H, ddd,J=12.4, 3.8, 2.0), 1.57(3H, d, J=7.0), 1.43(3H, d,J=6.2), 1.30(1H, ddd, J=12.5, 12.4, 10.9);13C-NMR(CDCl3, 75 MHz) δ: 187.8, 180.8, 173.5, 157.6, 149.1,138.5, 134.8, 133.5, 129.6, 119.5, 117.1, 77.5, 72.1,71.3, 68.6, 67.1, 66.4, 66.2, 40.2, 36.9, 28.1, 18.9,18.8。FAB-MS m/z: 458 [M+H]+。以上数据与Medermycin波谱数据一致[4]。
化合物(3), 黄色固体, C25H31NO9。1H-NMR(CDCl3, 300MHz) δ: 7.87(1H, d, J=7.7, ), 7.67(1H, d,J=7.7), 5.02(1H, q, J=6.9), 4.86(1H, dd, J=10.9, 2.07),4.66(1H, d, J=2.5), 4.35(1H, dt, J=6.5, 2.5), 3.75(3H,s), 3.52(1H, dp, J=9.0, 6.0), 3.18(2H, dd, J=9.0, 9.0),2.82(2H, d, J=6.5), 2.73(1H, ddd, J=12.4, 9.0, 3.5),2.32(6H, s), 2.25(1H, ddd, J=12.4, 3.5, 2.0), 1.56(3H, d,J=6.9), 1.42(3H, d, J=6.0), 1.28(1H, ddd, J=12.4, 12.4,10.7).13C-NMR (CDCl3, 75 MHz) δ: 189.3, 182.7,171.8, 157.4, 146.5, 141.2, 138.1, 133.1, 130.1, 119.2,114.1, 77.3, 72.0, 71.3, 67.7, 67.1, 66.7, 59.2, 51.8,40.0, 35.5, 28.3, 17.6, 17.5。FAB-MS m/z: 490[M+H]+。以上数据与Menoxymycin-B波谱数据一致[4]。
化合物(4), 无色油状, C19H20O4。1H-NMR(CD3OD,300MHz) δ: 7.21 (1H, m, H-2), 7.63~7.70 (3H, m, H-3,H-4, H-5), 4.23 (2H, m, H2-8), 1.43~1.63 (4H, m, H2-9,H2-10), 0.97 (3H, m, H3-11), 5.55 (2H, s, H2-12), 8.03(1H, m, H-15), 7.44 (2H, m, H-16, H-18), 7.51 (1H, m,H-17), 7.95 (1H, m, H-19);13C-NMR (CD3OD, 75 MHz)δ: 138.0 (s, C-1), 121.7(d, C-2), 129.1(d, C-3), 126.5(d,C-4), 130.0(d, C-5), 132.4(s, C-6), 166.2 (s, C-7),66.1(t, C-8), 31.3 (t, C-9), 19.0(t, C-10), 13.0(q, C-11),63.0(t, C-12), 167.3(s, C-13), 127.0(s, C-14), 129.9(d,C-15), 128.0(d, C-16), 131.1(d, C-17), 128.0(d, C-18),129.9(d, C-19); EIMS m/z: 312.0 [M]+。以上数据与Spatozoate波谱数据一致[5]。
化合物(5), 黄色粉末, C7H7NO2。1H-NMR(CD3OD,300 MHz) δ: 7.33(1H, dd, J= 5.9, 6.9Hz, H-3), 6.65(2H,m, H-4, H-5), 7.91(1H, d, J= 5.9 Hz, H-6);13C-NMR(CD3OD, 75 MHz) δ: 170.3(s, CO), 110.8(s, C-1),152.6(s, C-2), 134.7(d, C-), 132.3(d, C-), 117.2(d, C-),115.8(d, C-); EIMS: m/z(%) 137.0(65), 119.1(100),93.0(4), 92.1(50), 71.1(3), 66.0(2), 65.0(11), 58.0(5),43.0(7), 41.0(3)。以上数据与邻氨基苯甲酸波谱数据一致[6]。
化合物(6), 无色油状物, C10H11NO。1H-NMR(CD3OD, 300 MHz) δ: 8.05 (1H, br s, NH), 7.11(1H, s,H-2), 7.66(1H, d, J= 8.2 Hz, H-4), 7.15(1H, dd, J= 8.2,1.5 Hz, H-5), 7.23 (1H, dd, J= 8.2, 1.5 Hz, H-6),7.40(1H, d, J=8.2 Hz, H-7), 3.00(2H, t, J= 6.6Hz,H2-8), 3.90(2H, t, J= 6.6 Hz, H2-9);13C-NMR (CD3OD,75 MHz)δ: 123.1(d, C-2), 112.0(s, C-3), 127.2 (s,C-3a), 121.0(d, C-4), 123.2 (d, C-5), 124.5(d, C-6),1120.1 (d, C-7), 133.0(s, C-7a), 28.6 (t, C-8), 66.6(t,C-9)。EIMS m/z: 161.1 [M]+。以上数据与3-吲哚乙醇波谱数据一致[7]。
化合物(7), 无色油状物, C13H10N2O。1H-NMR(CD3OD, 300 MHz)δ: 8.68(1H, d, J= 6.0Hz, H-4),8.23(2H, m, H-8, H-9), 7.29(1H, m, H-6), 7.60(2H, m,H-7, H-5), 2.95(3H, s, H3-12);13C-NMR (CD3OD,75MHz)δ: 135.4(s, C-2), 123.3(s, C-3), 123.4(s, C-3a),122.2(d, C-4), 122.1(d, C-5), 121.8 (d, C-6), 111.9(d,C-7), 140.0(sC-7a), 120.5(d, C-8), 137.1(d, C-9),141.5(s, C-10), 201.1(s, C-11), 26.0(q, C-12)。EIMS m/z: 210.0 [M]+。以上数据与1-乙酰基咔啉波谱数据一致[8-10]。
化合物(8), 淡黄色油状物, C8H10O2;1H-NMR(CD3OD, 300 MHz)δ: 7.14(2H, J= 8.0 Hz, H-2, H-6),6.80(2H, d, J= 8.0 Hz, H-3, H-5), 2.81(2H, t, J= 6.2 Hz,H2-7), 3.80(2H, t, J= 6.2 Hz, H2-8);13C-NMR (CD3OD,75 MHz)δ: 156.9(s, C-1), 118.0(d, C-2), 129.8(d, C-3),138.7 (s, C-4), 129.8(d, C-5), 118.0(d, C-6), 39.3(t,C-7), 63.5(t, C-8); EIMS m/z: 138.1 [M]+。以上数据与对羟基苯基乙醇波谱数据一致[11-12]。
化合物(9), 淡黄色油状物, C10H9NO2。1H-NMR(CD3OD, 300 MHz)δ: 7.30(1H, s, H-2), 7.64(d1H, d,J= 1.8, 7.5 Hz, H-4), 7.00(1H, m, H-5), 7.17(1H, m,H-6), 7.30(1H, dd, J= 1.8, 7.5 Hz, H-7), 3.77(1H, s,H-8);13C-NMR (CD3OD, 75 MHz)δ: 124.9(d, C-2),109.0 (s, C-3), 128.0(s, C-3a), 120.1 (d, C-4), 120.3(d,C-5), 122.0(d, C-6), 112.3(d, C-7), 137.5(s, C-7a),32.4(t, C-8), 173.9(s, C-9); EIMS m/z: 175.1 [M]+。以上数据与吲哚-3-乙酸波谱数据一致[13]。
化合物(10), 淡黄色油状物, C9H7NO2。1H-NMR(CD3OD, 300 MHz)δ: 8.10(1H, m, H-4), 7.92(1H, s,H-2), 7.41(1H, m, H-7), 7.20(2H, m, H-5,H-6);13C-NMR (CD3OD, 75.5 MHz)δ: 168.3(s, CO),129.0 (d, C-2), 112.0(s, C-3), 133.2(s, C-3a), 122.0(d,C-4), 123.2(d, C-5), 123.1(d, C-6), 113.7(d, C-7),137.9 (s, C-7a)。以上数据与吲哚-3-甲酸波谱数据一致[14-16]。
3 结论
本研究以海洋链霉菌Streptomyces sp. L211为研究对象, 先期从中分离了 15个化合物, 并鉴定了其中的 10个, 研究表明化合物 Kalamycin (1),Medermycin (2), Menoxymycin-B (3)均表现出不同的抗肿瘤活性[3-4]。目前, 本课题组正在对其他海洋放线菌次生代谢产物进行深入研究, 相信会有更多有活性的代谢产物被发现。
[1] Amagata T, Xiao J, Chen Y P, et al. Creation of an HDAC-Based Yeast Screening Method for Evaluation of Marine-Derived Actinomycetes: Discovery of Streptosetin A[J]. Journal of Natural Products, 2012,75(12): 2193-2199.
[2] Sato S, Iwata F, Yamada S, et al. Neomaclafungins A–I:Oligomycin-Class Macrolides from a Marine-Derived Actinomycete[J]. Journal of Natural Products, 2012,75(11): 1974-1982.
[3] Zhang Y H, Qin M, Li F C, et al. Isolation and identification of metabolites produced by marine Streptomyces sp.S007[J]. Nat Prod Res Dev, 2011, 23:410-414.
[4] Yoichi H, Ishigami K, Shin-Ya K, et al. Menoxymycins A and B, antitumor antibiotics generating active oxygen in tumor cells[J]. J Antibiot, 1994, 47: 1344-1347.
[5] Atta-ur-Rahman M, Iqbal C, Safdar H, et al. Spatozoate and varninasterol from the brown alga Spatoglossum variabile[J]. Phytochemistry, 1999, 52: 495-499.
[6] Sindler-Kulyk M, Neckers D C. Photochemistry of 2-phenylbenzothiazole with ethoxyacetylene and ethoxypropyne. Synthesis of 1, 5-benzothiazepines[J]. Journal of Organic Chemistry, 1982, 47(25): 4914-4919.
[7] Gordon B B, Arthur C G. Indole Compounds Synthesized by Diplodia natalensis[J]. Plant physiology, 1962,37: 439-445.
[8] Zhang Y, Morikawa T, Nakamura S, et al. Bioactive constituents from chinese natural medicines. XXV.New flavonol bisdesmosides, sarmenosides I, II, III,and IV, with hepatoprotective activity from Sedum sarmentosum (Crassulaceae) [J]. Heterocycles, 2007,71(7): 1565-1576.
[9] Morikawa T, Zhang Y, Nakamura S, et al. Bioactive constituents from Chinese natural medicines. XXII.Absolute structures of new megastigmane glycosides,sedumosides E1, E2, E3, F1, F2, and G, from Sedum sarmentosum (Crassulaceae)[J]. Chemical & Pharmaceutical Bulletin, 2007, 55(3): 435-441.
[10] Ohmoto T, Koike K. Studies on the constituents of Picrasma quassioides Bennet. I. On the alkaloidal constituents[J]. Chemical & Pharmaceutical Bulletin,1982, 30(4): 1204-1209.
[11] Huang Q, Tezuka Y, Hatanaka Y, et al. Studies on metabolites of mycoparasitic fungi. III. new sesquiterpene alcohol from Trichoderma koningii[J]. Chemical & Pharmaceutical Bulletin, 1995, 43: 1035-1038.
[12] William A A, Latchezar S T, Phenolic and polyketide metabolites of the aspen blue stainfungus Ophiostoma crassivaginata[J]. Phytochemistry, 1995, 38: 371-372.
[13] Alejandro F B, Juan E O, Juan A P. Acidic metabolites from Phycomyces blakesleeanus[J]. Phytochemistry,1996, 42: 1427-1433.
[14] 刘海河, 林文翰, 赵喜, 等.南海红树内生真菌#HA-094化学成分的研究[J].黑龙江医药, 2007, 20(6): 564-565.
[15] Shaaban K A, Shaaban M, Facey P, et al. Electrospray ionization mass spectra of piperazimycins A and B and butyrolactones from a marine-derived Streptomyces sp.[J]. Journal of Antibiotics, 2008, 61(12): 736-746.
[16] Eggers M E, Jog P V, Bates D K. Intramolecular sulfoxide electrophilic sulfenylation in 2- and 3-indoleanilides[J]. Tetrahedron, 2007, 63(49): 12185-12194.
