勐仑翅子树化学成分的研究
2014-10-12马艳芳蒋金和陈业高
马艳芳,蒋金和,詹 睿,刘 莹,陈业高
(云南师范大学 化学化工学院,云南 昆明 650500)
勐仑翅子树(Pterospermum menglunense Hsue)为梧桐科翅子树属植物,常绿乔木,濒危种,分布于云南南部西双版纳勐仑,生于石灰岩山林疏林中[1].本品味微苦,民间用于散瘀止血、补益,治外伤及跌打损伤肿痛.翅子树属植物具有良好的免疫抑制[2],抗氧化[3],抗溃疡[4]及止血[5]等功效.药理实验表明,翅子树叶的乙醇提取物有较强抑制大肠杆菌生长和缩短小白鼠凝血时间的作用[6].勐仑翅子树的化学成分尚未见报道,为进一步开发其药用价值,阐述其有效成分,本文对勐仑翅子树的化学成分进行了分离鉴定.采用各种分离方法包括硅胶柱层析、MCI柱层析、凝胶层析和和C-18反相柱层析等分离方法,从中分得9个化合物.经过波谱分析(核磁共振氢谱、碳谱)确定它们的结构分别为teraxeryl acetate(1),β-sitosterol(2),7β-hydroxysitosterol(3),daucosterol(4),24-ethylcholesta-7,22-diene-3β,5α,6β-triol(5),24(S)-24-enthyl-5α-cholestane-3β,5,6β-triol(6),6β-hydroxy stigmast-4-en-3-one(7),(22E,24R)-23-methylergosta-7,22-diene-3β,5α,6β-triol(8),glycerol monopalmitate(9).所有化合物均为首次从该种植物分离得到,其中(3)、(5)~(9)为首次从该属植物中分离得到.其结构见图1.
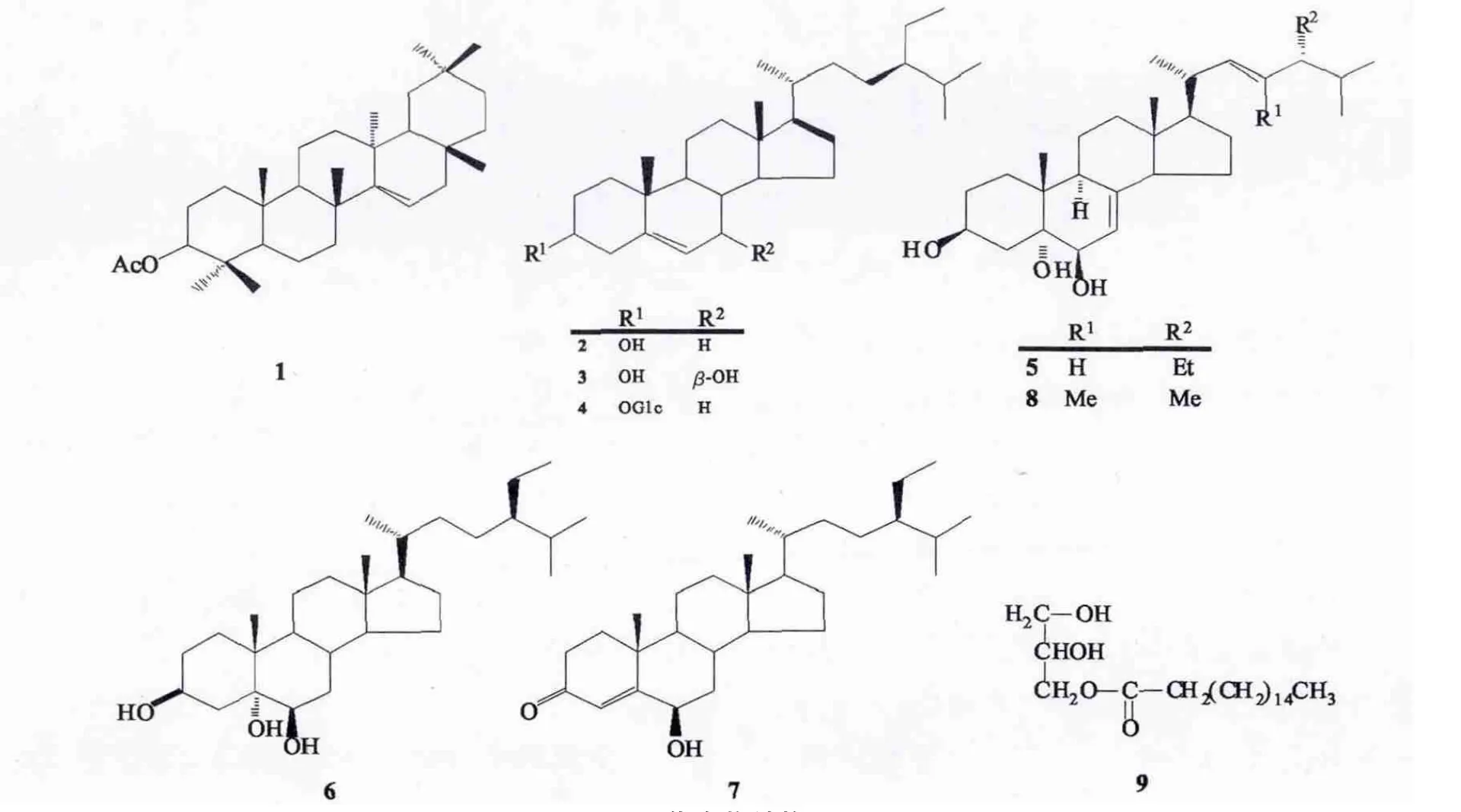
图1 化合物结构图Fig.1 Structure diagram of compound
1 药材和仪器
1.1 仪器
Bruker DRX-500MHz超导核磁共振仪(瑞士Bruker公司);ZF-Ⅱ型紫外分析仪(上海顾村中实仪器厂);EYELA IV-1100型旋转蒸发仪(上海爱朗仪器有限公司).
1.2 试剂
层析硅胶(青岛海洋化工厂出品),高效薄层层析硅胶G板(烟台化工研究院),Sephadex LH-20:20-80 m(Pharmacia Fine Chemical Co.,Ltd.),MCI(CHP20P,日本三菱公司);反相RP-18(德国Merck公司).显色剂:10%硫酸乙醇溶液.所用溶剂为工业纯,重蒸,其它试剂为化学纯或分析纯.
1.3 药材
勐仑翅子树枝叶采自云南西双版纳,由中国医学科学院药用植物开发研究所云南分所彭朝中先生采集,并鉴定为Pterospermum menglunense Hsue的枝叶.
2 试验方法
干燥的勐仑翅子树枝叶部分(9.0 kg),用工业甲醇室温浸提4次,浸出液减压浓缩得甲醇提取物,为黑色浸膏.浸膏用水悬溶,乙酸乙酯萃取,萃取液减压浓缩,得乙酸乙酯浸膏147 g.乙酸乙酯部分进行MCI柱层析(甲醇/水 8:2→10:0,丙酮),依此得到甲醇/水 8:2(A)28 g,甲醇/水 9:1(B)34 g,甲醇(C)74 g和丙酮部分.C部分经硅胶柱层析,凝胶柱层析和C-18反相柱层析反复分离纯化,得化合物1(220 mg),2(190 mg),3(5.0 mg),4(6.8 mg),5(3.0 mg),6(6.0 mg),7(9.0 mg),8(6.0 mg)和9(18 mg).
3 结果与讨论
从勐仑翅子树枝叶分离得到9个成分,利用现代核磁共振波谱法,结合理化分析进行鉴定,9个化合物的结构分别为teraxeryl acetate(1),β-sitosterol(2),7β-hydroxysitosterol(3),daucosterol(4),24-ethylcholesta-7,22-diene-3β,5α,6β-triol(5),24(S)-24-enthyl-5α-cholestane-3β,5,6β-triol(6),6β-hydroxy stigmast-4-en-3-one(7),(22E,24R)-23-methylergosta-7,22-diene-3β,5α,6β-triol(8),glycerol monopalmitate(9).所有化合物均为首次从该种植物分离得到,其中(3)、(5)~(9)为首次从该属植物中分离得到.
化合物1:无色针状结晶,分子式为C32H52O2,溶于氯仿.1H-NMR(500 MHz,CDCl3):δ 5.54(1H,dd,J=2.5,2.5 Hz,H-15),4.47(1H,dd,J=5.5,5 Hz,H-3)表明有烯氢质子,2.05(3H,s,-COCH3),1.91(1H,dd,J=15 Hz,H-16a),1.09(3H,s,H-26),0.96(3H,s,H-23),0.91(3H,s,H-30),0.88(3H,s,H-30),0.86(3H,s,H-29),0.83(3H,s,H-24);13C-NMR(CDCl3,125 MHz):δ 171.0(s,-COCH3),158.0(s,C-14),116.9(d,C-15),81.0(d,C-3),55.6(d,C-5),49.2(d,C-9),48.8(d,C-18),41.2(t,C-19),39.0(d,C-8),38.9(s,C-4),37.9(s,C-13),37.7(t,C-1),37.6(s,C-10),37.4(t,C-22),36.7(t,C-12),35.8(s,C-17),35.1(t,C-21),33.7(t,C-16),33.4(q,C-29),33.1(t,C-7),29.9(q,C-27),29.8(q,C-28),28.8(s,C-20),28.0(q,C-23),25.9(t,C-2),21.3(q,C-30;q,-COCH3),18.8(t,C-6),17.5(t,C-11),16.6(q,C-24),15.4(q,C-25).数据与文献[7]报道的化合物teraxeryl acetate完全一致,因此化合物1的结构确定为teraxeryl acetate.
化合物2:无色针状结晶,分子式为C29H50O,易溶于氯仿,难溶于甲醇.10%浓硫酸-乙醇显色为紫红色,与β-Sitosterol标准品进行TLC展开,多种不同的展开剂系统的Rf值均相同.根据以上理化性质,结合文献[8]报道,鉴定化合物2为β-Sitosterol.
化合物3:无色粉末,分子式为C29H50O2,可溶于氯仿、甲醇.1H-NMR(500 MHz,CDCl3):δ 5.32(1H,brs,H-6),3.87(1H,d,J=7.5 Hz,H-7),3.57(1H,m,H-3),1.07(3H,s,H-19),0.94(3H,d,J=7.0 Hz,H-21),0.87(2H,t,J=7.3 Hz,H-28),0.84(3H,d,J=6.5 Hz,H-26),0.81(3H,d,J=6.5 Hz,H-27),0.72(3H,s,H-18);13C-NMR(500 MHz,CDCl3):δ 143.5(s,C-5),125.5(d,C-6),73.4(d,C-7),71.5(d,C-3),56.0(d,C-14),55.4(d,C-17),48.3(d,C-9),45.9(d,C-24),42.3(d,C-8),42.2(t,C-4),41.8(s,C-13),39.6(t,C-12),37.0(t,C-1),36.9(s,C-10),36.5(d,C-20),36.1(t,C-22),31.6(t,C-2),29.2(d,C-25),28.6(t,C-16),26.4(t,C-15),26.1(t,C-23),23.1(t,C-28),21.1(t,C-11),19.8(q,C-26),19.2(q,C-27),19.0(q,C-19),18.8(q,C-21),12.0(q,C-18),11.8(q,C-29).数据与文献[9]报道的化合物7β-hydroxysitosterol完全一致,因此化合物3的结构确定为7βhydroxysitosterol.
化合物4:无色粉末,分子式为C35H60O6,可溶于氯仿、丙酮.10%浓硫酸-乙醇显色为紫红色,与daucosterol标准品进行TLC展开,多种不同的展开剂系统的Rf值均相同.根据以上理化性质,结合文献[10]报道,鉴定化合物4为daucosterol.
化合物5:无色粉末,分子式为C29H48O3,可溶于吡啶.1H-NMR(500 MHz,C5D5N):δ 5.73(1H,brs,H-7),5.20(1H,dd,J=15.0,6.8 Hz,H-23),5.18(1H,dd,J=12,6.8 Hz,H-22),4.83(1H,m,H-3),4.32(1H,d,J=4.5 Hz,H-6),3.05(1H,t,J=12.0 Hz,H-4),2.56(1H,m,H-9),1.71(1H,m,H-16β),1.69(2H,m,H-11),1.53(3H,s,H-19),1.26(1H,m,H-16α),1.24(1H,m,H-14),1.05(3H,d,J=6.5 Hz,H-21),0.93(1H,d,J=6.6 Hz,H-22),0.85(3H,d,J=4.0 Hz,H-26),0.83(3H,d,J=4.5 Hz,H-27),0.64(3H,s,H-18);13C-NMR(C5D5N,125 MHz):δ 141.3(s,C-8),136.0(d,C-22),131.8(d,C-23),120.2(d,C-7),75.9(s,C-5),74.0(d,C-6),67.3(d,C-3),55.9(d,C-17),55.0(d,C-14),43.5(d,C-9),43.5(s,C-13),43.0(d,C-24),41.7(t,C-4),40.6(d,C-20),39.6(t,C-12),37.8(s,C-10),33.6(d,C-25),33.1(t,C-2),32.4(t,C-1),29.7(t,C-28),28.2(t,C-16),23.2(t,C-15),22.1(t,C-11),21.1(q,C-26),19.9(q,C-21),19.6(q,C-27),18.5(q,C-19),17.6(q,C-29),12.3(q,C-18).数据与文献[11]报道的化合物24-ethylcholesta-7,22-diene-3β,5α,6β-triol完全一致,因此化合物5的结构确定为24-ethylcholesta-7,22-diene-3β,5α,6β-triol.
化合物6:无色粉末,分子式为C29H52O3,可溶于吡啶.1H-NMR(500 MHz,C5D5N):δ 5.24(m,1H,H-3),4.14(m,1H,H-6),0.69(s,3H,H-18),0.80(d,3H,J=6.5 Hz,H-26),0.82(d,3H,J=7.0 Hz,H-27),0.85(t,3H,J=7.5 Hz,H-29),0.9l(d,3H,J=6.6 Hz,H-21),1.18(s,3H,H-19)表明有6个甲基质子信号.13C-NMR(C5D5N,125 MHz):δ 76.2(d,C-6),75.8(s,C-5),67.3(d,C-3),56.5(d,C-14),56.5(d,C-17),46.0(d,C-24),46.0(d,C-9),43.0(s,C-13),42.8(t,C-4),40.6(t,C-12),39.0((s,C-10),36.4(t,C-20),35.6(t,C-7),34.1(t,C-22),33.2(t,C-2),32.4(t,C-1),31.1(d,C-8),29.3(d,C-25),28.6(t,C-16),26.3(t,C-23),24.6(t,C-15),23.3(t,C-28),21.7(t,C-11),19.9(q,C-27),19.1(q,C-26),18.9(q,C-21),17.1(q,C-19),12.3(q,C-18),12.0(q,C-29).数据与文献[12]报道的化合物24(S)-24-enthyl-5α-cholestane-3β,5,6β-triol完全一致,因此化合物6的结构确定为24(S)-24-enthyl-5α-cholestane-3β,5,6β-triol.
化合物7:无色结晶,分子式为C29H48O2,可溶于吡啶.1H-NMR(500 MHz,C5D5N):δ 6.04(s,1H,H-4),4.54(s,1H,H-6),2.56(m,2H,H-7),1.88(m,1H,H-25),1.53(s,3H,H-19),1.38(s,1H,H-20),1.30(m,2H,H-7),1.26(m,2H,H-28),0.98(d,J=6.5 Hz,3H,H-21),0.88(s,3H,H-29),0.87(d,J=6.56 Hz,3H,H-27),0.85(d,J=7 Hz,3H,H-26),0.71(s,3H,H-18);13C-NMR(C5D5N,125 MHz):δ 199.4(s,C-3),169.6(s,C-5),125.6(d,C-4),72.3(d,C-6),56.0(d,C-14),55.9(d,C-17),53.9(d,C-9),45.9(d,C-24),42.5(s,C-13),39.7(t,C-12),39.5(t,C-7),38.2(s,C-10),37.3(t,C-1),36.2(d,C-20),34.5(t,C-2),34.0(t,C-22),30.1(d,C-8),29.3(d,C-25),28.3(t,C-16),26.2(t,C-23),24.3(t,C-15),23.2(t,C-28),21.1(t,C-11),19.8(q,C-26),19.4),q,C-19),19.0(q,C-27),18.8(q,C-21),11.9(q,C-18).数据与文献[13]报道的化合物6β-hydroxy stigmast-4-en-3-one完全一致,因此化合物7的结构确定为6β-hydroxy stigmast-4-en-3-one.
化合物8:无色粉末,分子式为C29H48O3,可溶于氯仿,甲醇,吡啶.1H-NMR(500 MHz,C5D5N):δ 5.32(dd,1H,J=5.5,2.6 Hz,H-7),4.90(dd,J=9.5,1.5 Hz,1H,H-22),4.08(m,1H,H-3),3.63(m,1H,H-6),2.36(m,1H,H-20),2.15(dd,1H,J=12.8,12.1 Hz,H-4),1.51(d,3H,J=1.5 Hz,H-29),1.09(s,3H,H-19),0.95(d,3H,J=6.6 Hz,H-21),0.94(d,3H,J=7.0 Hz,H-28),0.85(d,3H,J=6.6 Hz,H-26),0.79(d,3H,J=6.6 Hz,H-27),0.62(s,3H,H-18);13C-NMR(C5D5N,125 MHz):δ 143.2(s,C-8),135.7(s,C-23),131.8(d,C-22),115.8(d,C-7),75.5(s,C-5),73.9(d,C-6),67.1(d,C-3),57.0(d,C-17),54.5(d,C-14),43.5(d,C-9),43.0(s,C-13),39.3(t,C-12),39.1(t,C-4),37.0(s,C-10),34.5(d,C-20),33.0(t,C-1),30.7(d,C-25),30.5(t,C-2),27.0(t,C-16),22.6(t,C-11),22.0(t,C-15),21.4(q,C-27),20.6(q,C-26),19.9(q,C-21),18.7(q,C-19),17.0(q,C-28),13.1(q,C-29),12.2(q,C-18).数据与文献[14]报道的化合物(22E,24R)-23-methylergosta-7,22-diene-3β,5α,6β-triol完全一致,因此化合物8的结构确定为(22E,24R)-23-methylergosta-7,22-diene-3β,5α,6β-triol.
化合物9:无色粉末,分子式为C20H40O4,可溶于氯仿、丙酮.1H-NMR(500 MHz,CDCl3):δ 4.16(d,1H,J=7.0 Hz,1’-OH),4.07(m,1H,3’-OH),3.84(m,1H,H-1’),3.58(dd,2H,J=2.5,2.5 Hz,H-2’),2.38(m,2H,H-1’),1.38(m,28H,H-2~H-15),0.92(t,3H,H-16);13C-NMR(CDCl3,125 MHz):δ 174.4(s,C-1),70.3(d,C-2’),65.2(t,C-1’),63.4(t,C-3’),34.2(t,C-2),31.2(t,C-14),29.1~79.7(t,C-4~C-13),24.9(t,C-3),22.7(t,C-15),14.1(q,C-16).数据与文献[15]报道的化合物glycerol monopalmitate完全一致,因此化合物9的结构确定为glycerol monopalmitate.
[1]中国科学院中国植物志编辑委员会.中国植物志[M].北京:北京科学出版社,1984:177.
[2]Manisha P,Nasreen B,Preety D,et al.Immunosuppressive activity of hexane and ethanolic extracts of Pterospermum acerifolium seeds in BALB/c mice[J].Med Chem Res,2011,20,1667-1673.
[3]Shweta S,Ritesh T,Khadabadi S S,et al.In vitroantioxidant activity and total phenolic,flavonoidcontents of the crude extracts of Pterospermum acerifolium wild leaves(Sterculiaceae)[J].J Chem Pharm Res,2010,2(3):417-423.
[4]Manna A K,Behera A K,Jena J,et al.The antiulcer activity of Pterospermum acerifoliumbark extract in experimental ani-mal[J].J.Pharm Res,2009,2(5):785-788.
[5]Manna A K,Bhunia S K,Nanda U,et al.Wound healing properties of Pterospermum acerifolium wild[J].J PharmRes,2010,3(3):537-538.
[6]钟永利,苏镜娱,曾陇梅,等.翅子树叶中的三萜化合物[J].高等学校化学学报,1993,14(2):214-216.
[7]石妍,李帅,李红玉,等.翻白叶树根化学成分的研究[J].中国中药杂志,2008,33(16):1994-1996.
[8]戚欢阳,王瑞,刘勇,等.白条党参化学成分研究[J].中药材,2011,34(4):546-548.
[9]侯朋艺,黄健,孙博航,等.新疆雪莲化学成分的分离与鉴定[J].沈阳药科大学学报,2011,28(2):120-123.
[10]鲍长余,范超君,陈湛娟,等.海南产三叉苦的化学成分研究[J].海南师范大学学报:自然科学版,2012,25(1):66-69.
[11]Lee H S,Hwang I H,Kim J A,et al.Isolation of Protein Tyrosine Phosphatase 1B Inhibitory Constituents from the Sclerotia of Polyporus umbellatus Fries[J].Bull.Korean Chem.Soc.,2011,32(2):697-700.
[12]刘青,刘珍伶,田瑄,等.羽裂蟹甲草中的甾醇类化合物[J].中国中药杂志,2008,33(9):1035-1038.Patricia G,Muriel S,Heinz R,et al.
[13]Ketosteroids and hydroxyketosteroids,minor metabolites of sugarcane wax[J].Steroids,2007,71(8):647-652.
[14]Yaoita Y,Endo M,Tani Y,et al.Sterol Constituents from Seven Mushrooms[J].Chem Pharm Bull,1999,47(6):847-851.
[15]孙彦君,李占林,陈虹,等.桃儿七化学成分研究[J].中药材,2012,35(10):1607-1609.
