Interferon-alpha-2b induces autophagy in hepatocellular carcinoma cells through Beclin1 pathway
2014-09-26JunZhaoMingLiWangZengLiDongMeiGaoYuCaiJunChangShiPingWang
Jun Zhao, Ming-Li Wang, Zeng Li, Dong-Mei Gao, Yu Cai, Jun Chang, Shi-Ping Wang
1Department of Parasitology, Xiangya Medical School, Central South University, Changsha 410078, China; 2Department of Microbiology, Anhui Medical University, Hefei 230032, China; 3Department of Clinical Laboratory, the Third Affiliated Hospital of Anhui Medical University, Hefei 230032, China; 4Department of Orthopedics, The First Affiliated Hospital of Anhui Medical University, Hefei 230032, China
Introduction
Interferon-alpha (IFN-α) is cytokines belonging to the type I IFN group.They exert many effects on cell functions1,2.The IFN-α family contains at least 13 functional IFN subtypes,all of which share the same receptor system and exert similar biological activities3.Study of 50 years on IFN-α has revealed that these cytokines exhibit a variety of biological effects which are different from those on viral replication, such as antitumor activity.IFN-α cytokines are widely expressed and secreted as the first line of defense against several types of tumors.They have been used in over 40 countries for the treatment of more than 14 types of cancer, including some hematological malignancies(hairy cell leukemia, chronic myeloid leukemia, some B- and T-cell lymphomas) and certain solid tumors, such as melanoma,renal carcinoma and Kaposi’s sarcoma.IFN-α can directly inhibit the proliferation of normal and tumor cells in vitro and in vivo,and can exert other direct effects on tumor cells4-6.
In the present study we investigated the effect of IFN-α2b on autophagy in hepatocellular carcinoma cells and related mechanisms.
Materials and methods
Cell culture
Hepatocellular carcinoma HepG2 cell line was procured from American type culture collection (ATCC).We propagated HepG2 cells in Dulbecco’s modi fied Eagle’s medium (GBICO)supplemented with 10% fetal bovine serum (GBICO) in a humidi fied incubator containing 5% CO2at 37 ℃.Human IFN-α2b (Sigma-Aldrich) was diluted in serum-free medium.
Acridine orange staining for autophagy
Autophagy is characterized by the formation and promotion of acidic vesicular organelles (AVOs).HepG2 Cells were treated with various concentrations of IFN-α2b in 6-well plates.After 48 h post treatment, then incubated with 1 mg/mL acridine orange (Sigma) for 15 min.Pictures were obtained with a fluorescence microscope.
GFP-LC3 dotted assay
Cells were transiently transfected with GFP-LC3 (Origene Tech.Inc, MD, USA) vector using Lipofectamine LTX and PLUS Reagents (Invitrogen Corporation) according to the manufacturer’s instructions.After 24 h, the cells were exposed to IFN-α2b for 48 h as indicated, and examined under fluorescence microscope.The induction of autophagy was quantified by counting the percentage of cells in each group with a number of LC3 aggregates.
Transmission electron microscopy
Cells were fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer for 1 h.After fixation, the samples were post- fixed in 1%OsO4in the same buffer for 30 min.Ultrathin sections were then observed under a transmission electron microscope.
Western blot
Western blotting procedure was performed as described previously7.Briefly, cells were lysed in appropriate volume of lysis buffer (Sigma Aldrich).Fifty micrograms of protein samples were separated by SDS-PAGE and transferred onto nitrocellulose membrane.The membranes were immunoblotted with primary antibodies purchased from Santa Cruz Biotechnology, Inc.(Santa Cruz, CA, USA).Blots were incubated with horseradish peroxide-conjugated goat anti-rabbit or goat anti mouse secondary antibodies purchased from Santa Cruz Biotechnology.All experiments were performed and verified using at least three biological replicates.
Statistical analysis
The experimental data were expressed as mean ± SD.Group means were compared by t-test using the statistical software program SPSS 13.0.P values <0.05 were considered to be statistically significant.
Results
HepG2 cells were treated with IFN-α2b.IFN-α2b was found to trigger the accumulation of acidic vesicular and autolysosomes in HepG2 cells (Figure 1A).The acridine orange HepG2 cell ratios were (4.3±1.0)%, (6.9±1.4)%, and (13.1±2.3)% after treatment with 100, 1,000, and 10,000 IU/mL IFN-α2b, respectively(Figure 1B).

Figure 1 Modulation of autophagy by IFN-α2b in HepG2 cells.Cells were treated with IFN-α2b for 48 h at concentrations of 100, 1,000, and 10,000 IU/mL.Cells were then stained with acridine orange.1, control group; 2, cells treated with 100 IU/mL IFN-α2b; 3, cells treated with 1,000 IU/mL IFN-α2b; 4, cells treated with 10,000 IU/mL IFN-α2b.(A)autophagic vacuoles were observed and imaged on a fluorescence microscope;(B) acridine orange staining positive cells (%).1, control group; 2, cells treated with 100 IU/mL IFN-α2b; 3, cells treated with 1,000 IU/mL IFN-α2b; 4, cells treated with 10,000 IU/mL IFN-α2b (#P<0.05 relative to control values and*P<0.01 relative to control values).
GFP-LC3 plasmid was transfected into HepG2 cells for observation and quantification of the redistribution of autophagy marker LC3 from a diffused to punctate pattern after treatment with IFN-α2b for 48 h.Similarly, as shown in Figure 2,a markedly punctate pattern appeared among HepG2 cells treated with 10,000 IU/mL IFN-α2b for 48 h, but there was only diffuse and weak fluorescent GFP-LC3 puncta among control cells.HepG2 cells treated with 10,000 IU/mL IFN-α2b for 48 h developed autophagosome-like characteristics, including singleor double-membrane vacuoles containing intact and degraded cellular debris (Figure 3).

Figure 2 IFN-α2b induced punctuation of GFP-LC3 distribution in HepG2 cells.At 24 h after the transient transfection of GFP-LC3, cells were treated with IFN-α2b for 48 h and then analyzed for fluorescence.A.Images were captured using a fluorescence microscope.1, control group; 2, cells treated with 100 IU/mL IFN-α2b; 3, cells treated with 1,000 IU/mL IFN-α2b; 4, cells treated with 10,000 IU/mL IFN-α2b; B.LC3 positive cells (%).1, control group; 2, cells treated with 100 IU/mL IFN-α2b; 3, cells treated with 1,000 IU/mL IFN-α2b; 4,cells treated with 10,000 IU/mL IFN-α2b (*P<0.01 relative to control values).
The autophagy in HepG2 cells was also confirmed by immunoblotting, which showed the degree of accumulation of LC3 to be correlated with the number of autophagosomes relative to the amount of endogenous LC3-II protein.Consistent with the data obtained from GFP-LC3-transfected cells, Western blot recorded a strong increase in the amount of endogenous LC3-II in HepG2 cells after treatment with 10,000 IU/mL IFN-α2b for 48 h (Figure 4).The molecular mechanism underlying autophagy induction was determined using IFN-α2b.The protein expression of Beclin1 was found to be up-regulated by IFN-α2b.These results indicated that IFN-α2b induced HepG2 cell autophagy exerted its effects through the Beclin1 pathway.

Figure 3 Transmission electron images of HepG2 cells treated with 10,000 IU/mL IFN-α2b.The arrowhead indicates single- or double-membrane vesicles containing intact and degraded cellular debris.(A) Control; (B) Cells treated with 10,000 IU/mL IFN-α2b.
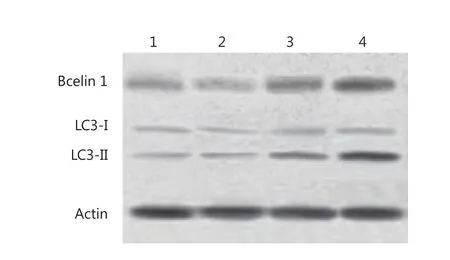
Figure 4 Modification of LC3 and Bcelin1 in HepG2 cells after treatment with IFN-α2b.1, control group; 2, cells treated with 100 IU/mL IFN-α2b; 3,cells treated with 1,000 IU/mL IFN-α2b; 4, cells treated with 10,000 IU/mL IFN-α2b.
Discussion
Hepatocellular carcinoma is the fifth most common cancer in the world.However, the potentially curable method is only possible for a small proportion of those aラicted, for the rest,palliative treatment is indicated.In this setting, type I IFN has emerged as an alternative treatment modality for hepatocellular carcinoma8,9.
Several biological functions of type I IFN, including its regulation of innate and adaptive immunity and its antiangiogenic and proapoptotic effects, make it an obvious candidate for anticancer therapy.Indeed, type I IFN has been used with some success for the treatment of several types of cancer, including hematological malignancies and solid tumors10.It was recently shown that IFN-α2c could induce autophagy in HeLa S3, MDAMB-231, T98G and A549 cell lines11.But IFN-α2c is rarely used in the clinical treatment of cancer, and IFN-α2b is the main drug treatment for cancer.
Autophagy is a self-degradation process whereby cytosolic components and organelles are sequestered in double membrane-bound vesicles and delivered to lysosomes for degradation and recycling.In normal tissue, autophagy maintains cellular homeostasis by clearing damaged organelles or misfolded proteins.However, the role of autophagy in cancer is complex and paradoxical as it is an adaptive process that is responsive to changes in the cellular microenvironment.Thus, autophagy can either suppress or support the growth of tumor cells depending on the cellular context12.
Given the critical roles of autophagy in tumor progression and maintenance, various preclinical and clinical studies have been undertaken to develop therapeutic agents targeting autophagy.As most anticancer agents inevitably cause cellular stress,autophagy is often activated in cancer cells after drug treatment.Indeed, many therapies targeting growth factor signaling-either singly or in combinations targeting two different pathwayslead to autophagy induction.For example, several allosteric and catalytic inhibitors of mTOR, PI3K-AKT, and the tyrosine kinase signaling and activators of energy sensing pathway induce autophagy in cells.It was originally proposed that autophagic cell death is part of the mechanism of action of anticancer drugs13.Beclin 1, which was the first gene to be identified positively associated with mammalian autophagy, plays a central role in coordinating the cytoprotective function of autophagy and in opposing apoptosis14.
In this study, hepatocellular carcinoma cells were treated with IFN-α2b.Autophagy was assessed by acridine orange staining,GFP-LC3 dotted assay, transmission electron microscopy and immunoblotting.We found that autophagy can be induced in a dose-dependent manner by treatment with IFN-α2b in HepG2 cells.The dependence of IFN-α2b induced autophagy on the Beclin1 pathway was also assessed, and the Beclin1 signaling pathway was stimulated by IFN-α2b.
Acknowledgements
This work was supported by grants from the Scientific Support Project of Anhui Province Education Department of China(Grant No.KJ2012ZD08 and KJ2012Z162), the National Scientific and Technological Support Projects of China (Grant No.81101273), and the National Natural Science Foundation of China (Grant No.30872253).
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Zhang W, Rao HY, Feng B, Liu F, Wei L.Effects of interferon-alpha treatment on the incidence of hyperglycemia in chronic hepatitis C patients: a systematic review and meta-analysis.PLoS One 2012;7:e39272.
2.Tough DF.Modulation of T-cell function by type I interferon.Immunol Cell Biol 2012;90:492-497.
3.Moll HP, Maier T, Zommer A, Lavoie T, Brostjan C.The differential activity of interferon-alpha subtypes is consistent among.Cytokine 2011;53:52-59.
4.Harris J.Autophagy and cytokines.Cytokine 2011;56:140-144.
5.Ota K, Matsumiya T, Sakuraba H, Imaizumi T, Yoshida H,Kawaguchi S, et al.Interferon-alpha2b induces p21cip1/waf1 degradation and cell proliferation in HeLa cells.Cell Cycle 2010;9:131-139.
6.Zhang T, Sun HC, Zhou HY, Luo JT, Zhang BL, Wang P, et al.Interferon alpha inhibits hepatocellular carcinoma growth through inducing apoptosis and interfering with adhesion of tumor endothelial cells.Cancer Lett 2010;290:204-210.
7.Cao X, Liu B, Cao W, Zhang W, Zhang W, Zhang F, et al.Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells.Chin J Cancer Res 2013;25:212-222.
8.Zhuang PY, Shen J, Zhu XD, Zhang JB, Tang ZY, Qin LX, et al.Direct transformation of lung microenvironment by interferon-α treatment counteracts growth of lung metastasis of hepatocellular carcinoma.PLoS One 2013;8:e58913.
9.Nagano H, Kobayashi S, Marubashi S, Wada H, Eguchi H,Tanemura M, et al.Combined IFN-α and 5-FU treatment as a postoperative adjuvant following surgery for hepatocellular carcinoma with portal venous tumor thrombus.Exp Ther Med 2013;5:3-10.
10.Pasquali S, Mocellin S.The anticancer face of interferon alpha (IFN-alpha): from biology to clinical.Curr Med Chem 2010;17:3327-3336.
11.Schmeisser H, Fey SB, Horowitz J, Fischer ER, Balinsky CA,Miyake K, et al.Type I interferons induce autophagy in certain human cancer cell lines.Autophagy 2013;9:683-696.
12.Choi KS.Autophagy and cancer.Exp Mol Med 2012;44:109-120.
13.Kung CP, Budina A, Balaburski G, Bergenstock MK, Murphy M.Autophagy in tumor suppression and cancer therapy.Crit Rev Eukaryot Gene Expr 2011;21:71-100.
14.Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM,Piacentini M, et al.Beclin1: a role in membrane dynamics and beyond.Autophagy 2012;8:6-17.
杂志排行
Cancer Biology & Medicine的其它文章
- Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention
- Uptake of prostate cancer screening and associated factors among Chinese men aged 50 or more: a population-based survey
- pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment
- Mechanistic considerations for the use of monoclonal antibodies for cancer therapy
- Cancer metabolic reprogramming: importance, main features,and potentials for precise targeted anti-cancer therapies
- Instructions for Authors
