Parasitic plant in natural Boswellia papyrifera stands at Humera, Northern Ethiopia
2014-09-06AbrahamYirguAlemuGezahgneHabtemariamKassaMinilikTsega
Abraham Yirgu · Alemu Gezahgne · Habtemariam Kassa Minilik Tsega
ORIGINAL PAPER
Parasitic plant in natural Boswellia papyrifera stands at Humera, Northern Ethiopia
Abraham Yirgu · Alemu Gezahgne · Habtemariam Kassa Minilik Tsega
Received: 2013-03-29; Accepted: 2013-07-15
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
In Boswellia papyrifera (Del.) Hochst natural stands, we studied the association of parasitic plants with B. papyrifera trees from which frankincense was tapped and marketed for domestic and export markets. Data on the rate of infection of parasitic plants on B. papyrifera was collected in three transects located at separate locations around Baha kar, northern Ethiopia. Each transect had ten circular sample plots of 400 m2and separated by 100 m. Species composition, DBH, height, crown diameter, number of main, secondary and tertiary branches and number of parasitic plants on individual trees were recorded. Sixteen tree species were recorded in the combined sample plots. The parasitic plant associated with B. papyrifera was identified as Tapinanthus globiferus. This parasite infected 38% of Boswellia trees in sample plots. The infection rate of the parasitic plant varied from 1 to 33 per Boswellia tree. The infection of T. globiferus on B. papyrifera was predominantly limited to tertiary small branchlets arising from secondary branches; parasitic plants were absent on thick main and secondary branches. In all plots, infectionof T. globiferus was exclusively limited to Boswellia trees. The influence of T. globiferus parasitism on growth of Boswellia trees and its influence on yield of incense production needs further investigation. Management of natural stands for frankincense production should include measures to reduce infection by T. globiferus.
Boswellia papyrifera, parasitic plant, Tapinanthus globiferus, Humera, Ethiopia
Introduction
Boswellia papyrifera (Del.) Hochst is a deciduous, multipurpose tree species that attains heights to 16 m and has thick branches tipped with clusters of leaves and a rounded crown (Groenendijk et al. 2012; Bekele 1993). The genus is widely grown in arid regions of tropical Africa (Ogbazghi et al. 2006) such as Nigeria, Cameroun, Central African Republic, Chad, Sudan, Uganda, Eretria and Ethiopia (Vollesen 1989), and in parts of the Arabian and Indian sub-continent (Ogbazghi et al. 2006). In Ethiopia it is distributed in Tigray, Amhara, Oromiya, Afar and Benishangul-Gumuz Regional States often on steep rocky slopes, lava flows or sandy river valleys (Lemenih and Kassa 2011; Bekele 1993; Vollesen 1989). Despite its ecological and economic importance (Kassa et al. 2011; Worku et al. 2011; Rijkers et al. 2006; Gebrehiwot et al. 2003), the population of B. papyrifera declined at alarming rate as a result of expansion of agriculture, overgrazing, fire, over-tapping and improper tapping practices, and insect infection (Rijkers et al. 2006; Gebrehiwot et al. 2003).
Parasitic plants are taxonomically diverse (Shen et al. 2006) and can be classified as root or shoot parasites or hemi- or holo parasites, based on their site of attachment and presence or absence of chloroplasts, respectively. In either case the growth of parasitic plants partially or completely relies on the translocation of host plant for dissolved minerals, nutrients and water through penetration of host’s vascular systems by haustoria development (Watson 2009; Press and Phoenix 2005).
The association between parasitic flowering plants and Bos-wellia papyrifera trees in Ethiopia has not been adequately studied. Therefore, we aimed to identify parasitic species, quantify levels of infections, and describe the association of tree characteristics with infection of B. papyrifera in natural stands at Humera, northwestern Ethiopia.
Materials and methods
Study area
A field survey was conducted in September−October 2011 and 2012 in Baha ker sub-district, 50 km East of Humera town, in Qefta Humera District of western Tigray Zone, Ethiopia (Fig. 1). It was estimated that about 97,500 ha of the district were predominately covered by Boswellia papyrifera species. The area is characterized by a pronounced dry season with mean annual rainfall of 952 mm and temperature of 22.3°C (Gebrehiwot et al. 2003). In some localities, the forest of B. papyrifera was encroached by the expansion of mechanized sesame and cotton farming. This study was conducted at 797−860 m asl. At the zonal level soil types included Vertisols, Leptosols, Regosols, Luvisols and Fluvisols.
Fig. 1: Study area
Sample collection
In 2011, a reconnaissance survey was completed to identify representative sites with large Boswellia population stands in Baha kar sub-district. In the following year, three 1-km long transect lines were demarcated at three separate localities, each with 10 circular plots of 400 m2separated by 100 m from edge to edge. Tree height, crown diameter and diameter at breast height (DBH) were recorded within each study plot. Numbers of dead trees, presence or absence of tapping scars, numbers of main and secondary branches, and locations of parasitic plant infection with respect to the branches of each tree were recorded for B. papyrifera. In this study branches that arose from the main bole of the stem were defined as main; those arising from main as secondary and those arising from secondary as tertiary branches. Specimens of the parasitic plant were collected and send to the National Herbarium of Addis Ababa University for species identification.
Statistical analysis
The proportional presence of parasitic plants was calculated for B. papyrifera host trees. Levene’s test was used to test the homogeneity of variance of measured responses for trees with and without parasitic plants. Independent sample t-test was used to compare the proportion of infected and non-infected sample trees as a function of DBH, height, crown diameter, number of main and secondary branches. Pearson correlation was used to assess the relationship between presence of parasitic plants and the above parameters. One-way analysis of variance (ANOVA) was used to compare species at densities >5 trees per hectare with respect to DBH, height and crown diameter. Where the overall ANOVA detected significant difference, Post Hock mean separation procedure was used to separate the means. Data were analyzed using SPSS ver.16.
Results
Species composition in sample plots
In the 30 sample plots we identified a total of 330 trees of 16 species and 10 families (Table 1). Plots were dominated by B. papyrifera and the numbers of dead trees were three, two and one of B. papyrifera, S. kunthianum and A. seyal, respectively. All B. papyrifera trees in the sample plots had scars due to several rounds of tapping to produce frankincense. Beginning in 2011 all Boswellia trees administered by the National Gum and Resin Enterprise of Ethiopia that have been tapped for several years in succession are required to be rested for a period of five years without tapping.
Identification and characteristics of the parasitic plant
The plant parasitizing B. papyrifera was identified as Tapinanthus globiferus (A.Rich.) Tiegh. It has alternate to sub-opposite leaves with chlorophyll. The sub-globose green fruits have white spots when immature and changing to bright red berries as the plant matures. The flowers and berries of T. globiferus remain intact for some time in the dry periods where B. papyrifera shades T. globiferus leaves. Thus berries were available to birds as forage. We frequently observed initiation of several clumps of black, massive, woody swellings of T. globiferus on branches of B. papyrifera(Fig. 2). Severely infected branches were frequently dead and later fell to the ground.

Table 1: List of species in the study plots
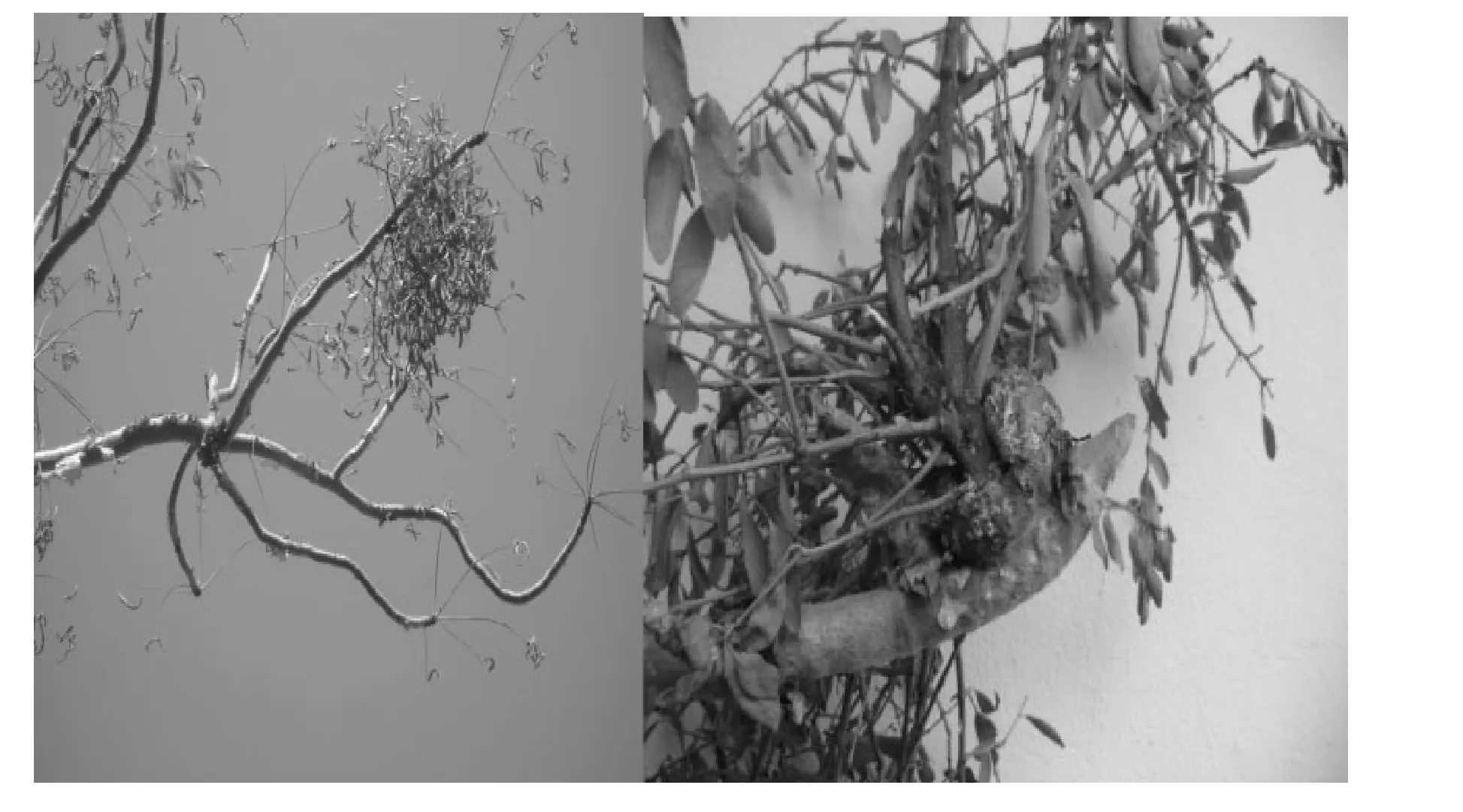
Fig. 2: Tapinanthusglobiferusinfection on branches of B. papyrifera
Parasitic plants on Boswelliapapyrifera
Of 170 B. papyrifera trees in all sample plots, 38% were infected by T. globiferus. In the preliminary non-structured random sampling conducted in 2011 at two of the three plots which were later selected for sampling sites and 41% (146/356) of Boswellia trees were similarly infected by this parasite. In both samples, infection of T. globiferus was exclusive to B. papyrifera. Infection was entirely restricted to tertiary branches, whereas none of the main and secondary branches were parasitized. Even among tertiary branches, most infections were restricted to the distal branchlets of the host trees.
Although the bivariate correlation analysis showed that pres-ence of parasitic plants on tertiary branches was significantly correlated with DBH and crown diameter (Table 2).Further analysis with the effect of DBH constant clearly showed that the presence or absence of parasitic plants on B. papyrifera was not related to height, crown diameter, or number of main and secondary branches at p<0.05 (Table 3). The absence of at least one parasitic plant in the main and secondary branches of B. papyrifera prevented calculation of bivariate correlation.
Tree heights in sample plots were similar for B. papyrifera, A. leiocarpusand S. kunthianum. Crown diameters differed significantly between B. papyrifera, A. leiocarpus, C. collinum, C. molle and Dichrostachys cinerea. A. leiocarpusand Terminalia schimperiana differed significantly in DBH (Table 4).
In Baha kar sub-district a number of avian species have been recorded, including Abyssinian Roller (Coracias abyssinica), Black-headed Oriole (Oriolus larvatus) and weavers such as Northern Red Bishop (Euplectes capensis). Among these C. abyssinica frequently landed and spent time on the branches of B. papyrifera. Bird nesting was commonly observed on the branches of Ziziphus species that grew along riversides. None of the Boswellia trees within or outside the study area had any bird nests.
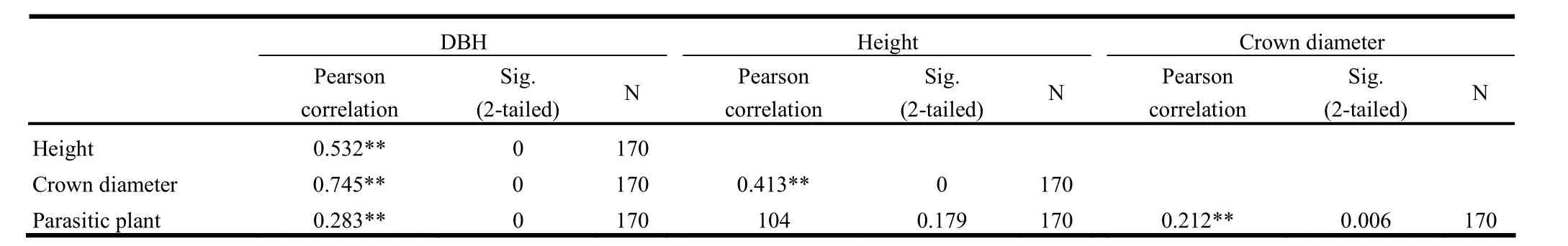
Table 2: Bivariate correlations
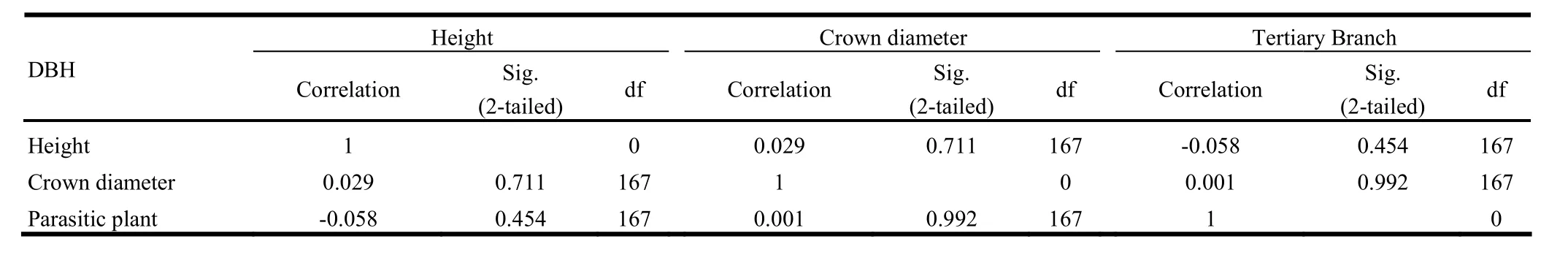
Table 3: Partial correlations

Table 4: Crown diameter multiple comparisons of species with respect to DBH, height and crown diameter
Discussion
In Baha kar sub-district nearly 40% of B. papyrifera treeswere infected by T. globiferus. Nigussie (2008) studied the same host species in another district and sub-district of Tigray and reported that T. globiferus infected Boswellia but affected different percentages of trees, viz. 13.9% in open grazed land of Qafta-Humera-Tekeze District and 63.8% in open forest and 57.4% in closed forest of Tanqua-Abergele-Jijike District. Our findings on the importance of tree height and crown diameter of B. papyrifera in T. globiferus infection agreed with Nigussie (2008) and disprove the hypothesis that host characteristics areunrelated to infection of T. globiferus in Bah kar. The greater infection of parasitic plants on the tertiary branches than on the main and secondary branches of B. papyrifera might be due to the physiological, biochemical and physical compatibility of branches and suitability of diameters of the host branch or twigs for landing and perching of fruit dispersing birds (Roxburgh and Nicolson 2005; Lopez de Buen and Ornelas 2002; Norton and Ladley 1998; Yan and Reid 1995).
T. globiferus showed greater host specificity in Ethiopia than in Burkina Faso. In Burkina Faso, T. globiferus infected nearly all woody species that are common to both countries. In Ethiopia, however, no infection was observed except in B. papyrifera. This inconsistency between the two countries might be linked to differences in host abundance (Devkota et al. 2010), tree height (Aukema and Martinez del Rio 2002), physiological, biochemical and physical compatibility of branches (Roxburgh and Nicolson 2005; Lopez de Buen and Ornelas 2002; Norton and Ladley 1998; Yan and Reid 1995), host suitability for haustorial penetration and growth (Yan 1993), abundance, availability and behavior of avian dispersers (Roxburgh and Nicolsan, 2005; Devkota et al. 2010; Martinez del Rio et al. 1996; Lopez de Buen and Ornelas 2001); the length of time the host and the parasite have been together (Norton and De Lang 1999); conspicuousness and nutritional value of mistletoe seeds (reviewed in Lopez de Buen and Ornelas 2001; Ladley and Kelly 1996); or forest disturbance and climatic factors such as light, temperature, and moisture (Devkota et al. 2010). Differences in crown diameters between trees and the availability of fruits of T. globiferus on infected B. papyrifera branches during the period of scarcity of fruits and seeds enable seed-dispersing birds to frequently visit, and re-infect Boswellia trees by depositing more T. globiferusfruits on its branches than on branches of other tree species (Gill and Hawksworth 1961; Kartoolinejad et al. 2007).
Conclusion and recommendation
In Ethiopia, several factors are now affecting the survival rate of B. papyrifera in its natural range. The additional pressure exerted by T. globiferus along with 8−12 rounds of tapping of the host during the dry and leafless season (Groenendijk et al. 2012; Gebrehiwot et al. 2003) definitely impair the growth, vigor and disease susceptibility of B. payrifera in natural stands (Watson 2009; Puustinen and Mutikainen 2001; Luttge et al. 1998). Furthermore the infection of T. globiferus on branches that produce seeds might have reduce the quality and/or quantity of seeds produced by the host. Therefore, in addition to immediate interception of the infection through pruning of infected branches, better understanding is needed of the ecophysiology of the mistletoe/host-association and its relation to environmental factors during periods of drought stress. Further study is also needed of fruit dissemination by birds.
Acknowledgment
We would like to thank Tinsae Bahiru, Mengistu Wondafrash, Merehatsedk Abebe, Temesgen Yohannes, Betelhem Fufa, Ermias, Worku Zewdie, Mersha Alemu, Girmay Fitewi and the staff of Bah akar District Natural Gum Enterprise for their support.
Aukema JE, Martinez del Rio. 2002. Where does a fruit-eating bird deposit mistletoe seeds? Seed deposition patterns and an experiment. Ecology, 83: 3489−3496.
Bekele A, Birnie A,Tengas B. 1993. Useful trees and shrubs of Ethiopia: identification, propagation, and management for agricultural and pastoral communities. Regional Soil Conservation Unit, Technical Handbook No.5. Naibori: Swedish International Development Cooperation Agency, p. 114.
Boussim IJ, Guinko S, Tuquet C, Sallé G. 2004. Mistletoes of the agroforestry parklands of Burkina Faso. Agroforestry Systems, 60 (1): 39−49.
Devkota MP, Joshi GP, Parajuli P. 2010. Diversity, distribution and host range of mistletoe in protected and unprotected areas of Central Nepal Himalas. Banko Janakari, 20:14−20
(4)课后服务经费来源。很多国家已构建起政府拨款、企业援助和家庭有限支出三大板块为主体的经费支撑模式。法国和美国的经费来源主要是学生父母缴纳的学杂费、联邦政府和地方政府的公共资金和社会捐赠;澳大利亚主要由国家政府、州政府和区域行政部门承担,还为家庭经济困难者提供政府援助;欧盟国家的支付方式则根据各国的财政情况而定,具体有免费、低收费和高收费三种模式,如爱沙尼亚、立陶宛和希腊的课后服务不收费,而爱尔兰和英国课后服务被视为私人服务,收费较高。
Devkota MP. 2005. Biology of mistletoes and their status in Nepal Himalaya. Himalayan Journal of Science, 3:85−88.
Gebrehiwot K, Muys B, Haile M, Mitloehner R. 2003. Introducing Boswelliapapyrifera (Del.) Hochst and its non-timber forest product, frankincense. International Forestry Review, 5:348–353.
Gill LS, Hawksworth FG. 1961. The mistletoes: A literature review. Washington D.C.: U.S. Dept. Agr. Tech. Bul.. 1242. U.S. Government Printing Office.
Groenendijk P, Eshete A, Sterck FJ, Zuidema PA, Bongers F. 2012. Limitations to sustainable frankincense production: blocked regeneration, high adult mortality and declining populations. Journal of Applied Ecology, 49: 164−173.
Kartoolinejad D, Hosseini SM, Mirnia SK,Akbarinia M,Shayanmehr F. 2007. The relationship among infection intensity of Viscum album sp. with some ecological parameters of host trees. International Journal of Environmental Research, 1(2): 143−149.
Kassa H,Tefera B, Fitwi G. 2011. Preliminary value chain analysis of gum and resin marketing in Ethiopia: Issues for policy and research. CIFOR Brief No. 4, March 2011. Bogor, Indonesia: CIFOR.
Ladley JJ, Kelly D. 1996. Dispersal, germination and survival of New Zealand mistletoes (Loranthaceae): Dependence on birds. New Zealand Journal of Ecology, 20: 69−79
Lemenih M, Kassa H. 2011. Management Guide for Sustainable Production of Frankincense: A Manual for Extension Workers and Companies Managing Dry Forests for Resin production and marketing. Bogor, Indonesia: CIFOR, p. 30.
Lopez de Buen LL, Ornelas JF. 2002. Host compatibility of the cloud forest mistletoe Psittacanthus schiedeanus (Loranthaceae) in Central Veracruz, Mexico. American Journal of Botany, 89(1): 95−102.
Luttge U, Haridasan M, Fernandes GW, de Mattos EA, Trimborn P, Franco AC, Caldas LS, Ziegler H. 1998. Photosynthesis of mistletoes in relation to their hosts at various sites in tropical Brazil. Tree, 12: 167−174.
Martinez del Rio C, Silva A, Medel R, Hourdequin M. 1996. Seed dispersersas disease vectors: bird transmission of mistletoe seeds to plant hosts. Ecology, 77: 912−921.
Nigussie A. 2008. The Damage of Long Horn Beetle (from Cerambycidea family) on Dry Deciduous Boswellia Woodlands in Central and Western Tigray, Northern Ethiopia. MSc Thesis, Mekele: School of Graduate Studies Faculty of Dryland Agriculture and Natural Resources, Mekele University.
Norton DA, de Lange PG. 1999. Host specificity in parasitic mistletoes (Loranthaceae) in New Zealand. Functional Ecology, 13: 552−559.
Norton DA, Ladley JJ. 1998. Establishment and early growth of Alepis flavida in relation to Nothofagus solandri branch size. New Zealand Journal of Botany, 36: 213−217.
Ogbazghi W, Rijkers T, Wessel M, Bongers F. 2006. Distribution of the frankincense tree Boswellia papyrifera in Eritrea: the role of environment and land use. Journal of Biogeography, 33: 524−535.
Press MC, Phoenix GK. 2005. Impact of parasitic plants on natural communities. New Phytologist, 166: 737−751.
Puustinen S, Mutikainen P. 2001. Host-parasite-herbivore interactions: Implications of host cyanogenesis. Ecology, 82: 2059−2071.
Rijkers T, Ogbazghi W, Wessel M, Bongers F. 2006. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. Journal of Applied Ecology, 43: 1188−1195.
Roxburgh L, Nicolson SW. 2005. Patterns of host use in two African mistletoes: the importance of mistletoe-host compatibility and avian disperser behavior. Functional Ecology, 19: 865–873.
Shen H, Ye W, Hong L, Huang H, Wang Z, Deng X, Yang Q, Xu Z. 2006. Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biology, 8: 175−185.
Vollesen K. 1989. Burseraceae. In: Hedberg I, Edwards S (eds), Flora of Ethiopia Volume 3, Pittosporaceae to Araliaceae. Nordic Journal of Botany, 12(3): 442−443.
Watson DM. 2009. Determinants of parasitic plant distribution: the role of host quality. Botany, 87: 16−21
Worku A, Lemenih M, Fetene M, Teketay D. 2011. Socio-economic importance of gum and resin resources in the dry woodlands of Borana, Southern Ethiopia. Forests, Trees and Livelihoods, 20: 137−155.
Yan Z, Reid N. 1995. Mistletoe (Amyema miquelii and A. pendulum) seedling establishment on eucalypt hosts in Easter Australia. Journal of applied ecology, 32:778−784.
Yan Z. 1993. Resistance to haustorial development of two mistletoes, Amyemapreissii (MIQ.) Tieghem and Lysianaexocarpi (Behr) Tieghem ssp. Exocarpi (Loranthaceae), on hosts and nonhost species. International Journal of Plant Sciences, 154: 386−394.
DOI 10.1007/s11676-014-0539-x
Project funding: This work was financially supported bythe Austrian Development Agency for financing CIFOR’s project in Ethiopia entitled “Supporting Community Forestry to Improve Livelihoods and to Facilitate Sustainable Management of Dry Forests in Ethiopia” (Project No. 2008/03).
The online version is available at http://www.springerlink.com
Tel.: (+251) 911184266
E-mail:abrahamyirguw@gmail.com
Habtemariam Kassa
Centers for International Forestry Research, P.O. BOX 5689, Addis Ababa, Ethiopia.
Minilik Tsega
Ethiopian Institute of Agricultural Research, P.O. BOX 2003, Addis Ababa, Ethiopia.
Corresponding editor: Yu Lei
猜你喜欢
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
- Bamboo resources of Sikkim Himalaya: diversity, distribution and utilization
- Implications of crude oil pollution on natural regeneration of plant species in an oil-producing community in the Niger Delta Region of Nigeria
- Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
- Bio-amelioration of alkali soils through agroforestry systems in central Indo-Gangetic plains of India
