有机硅溶胶-凝胶防腐蚀涂层研究进展
2014-08-29彭叔森曾志翔陈建敏乌学东
彭叔森,曾志翔,韩 金,陈建敏,乌学东
(中国科学院宁波材料技术与工程研究所 中国科学院海洋新材料与应用技术重点实验室浙江省海洋材料与防护技术重点实验室, 浙江 宁波 315201)
1 前 言
溶胶-凝胶技术是一种由金属有机化合物、金属无机化合物或它们的混合物经水解缩合过程,逐渐凝胶化及进行相应后处理,而获得氧化物或其它化合物的工艺。现代溶胶-凝胶技术的历史可以追溯到1846年,法国科学家Ebelmen发现SiCl4与乙醇混合后在湿空气中会水解形成凝胶[1]。经过近两个世纪的发展,溶胶-凝胶技术已被广泛的用于制备体材料、纤维材料、薄膜材料及纳米粉体等[2]。早期用于金属表面上的溶胶-凝胶涂层从其作用上可以分为抗氧化涂层、耐蚀涂层、附着促进层、耐刮擦涂层及绝缘涂。Guglielmi[3]于1997年撰文总结了用于金属表面的溶胶-凝胶涂层的工作,从该文献可知涂层材料多为无机材料。无机溶胶-凝胶涂层的优点是具有高的热稳定性及在金属上有好的附着性能,其优异的附着性能也使得溶胶-凝胶涂层作为一种取代铬钝化和磷化膜的技术而被广泛研究。不过,尽管无机溶胶-凝胶涂层能够提供很好的腐蚀保护效果,然而其应用受限于:①无机氧化物涂层的高内应力、高脆性使得难以获得无裂痕的厚膜(>1 μm);②需要相对的高温(400~800 ℃)才能获得较好的性能[4]。
有机-无机杂化材料兼具了有机材料和无机材料的特性,而成为新材料的研发热点[6]。通过改变无机、有机组分可以连续的改变材料的性质而获得所需的性能。无机成分可以获得好的耐刮擦、耐久性能及在金属上优异的附着性能;而有机组份提高材料的致密性、韧性以及带官能基团等。溶胶-凝胶技术是一种制备有机-无机杂化材料的优异的手段,关于这方面的工作可以参阅Wilkes等人的综述文章[7-8]。为了解决纯无机溶胶-凝胶涂层的高脆性、降低其成膜温度,有机成分被添加到体系中以来获得优异的性能。目前主要有3种途径获得有机-无机杂化溶胶-凝胶涂层[9]:简单混合有机组分和无机溶胶-凝胶,两成分间无化学键作用(图1a);利用有机聚合物/低聚物中的官能团与无机前驱体反应,两者间通过化学键连结(图1b);使用烷基烷氧基硅烷作为溶胶-凝胶前躯体或与金属醇盐、Si(OR)4一起作为起溶胶-凝胶的前躯体,所得的主链为无机Si-O-Si或Si-O-M(M为Zr或Ti)结构,有机成份为侧基悬挂在硅原子上(图1c)。在这3种途径中,以有机硅为前驱体的方法使用最为广泛。这是因为烷基烷氧基硅烷的有机活性基团多种多样、Si-OR水解反应温和、多数烷基烷氧基硅烷为商业化、批量生化产的产品。近年来,有机硅溶胶-凝胶防腐蚀涂层已成为防腐蚀材料领域的研究热点。本文从有机硅溶胶的制备的基本反应、硅烷在金属表面上的成膜机理以及在不同金属上的应用等方面进行介绍。
2 有机硅溶胶-凝胶涂层
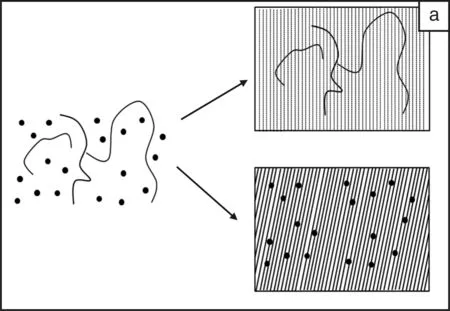
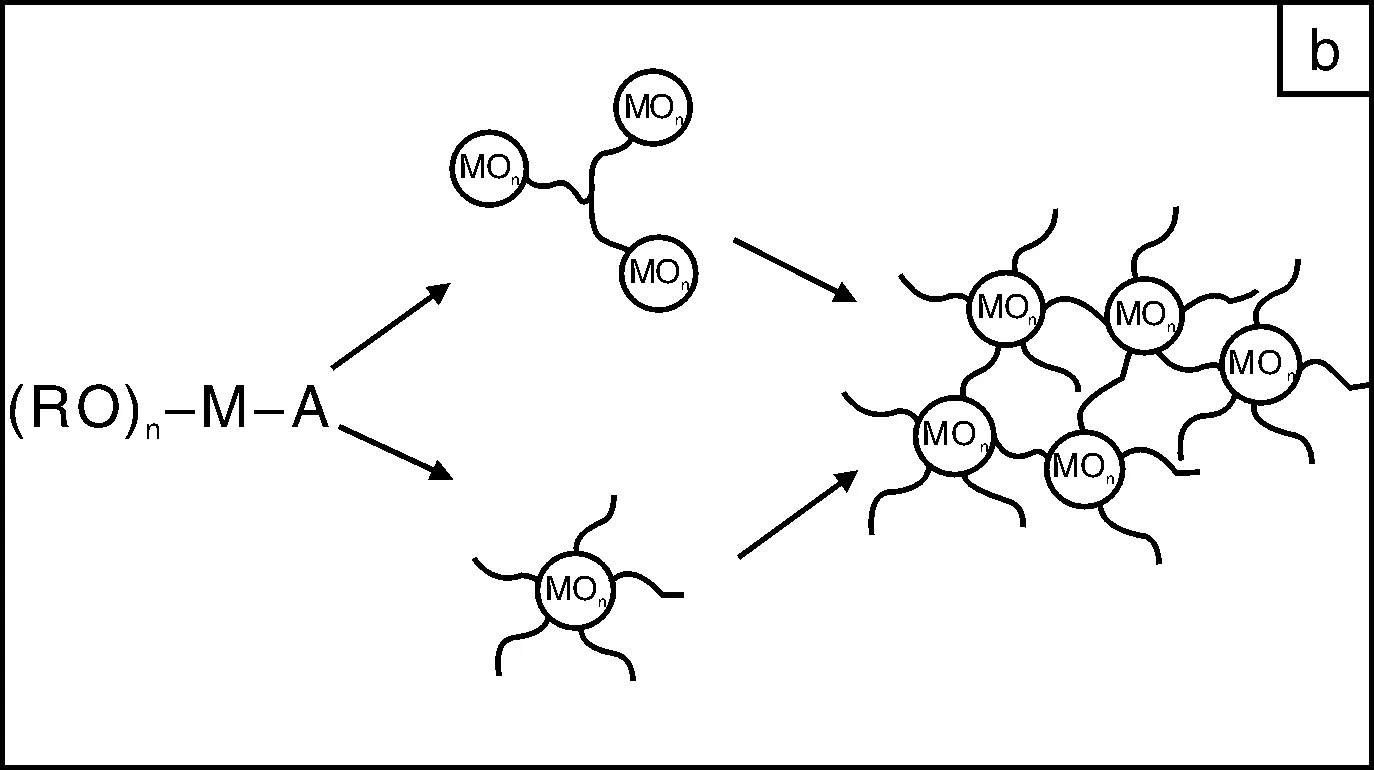
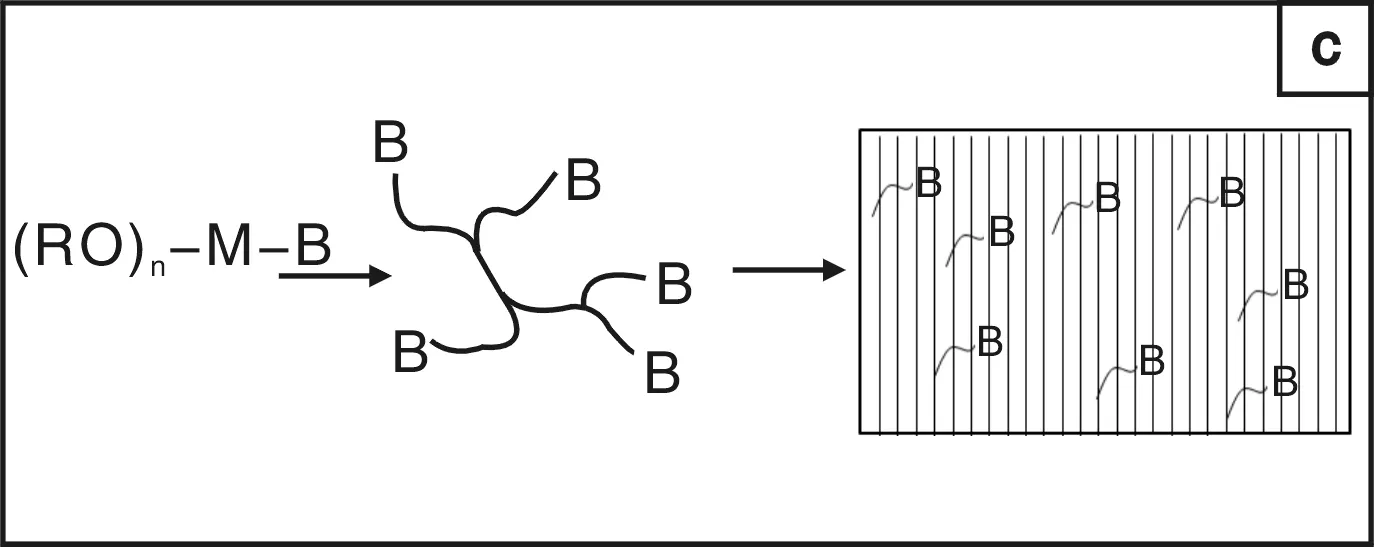
图1 溶胶-凝胶技术制备有机-无机杂化材料的结构示意图[5] Fig.1 Structural models of different classes of organic-inorganic hybrid materials formed by the sol-gel process[5]
有机硅溶胶-凝胶涂层是指以烷基烷氧基硅烷为前驱体通过溶胶-凝胶技术制备的涂层。烷基烷氧基硅烷是一类天然的有机-无机杂化分子:一端是可水解的Si-OR基团;另一端是各种有机基团如氨基、环氧、巯基、双键、异氰基官能等。这类有机硅化合物作为偶联剂而广泛应用于有机聚合物复合材料的制备、高分子化合物改性以及有机-无机杂化材料的合成等领域。图2给出了一些常见的烷基烷氧基硅烷和烷氧基硅烷的分子结构示意图。有机硅分子在溶胶-凝胶过程中主要涉及Si-OR的水解及Si-OH的缩合反应,下面专门进行介绍。
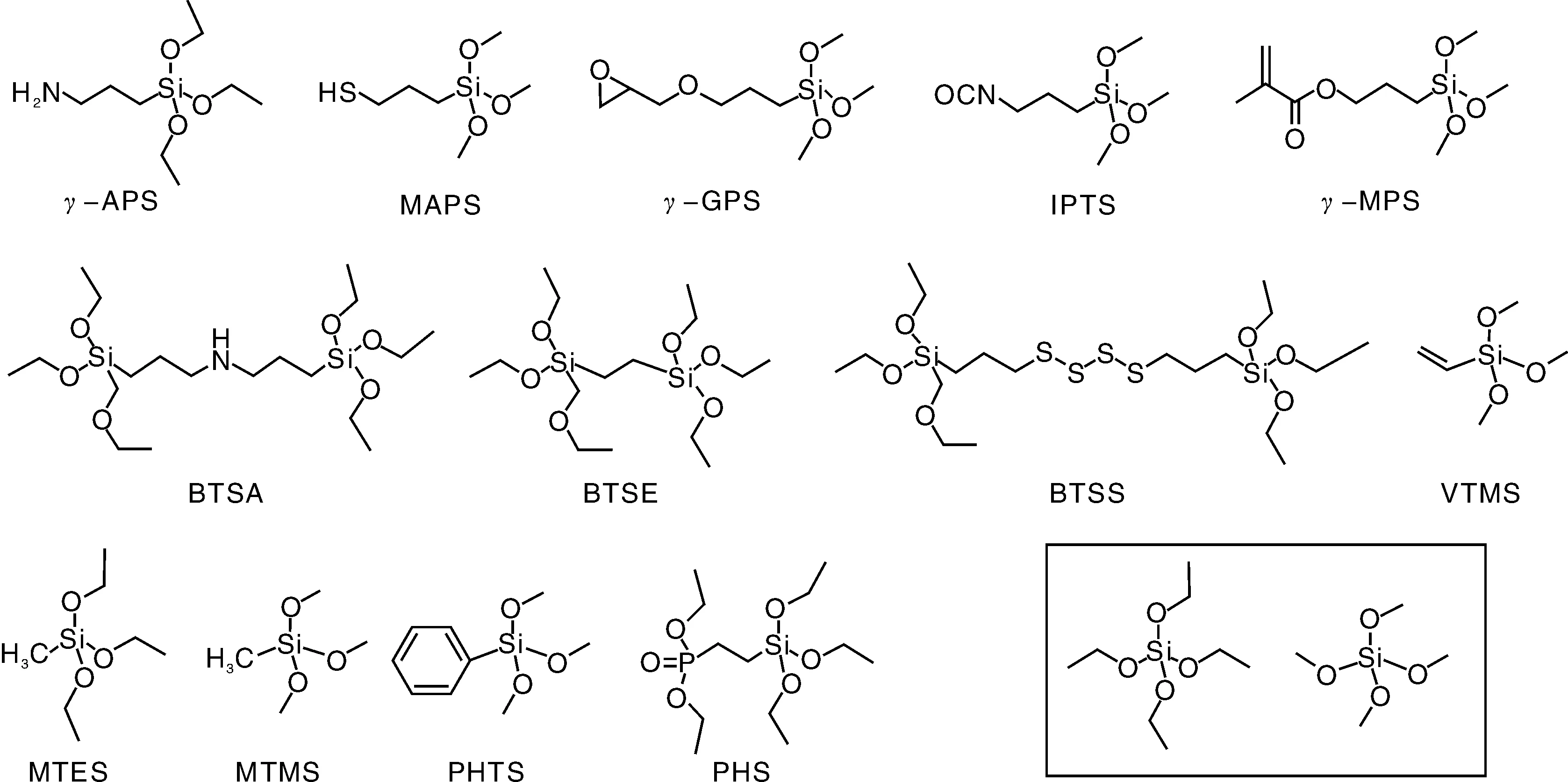
图2 一些常见烷基烷氧基硅烷和烷氧基硅烷的分子结构Fig.2 Molecular structure of alkylalkoxysilanes and alkoxysilanes
2.1 烷氧基硅烷和烷基烷氧基硅烷的反应
烷氧基硅烷在含水溶液中最为重要的反应是水解和缩合反应[10]。这两个反应会影响硅烷分子的溶解性、硅烷溶液的稳定性[11]、反应活性以及所得产物的结构,因此已有大量关于其反应机理的研究。第一篇相关的研究报道见于1844年,Ebelmen研究了四乙氧基硅烷(TEOS)的水解和缩合反应[12],并发现缩聚反应伴随水解反应的进行而进行。Konrad[13]系统的研究了水在四甲氧基硅烷(TMOS)水解中起到的作用,其结果显示当水量少于可水解基团的时,产物更偏向形成线性分子,而当水过量时产物偏向形成三维网状结构。Schmidt[14]撰文这方面的工作进行了详细的综述。
对烷基烷氧基硅烷的水解-缩合的研究也有不少的报道,如已有报道研究了甲基三乙氧基(MTES)[15],乙烯基三甲氧基硅烷(VTMS)和乙烯基三乙氧基硅烷(VTES)[15],3-缩水甘油氧基丙基三甲氧基硅烷(γ-GPS)[16],γ-GPS和胺丙基三乙基硅烷(γ-APS)混合体系[17],γ-UPS[17],甲基丙烯酰氧基丙基三甲氧基硅烷(γ-MPS)[18],苯基三乙氧基硅烷(PHTS)[19],胺丙基三乙氧基硅烷(γ-APS),1,2-二(三乙氧基硅基)乙烷(BTSE)[20],1,2-二(3-三甲氧基甲硅烷基丙基)胺(BTSA)和乙烯基三乙酰氧基(VTSA)的混合体系[21],VTSA[22],丙基三甲氧基硅烷(PTS)[23]的水解-缩合反应。研究手段多为IR,Raman,1H-NMR,29Si-NMR等。总的来说,影响烷基烷氧基硅烷的反应活性的因素[24]主要有烷氧基的体积效应[25]、连接在硅原子上的烷基、有机溶剂、溶液浓度、pH值及温度。
2.2 硅烷在金属表面上的成键机理
硅烷在金属表面的成键机理一般认为是硅醇能和金属表面上的羟基通过脱水反应形成Si-O-Me键,其反应如图3所示。该机理由Plueddemann[26]提出,也为已有的一些研究所证实。如Getting等[27]利用SIMS和XPS研究了γ-GPS在钢材上形成薄膜。SIMS结果显示在硅烷处理过的钢材上得到Fe-O-Si+峰,因此他们称硅烷在钢上形成Si-O-Fe键而结合到铁基体上。Watts等[28]用SIMS和XPS研究了γ-GPS在铁基体上形成的硅烷薄膜,同样发现Fe-O-Si+的存在。在γ-GPS处理过的铝合金表面上,Leung等[29]通过XPS分析得到了Si-O-Al的信号。Teo等[30]用SIMS研究了经BTSE处理的铝表面发现了Al-O-Si+的峰。Fang等[31]用SIMS和XPS研究了γ-GPS处理过的铝合金表面,获得了Al-O-Si+的信息。从文献报道来看,关于硅烷在金属表面上的成键机理还需进一步的研究,还需要更多的数据支持。
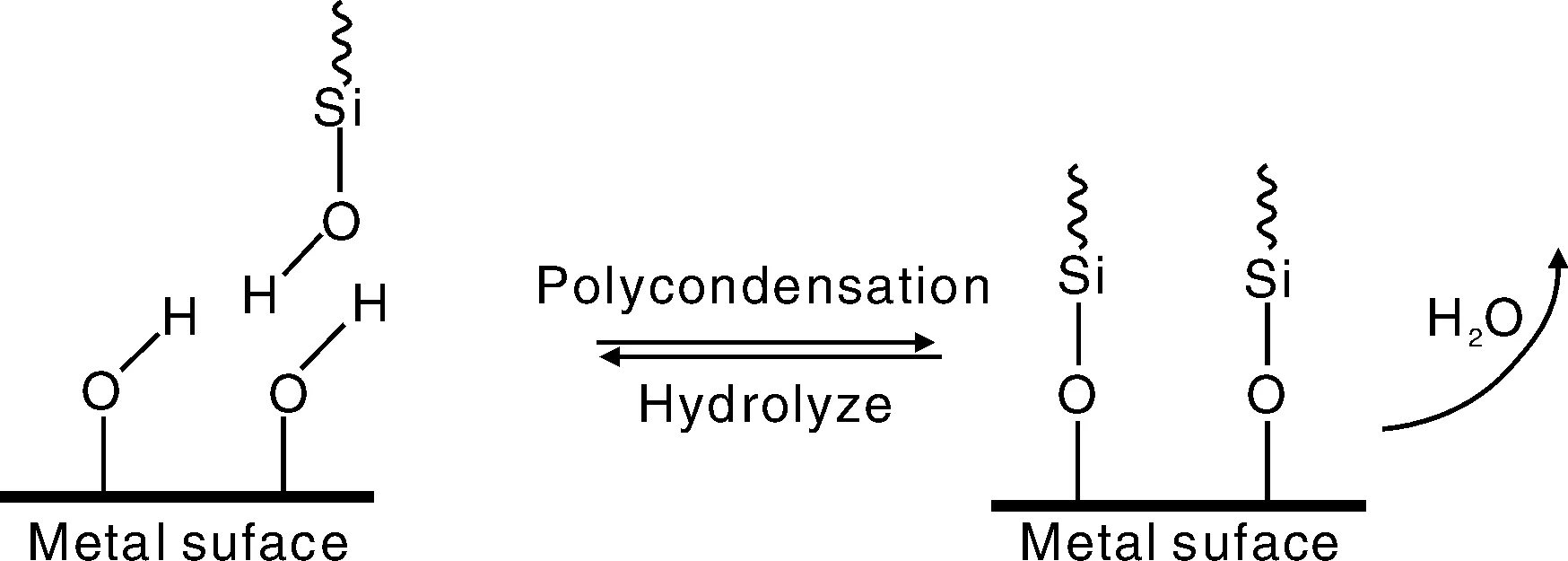
图3 硅羟基和金属表面上的羟基的反应示意图Fig.3 Schematic representation of condensation of silanol with hydroxyl groups on the metal surface
2.3 有机硅溶胶-凝胶涂层在不同金属上的应用
Schmidt等[32]在1994年撰文介绍了有机-无机杂化溶胶-凝胶涂层在不同场合下的应用,其中提到有机硅溶胶-凝胶涂层用作金属防腐涂层。1997年,Pilz等[33]采用多种硅烷制备了用于青铜户外保护的溶胶-凝胶涂层。1998年,Langenfeld等[34]研究了基于γ-GPS的溶胶-凝胶涂层对镁、铝、锌、黄铜的保护。1999年,Metroke等[35]以γ-GPS和TEOS为前驱体制备了杂化溶胶-凝胶涂层,并用盐雾测试评估了该涂层对铝合金的保护效果。进入2000年后,有机硅溶胶-凝胶涂层也得到了飞速发展。表1给出了常用硅烷及其它前驱体的缩写及化学名称。
表2总结了2000年后有关于有机硅溶胶-凝胶防腐蚀涂层的文献报道。文献按照金属基体进行了分类并列出了所用的前驱体(缩写及化学名称见表1)及添加物。从金属基体看有机硅溶胶-凝胶防腐蚀涂层主要应用于铝基、铁基、铜基及镁基材料。其中在铝基材料研究最多,在纯铝及各种型号的铝合金上都有相关的研究。铁基材料除纯铁外,还涉及碳钢、不锈钢及镀锌铁。在铜基材料上主要为紫铜。随着近年来镁合金的应用推广,利用有机硅溶胶-凝胶涂层对镁合金的防护研究也日趋增多。研究报道的多少在一定程度上也反映了有机硅溶胶-凝胶涂层在该金属基材料上应用情况。从应用上看,在铝合金最为成功,国外已有取代铬钝化的应用,在铜上的效果较差。这是因为涂层的保护性能取决于涂层在金属表面上的附着性能,而有机硅溶胶-凝胶涂层与金属间的附着取决于是否易形成Si-O-Metal键。已有的研究证实Si-O-Al易形成[31],而Si-O-Cu是难以形成的[36]。
从有机基团的角度可以将有机硅溶胶-凝胶涂层分为以下几大类:①有机基团为非活性基团的甲基、苯基,如以MTMS[37],PhTS[38]为前驱体制备的涂层等;②有机活性基团带有环氧基团,烷基烷氧基硅烷多为γ-GPS;③有机活性基团带有双键,烷基烷氧基硅烷多为γ-MPS或VTMS;④有机活性基团带有氨基,如以γ-APS为前驱体[39]。第一类适合作为单独的保护涂层,如MTES/TEOS的溶胶-凝胶涂层具有优异的耐高温特性[40]。而带有环氧、双键、氨基的涂层,能够和有机涂层通过这些基团形成化学键,因此除作为单独的保护涂层,还适合作为有机涂层的打底涂层提高有机涂层在金属上的附着性能。除这些基团,一些含有特殊基团的涂层也被设计。如Khramov等[41]以PHS和TEOS为前躯体制备了含有膦基的涂层,该涂层可以通过膦基和镁合金形成化学键;含巯基的涂层被用来获得在铜上有好的性能的[42-44]基于巯基可以和铜形成Cu-S-C键。
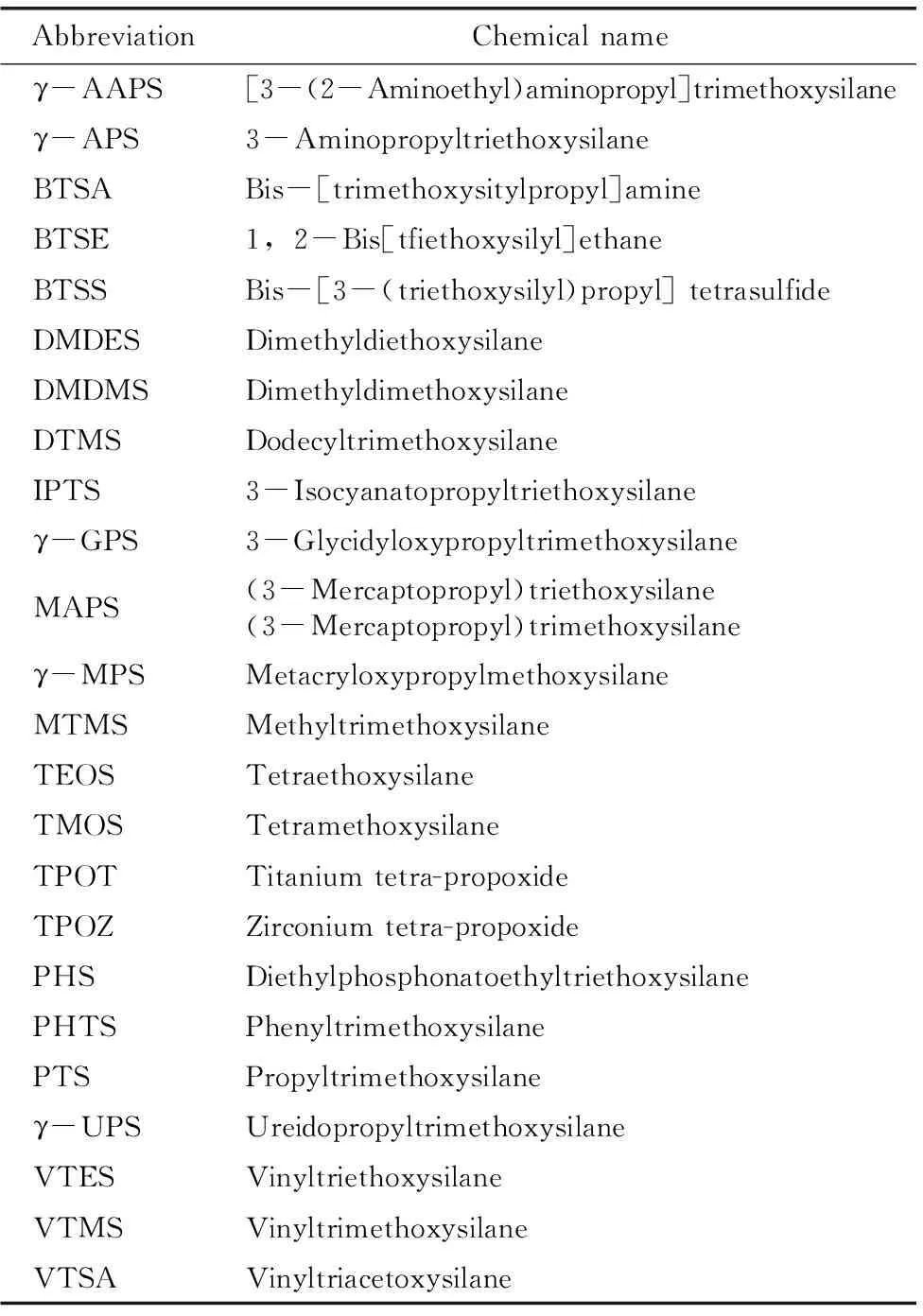
表1 常用硅烷及其它前驱体的缩写及化学名称
为了进一步提高有机硅溶胶-凝胶涂层的防腐蚀性能有机和无机缓蚀剂[45-46]被添加到涂层中。缓蚀剂的浓度在一定的范围内可以有效的增强涂层的性能,但是当浓度过高时会破坏溶胶-凝胶涂层的结构而降低性能[47]。一些报道还研究了微米、纳米颗粒对涂层性能的影响[48-49]。
2.4 有机硅溶胶-凝胶涂层在海洋防腐蚀的应用
尽管目前有关于有机硅溶胶-凝胶涂层的报道还集中于涂层的设计及对不同金属的保护效果的评价,直接针对海洋相关领域的应用研究还非常少见。但有机硅溶胶-凝胶涂层优异的耐热、力学性能、耐腐蚀、附着性能以及灵活的施工手段使得其在一些特殊的场合有大的应用前景。如对耐热、耐刮擦有一定要求,但一般有机涂层无法满足要求的场合,或者其它保护如喷涂技术无法施工的地方。当然,有机硅溶胶-凝胶涂层在海洋相关领域的应用还需结合实际情况具体分析和探讨。
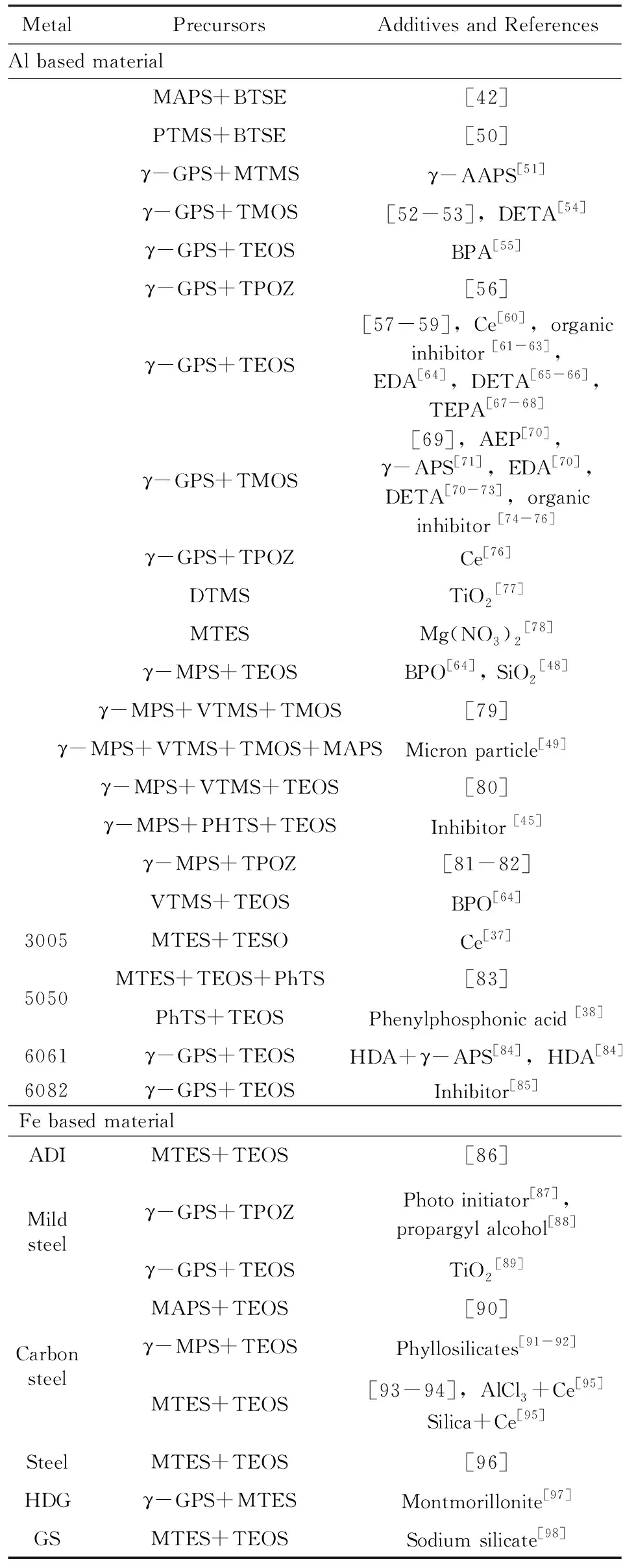
表2 不同金属表面上的有机硅溶胶-凝胶防蚀腐涂层
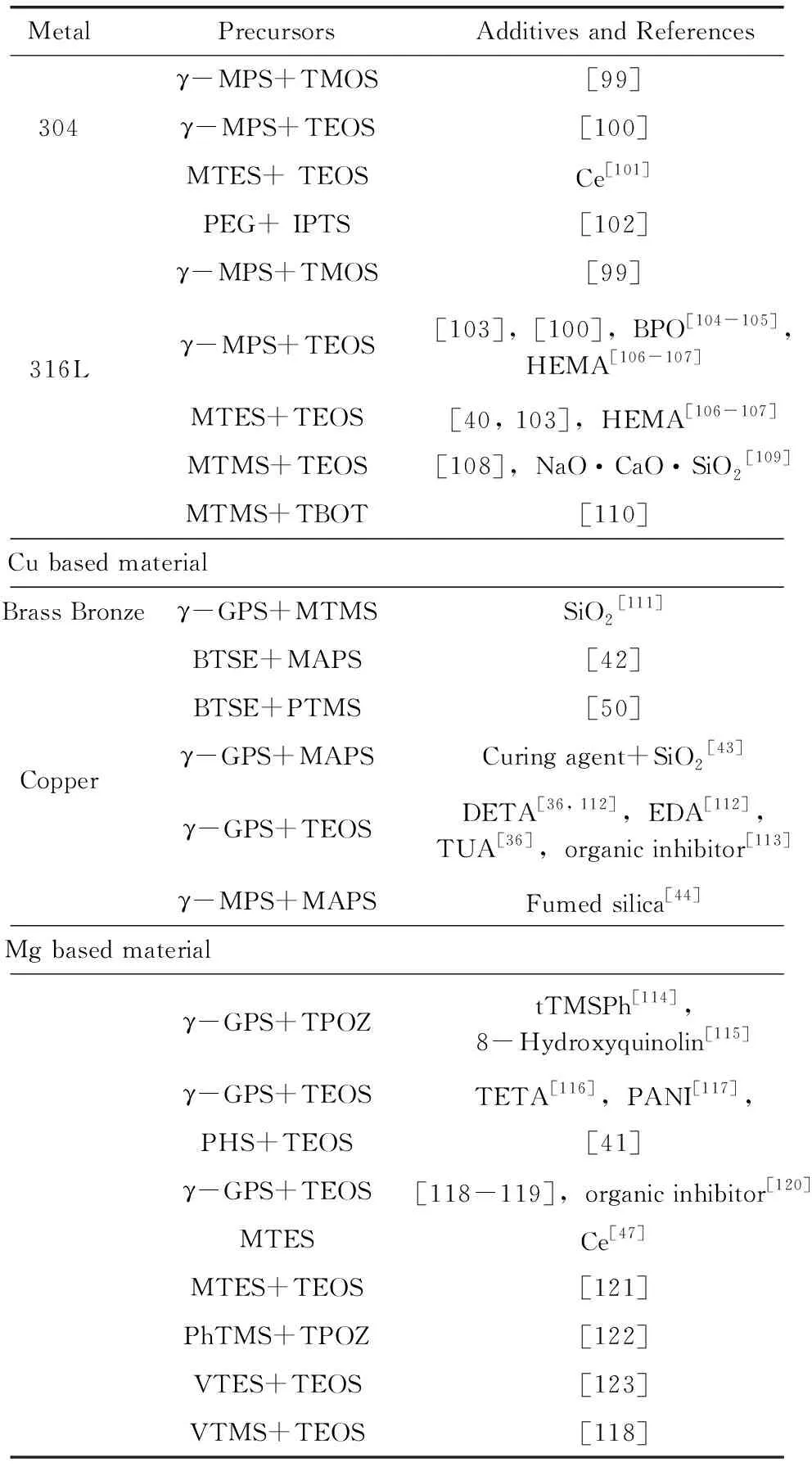
(接下表) (续表)
3 结 语
有机硅溶胶-凝胶涂层因其特殊的性能而广泛用于金属材料的防腐蚀,应用前景非常广阔。目前,市面上已有些商业化的产品。但有机硅溶胶-凝胶涂层也面临一些亟需解决问题及值得继续研究的方向:
(1)多数有机硅溶胶-凝胶涂层体系含有大量醇,醇的来源有两类:一是作为溶剂额外添加的,二是前驱体水解反应产生的。大量醇的存在不但会带来安全隐患,还面临VOC排放的问题。因此,开发水基/低VOC有机硅溶胶-凝胶涂层非常有意义。
(2)借鉴有机缓蚀剂在金属表面上化学吸附的工作原理,设计含有特殊官能基团的有机硅溶胶-凝胶涂层,通过有机基团和金属的相互作用增强涂层在金属表面(特别是铜)上的附着能力以及具有“自修复”功能的涂层,扩大有机硅溶胶-凝胶涂层的适用范围及提升其保护效果。
参考文献 References
[1] Brinker C J, Scherer G W.Sol-GelScience:thePhysicsandChemistryofSol-GelProcessing[M]. New York: Academic Press, 1990.
[2] Huang Jianfeng(黄剑锋).PrincipleandTechnologyofSol-Gel(溶胶-凝胶原理与技术) [M]. Beijing: Chemical Industry Press, 2005.
[3] Guglielmi M. Sol-Gel Coatings on Metals [J].JournalofSol-GelScienceandTechnology, 1997, 8 (1-3): 443-449.
[4] Wang D, Bierwagen G R. Sol-Gel Coatings on Metals for Corrosion Protection [J].ProgressinOrganicCoatings, 2009, 64 (4): 327-338.
[5] Zheludkevich M L, Salvado I M, Ferreira M G S. Sol-Gel Coatings for Corrosion Protection of Metals [J].JournalofMaterialsChemistry, 2005, 15 (48): 5 099-5 111.
[6] Sanchez C, Soler-Illia G J D A, Ribot F,etal. Designed Hybrid Organic-Inorganic Nanocomposites from Functional Nanobuilding Blocks [J].ChemistryofMaterials, 2001, 13 (10): 3 061-3 083.
[7] Wen J Y, Wilkes G L. Organic/Inorganic Hybrid Network Materials by the Sol-Gel Approach [J].ChemistryofMaterials, 1996, 8 (8): 1 667-1 681.
[8] Schottner G. Hybrid Sol-Gel-Derived Polymers: Applications of Multifunctional Materials [J].ChemistryofMaterials, 2001, 13 (10): 3 422-3 435.
[9] Mammeri F, Bourhis E L, Rozes L,etal. Mechanical Properties of Hybrid Organic-Inorganic Materials [J].JournalofMaterialsChemistry, 2005, 15 (35-36): 3 787-3 811.
[10] Osterholtz F D, Pohl E R. Kinetics of the Hydrolysis and Condensation of Organofunctional Alkoxysilanes: a Review [J].JournalofAdhesionScienceandTechnology, 1992, 6 (1): 127-149.
[11] Arkles B, Steinmetz J R, Zazyczny J,etal. Factors Contributing to the Stability of Alkoxysilanes in Aqueous-Solution [J].JournalofAdhesionScienceandTechnology, 1992, 6 (1): 193-206.
[12] Ebelmen. Untersuchungen ber Die Verbindungen Der Borsãure Und Kieselsäure Mit Aether [J].JustusLiebigsAnnalenderChemie, 1846, 57 (3): 319-355.
[13] Konrad E, Bächle O, Signer R. ber Polymere Kieselsäureester. 19. Mitteilung ber Hochpolymere Verbindungen [J].JustusLiebigsAnnalenderChemie, 1929, 474 (1): 276-295.
[14] Schmidt H, Scholze H, Kaiser A. Principles of Hydrolysis and Condensation Reaction of Alkoxysilanes [J].JournalofNon-CrystallineSolids, 1984, 63 (1-2): 1-11.
[15] Brunet F. Polymerization Reactions in Methyltriethoxysilane Studied through Si-29 Nmr with Polarization Transfer [J].JournalofNon-CrystallineSolids, 1998, 231 (1-2): 58-77.
[16] Premachandra J K, Van Ooij W J, Mark J E. Reaction Kinetics of Gamma-Ureidopropyltrimethoxysilane in the Water-Methanol System Studied by Ftir Spectroscopy [J].JournalofAdhesionScienceandTechnology, 1998, 12 (12): 1 361-1 376.
[17] Riegel B, Blittersdorf S, Kiefer W,etal. Kinetic Investigations of Hydrolysis and Condensation of the Glycidoxypropyltrimethoxysilane/Aminopropyltriethoxy-Silane System by Means of Ft-Raman Spectroscopy I [J].JournalofNon-CrystallineSolids, 1998, 226 (1-2): 76-84.
[18] Pantoja M, Velasco F, Broekema D,etal. The Influence of Ph on the Hydrolysis Process of Gamma-Methacryloxypropyltrimethoxysilane, Analyzed by Ft-Ir, and the Silanization of Electrogalvanized Steel [J].JournalofAdhesionScienceandTechnology, 2010, 24 (6): 1 131-1 143.
[19] Kuniyoshi M, Takahashi M, Tokuda Y,etal. Hydrolysis and Polycondensation of Acid-Catalyzed Phenyltriethoxysilane (Phtes) [J].JournalofSol-GelScienceandTechnology, 2006, 39 (2): 175-183.
[20] Diaz-Benito B, Velasco F, Martinez F J,etal. Hydrolysis Study of Bis-1,2-(Triethoxysilyl)Ethane Silane by Nmr [J].ColloidsandSurfacesA-PhysicochemicalandEngineeringAspects, 2010, 369 (1-3): 53-56.
[21] Metroke T, Wang Y M, Van Ooij W J,etal. Chemistry of Mixtures of Bis-Trimethoxysilylpropyl Amine and Vinyltriacetoxysilane: an Nmr Analysis [J].JournalofSol-GelScienceandTechnology, 2009, 51 (1): 23-31.
[22] Dubitsky Y, Zaopo A, Zannoni G,etal. H-1 Nmr Study of the Hydrolysis of Vinyltrialkoxysilanes [J].MaterialsChemistryandPhysics, 2000, 64 (1): 45-53.
[23] Li Fangwen (李方文), Wu Jianfeng (吴建锋), Xu Xiaohong (徐晓虹),etal. 丙基三甲氧基硅烷的水解 [J].ChemicalIndustryandEngineering(化学工业与工程), 2008, 25 (3): 203-207.
[24] Van Ooij W, Zhu D, Stacy M,etal. Corrosion Protection Properties of Organofunctional Silanes——an Overview [J].TsinghuaScience&Technology, 2005, 10 (6): 639-664.
[25] Pohl E R, Chaves A.StericallyHinderedSilanesforWaterborneSystems:aModelStudyofSilaneHydrolysis[C]. United States:CRC Press, 2004: 3-9.
[26] Pan G, Schaefer D W, Van Ooij W J,etal. Morphology and Water Resistance of Mixed Silane Films of Bis 3-(Triethoxysilyl) Propyl Tetrasulfide and Bis-Trimethoxysilylpropyl Amine [J].ThinSolidFilms, 2006, 515 (4): 2 771-2 780.
[27] Gettings M, Kinloch A J. Surface Analysis of Polysiloxane Metal Oxide Interfaces [J].JournalofMaterialsScience, 1977, 12 (12): 2 511-2 518.
[28] Davis S J, Watts J F. Organization of Methoxysilane Molecules on Iron [J].InternationalJournalofAdhesionandAdhesives, 1996, 16 (1): 5-15.
[29] Susac D, Leung C W, Sun X,etal. Comparison of a Chromic Acid and a Btse Final Rinse Applied to Phosphated 2024-T3 Aluminum Alloy [J].Surface&CoatingsTechnology, 2004, 187 (2-3): 216-224.
[30] Teo M, Kim J, Wong P C,etal. Pre-Treatments Applied to Oxidized Aluminum Surfaces to Modify the Interfacial Bonding with Bis-1,2-(Triethoxysilyl)Ethane (Btse)-Part I. High-Purity Al with Native Oxide [J].AppliedSurfaceScience, 2005, 252 (5): 1 293-1 304.
[31] Fang J, Flinn B J, Leung Y L,etal. A Characterization of the Gamma-Glycidoxypropyltrimethoxysilane and Aluminium Interface by Sims and Xps [J].JournalofMaterialsScienceLetters, 1997, 16 (20): 1 675-1 676.
[32] Kasemann R, Schmidt H. Coatings for Mechanical and Chemical Protection Based on Organic-Inorganic Sol-Gel Nanocomposites [J].NewJournalofChemistry, 1994, 18 (10): 1 117-1 123.
[33] Pilz M, Romich H. Sol-Gel Derived Coatings for Outdoor Bronze Conservation [J].JournalofSol-GelScienceandTechnology, 1997, 8 (1-3): 1 071-1 075.
[34] Langenfeld S, Jonschker G, Schmidt H. New Sol-Gel Based Coatings as Corrosion-and Wear-Protection on Non-Ferrous Metals [J].MaterialwissenschaftundWerkstofftechnik, 1998, 29 (1): 23-29.
[35] Metroke T L, Parkhill R L, Knobbe E T. Synthesis of Hybrid Organic-Inorganic Sol-Gel Coatings for Corrosion Resistance [J].Organic/InorganicHybridMaterialsII, 1999, 576: 293-298.
[36] Peng S, Zeng Z, Zhao W,etal. Synergistic Effect of Thiourea in Epoxy Functionalized Silica Sol-Gel Coating for Copper Protection [J].Surface&CoatingsTechnology, 2012, 213: 175-192.
[37] Pepe A, Aparicio M, Cere S,etal. Preparation and Characterization of Cerium Doped Silica Sol-Gel Coatings on Glass and Aluminum Substrates [J].JournalofNon-CrystallineSolids, 2004, 348: 162-171.
[38] Sheffer M, Groysman A, Starosvetsky D,etal. Anion Embedded Sol-Gel Films on Al for Corrosion Protection [J].CorrosionScience, 2004, 46 (12): 2 975-2 985.
[39] Osborne J H, Du Y J, Damron M,etal. Inorganic/Organic Hybrid Coatings for Aircraft Aluminum Alloy Substrates [J].ProgressinOrganicCoatings, 2001, 41 (4): 226-232.
[40] Gallardo J, Duran A, De Damborenea J J. Electrochemical and in Vitro Behaviour of Sol-Gel Coated 316l Stainless Steel [J].CorrosionScience, 2004, 46 (4): 795-806.
[41] Khramov A N, Balbyshev V N, Kasten L S,etal. Sol-Gel Coatings with Phosphonate Functionalities for Surface Modification of Magnesium Alloys [J].ThinSolidFilms, 2006, 514 (1-2): 174-181.
[42] Li Y S, Lu W, Wang Y,etal. Studies of (3-Mercaptopropyl)Trimethoxylsilane and Bis(Trimethoxysilyl)Ethane Sol-Gel Coating on Copper and Aluminum [J].SpectrochimicaActaPartA-MolecularandBiomolecularSpectroscopy, 2009, 73 (5): 922-928.
[43] Tan A L K, Soutar A M. Hybrid Sol-Gel Coatings for Corrosion Protection of Copper [J].ThinSolidFilms, 2008, 516 (16): 5 706-5 709.
[44] Soutar A, Rabaud B, Qian M,etal. Mechanical and Electrochemical Properties of Hybrid Sol-Gel Protective Coatings for Copper Substrates [J].SIMTechtechnicalreports, 2005, 6 (1): 50-54.
[45] Raps D, Hack T, Wehr J,etal. Electrochemical Study of Inhibitor-Containing Organic-Inorganic Hybrid Coatings on Aa2024 [J].CorrosionScience, 2009, 51 (5): 1 012-1 021.
[46] Barranco V, Carmona N, Galvan J C,etal. Electrochemical Study of Tailored Sol-Gel Thin Films as Pre-Treatment Prior to Organic Coating for Az91 Magnesium Alloy [J].ProgressinOrganicCoatings, 2010, 68 (4): 347-355.
[47] Correa P S, Malfatti C F, Azambuja D S. Corrosion Behavior Study of Az91 Magnesium Alloy Coated with Methyltriethoxysilane Doped with Cerium Ions [J].ProgressinOrganicCoatings, 2011, 72 (4): 739-747.
[48] Rosero-Navarro N C, Pellice S A, Castro Y,etal. Improved Corrosion Resistance of Aa2024 Alloys through Hybrid Organic-Inorganic Sol-Gel Coatings Produced from Sols with Controlled Polymerisation [J].Surface&CoatingsTechnology, 2009, 203 (13): 1 897-1 903.
[49] Metroke T L, Kachurina O, Knobbe E T. Particle-Doped Organic-Inorganic Hybrid Coatings as Corrosion Inhibiting Surface Treatments for Aluminum Alloys [J].MaterialsResearchSocietySymposiumProceedings, 2002, 726: 395-400.
[50] Li Y S, Tran T, Xu Y,etal. Spectroscopic Studies of Trimetoxypropylsilane and Bis(Trimethoxysilyl) Ethane Sol-Gel Coatings on Aluminum and Copper [J].SpectrochimicaActaPartA-MolecularandBiomolecularSpectroscopy, 2006, 65 (3-4): 779-786.
[51] Pathak S S, Khanna A S. Synthesis and Performance Evaluation of Environmentally Compliant Epoxysilane Coatings for Aluminum Alloy [J].ProgressinOrganicCoatings, 2008, 62 (4): 409-416.
[52] Voevodin N, Buhrmaster D, Balbyshev V,etal. Nonchromated Coating Systems for Corrosion Protection of Aircraft Aluminum Alloys [J].MaterialsPerformance, 2006, 45 (11): 48-51.
[53] Donley M S, Mantz R A, Khramov A N,etal. The Self-Assembled Nanophase Particle (Snap) Process: a Nanoscience Approach to Coatings [J].ProgressinOrganicCoatings, 2003, 47 (3-4): 401-415.
[54] Kasten L S, Balbyshev V N, Donley M S. Surface Analytical Study of Self-Assembled Nanophase Particle (Snap) Surface Treatments [J].ProgressinOrganicCoatings, 2003, 47 (3-4): 214-224.
[55] Zandi-zand R, Ershad-langroudi A, Rahimi A. Silica Based Organic-Inorganic Hybrid Nanocomposite Coatings for Corrosion Protection [J].ProgressinOrganicCoatings, 2005, 53 (4): 286-291.
[56] Feng Z, Liu Y, Thompson G E,etal. Sol-Gel Coatings for Corrosion Protection of 1050 Aluminium Alloy [J].ElectrochimicaActa, 2010, 55 (10): 3 518-3 527.
[57] Parkhill R L, Knobbe E T, Donley M S. Application and Evaluation of Environmentally Compliant Spray-Coated Ormosil Films as Corrosion Resistant Treatments for Aluminum 2024-T3 [J].ProgressinOrganicCoatings, 2001, 41 (4): 261-265.
[58] Tavandashti N P, Sanjabi S, Hahrabi T S. Corrosion Protection Evaluation of Silica/Epoxy Hybrid Nanocomposite Coatings to Aa2024 [J].ProgressinOrganicCoatings, 2009, 65 (2): 182-186.
[59] Metroke T L, Kachurina O, Knobbe E T. Spectroscopic and Corrosion Resistance Characterization of Glymo-Teos Ormosil Coatings for Aluminum Alloy Corrosion Inhibition [J].ProgressinOrganicCoatings, 2002, 44 (4): 295-305.
[60] Kasten L S, Grant J T, Grebasch N,etal. An Xps Study of Cerium Dopants in Sol-Gel Coatings for Aluminum 2024-T3 [J].Surface&CoatingsTechnology, 2001, 140 (1): 11-15.
[61] Khramov A N, Voevodin N N, Balbyshev V N,etal. Hybrid Organo-Ceramic Corrosion Protection Coatings with Encapsulated Organic Corrosion Inhibitors [J].ThinSolidFilms, 2004, 447: 549-557.
[62] Shi H W, Liu F C, Han E H. Characterization of Self-Assembled Nano-Phase Silane-Based Particle Coating [J].TransactionsofNonferrousMetalsSocietyofChina, 2010, 20 (10): 1 928-1 935.
[63] Khramov A N, Balbyshev V N, Mantz R A. Protection of Aluminum Alloys via Hybrid Sol-Gel Coatings with Encapsulated Organic Corrosion Inhibitors[J].ResearchthroughInnovationandTechnology, 2006: 661-666.
[64] Liu Y, Sun D Z, You H,etal. Corrosion Resistance Properties of Organic-Inorganic Hybrid Coatings on 2024 Aluminum Alloy [J].AppliedSurfaceScience, 2005, 246 (1-3): 82-89.
[65] Roussi E, Tsetsekou A, Tsiourvas D,etal. Novel Hybrid Organo-Silicate Corrosion Resistant Coatings Based on Hyperbranched Polymers [J].Surface&CoatingsTechnology, 2011, 205 (10): 3 235-3 244.
[66] Voevodin N N, Kurdziel J W, Mantz R. Corrosion Protection for Aerospace Aluminum Alloys by Modified Self-Assembled Nanophase Particle (Msnap) Sol-Gel [J].Surface&CoatingsTechnology, 2006, 201 (3-4): 1 080-1 084.
[67] Wu K H, Chang T C, Yang C C,etal. Dynamics and Corrosion Resistance of Amine-Cured Organically Modified Silicate Coatings on Aluminum Alloys [J].ThinSolidFilms, 2006, 513 (1-2): 84-89.
[68] Wu K H, Li M C, Yang C C,etal. Domain Size and Thermal Stability of Amine-Cured Hybrid Films as Corrosion Resistance Treatments for Aluminum Alloy [J].JournalofNon-CrystallineSolids, 2006, 352 (26-27): 2 897-2 904.
[69] Donley M S, Balbyshev V N, Oevodin N N V. Self-Assembled Nanophase Particle (Snap) Surface Treatments for Corrosion Protection of Aa2024-T3 [J].ProgressinOrganicCoatings, 2005, 52 (1): 34-38.
[70] Vreugdenhil A J, Gelling V J, Oods M E W,etal. The Role of Crosslinkers in Epoxy-Amine Crosslinked Silicon Sol-Gel Barrier Protection Coatings [J].ThinSolidFilms, 2008, 517 (2): 538-543.
[71] Khramov A N, Balbyshev V N, Voevodin N N,etal. Nanostructured Sol-Gel Derived Conversion Coatings Based on Epoxy-and Amino-Silanes [J].ProgressinOrganicCoatings, 2003, 47 (3-4): 207-213.
[72] Vreugdenhil A J, Balbyshev V N, Donley M S. Nanostructured Silicon Sol-Gel Surface Treatments for Al 2024-T3 Protection [J].JournalofCoatingsTechnology, 2001, 73 (915): 35-43.
[73] Metroke T L, Kachurina O, Knobbe E T. Spectroscopic and Corrosion Resistance Characterization of Amine and Super Acid-Cured Hybrid Organic-Inorganic Thin Films on 2024-T3 Aluminum Alloy [J].ProgressinOrganicCoatings, 2002, 44 (3): 185-199.
[74] Voevodin N N, Balbyshev V N, Khobaib M,etal. Nanostructured Coatings Approach for Corrosion Protection [J].ProgressinOrganicCoatings, 2003, 47 (3-4): 416-423.
[75] Khramov A, Voevodin N N, Balbyshev V N,etal. Sol-Gel-Derived Corrosion-Protective Coatings with Controllable Release of Incorporated Organic Corrosion Inhibitors [J].ThinSolidFilms, 2005, 483 (1-2): 191-196.
[76] Yasakau K A, Zheludkevich M L, Karavai O V,etal. Influence of Inhibitor Addition on the Corrosion Protection Performance of Sol-Gel Coatings on Aa2024 [J].ProgressinOrganicCoatings, 2008, 63 (3): 352-361.
[77] Li M, Yang Y Q, Liu L,etal. Electro-Assisted Preparation of Dodecyltrimethoxysilane/Tio(2) Composite Films for Corrosion Protection of Aa2024-T3 (Aluminum Alloy) [J].ElectrochimicaActa, 2010, 55 (8): 3 008-3 014.
[78] Varma P C R, Duffy B, Cassidy J. Influence of Magnesium Nitrate on the Corrosion Performance of Sol-Gel Coated Aa2024-T3 Aluminium Alloy [J].Surface&CoatingsTechnology, 2009, 204 (3): 277-284.
[79] Kachurina O, Metroke T L, Stesikova E,etal. Comparison of Single and Multilayer Coatings Based on Ormosil and Conversion Layers for Aluminum Alloy Corrosion Inhibition [J].JournalofCoatingsTechnology, 2002, 74 (926): 43-48.
[80] Metroke T L, Apblett A. Effect of Solvent Dilution on Corrosion Protective Properties of Ormosil Coatings on 2024-T3 Aluminum Alloy [J].ProgressinOrganicCoatings, 2004, 51 (1): 36-46.
[81] Varma P C R, Colreavy J, Cassidy J,etal. Effect of Organic Chelates on the Performance of Hybrid Sol-Gel Coated Aa 2024-T3 Aluminium Alloys [J].ProgressinOrganicCoatings, 2009, 66 (4): 406-411.
[82] Varma P C R, Colreavy J, Cassidy J,etal. Corrosion Protection of Aa 2024-T3 Aluminium Alloys Using 3, 4-Diaminobenzoic Acid Chelated Zirconium-Silane Hybrid Sol-Gels [J].ThinSolidFilms, 2010, 518 (20): 5 753-5 761.
[83] Sheffer M, Groysman A, Mandler D. Electrodeposition of Sol-Gel Films on Al for Corrosion Protection [J].CorrosionScience, 2003, 45 (12): 2 893-2 904.
[84] Tiwari A, Zhu J, Hihara L H. The Development of Low-Temperature Hardening Silicone Ceramer Coatings for the Corrosion Protection of Metals [J].Surface&CoatingsTechnology, 2008, 202 (19): 4 620-4 635.
[85] Lampke T, Darwich S, Nickel D,etal. Development and Characterization of Sol-Gel Composite Coatings on Aluminum Alloys for Corrosion Protection [J].MaterialwissenschaftundWerkstofftechnik, 2008, 39 (12): 914-919.
[86] Pepe A, Galliano P, Cere S,etal. Hybrid Silica Sol-Gel Coatings on Austempered Ductile Iron (Adi) [J].MaterialsLetters, 2005, 59 (17): 2 219-2 222.
[87] Kiruthika P, Subasri R, Jyothirmayi A,etal. Effect of Plasma Surface Treatment on Mechanical and Corrosion Protection Properties of Uv-Curable Sol-Gel Based Gpts-Zro(2) Coatings on Mild Steel [J].Surface&CoatingsTechnology, 2010, 204 (8): 1 270-1 276.
[88] Hosseini S M A, Jafari A H, Amalizadeh E J. Self-Healing Corrosion Protection by Nanostructure Sol-Gel Impregnated with Propargyl Alcohol [J].ElectrochimicaActa, 2009, 54 (28): 7 207-7 213.
[89] Madani S M, Ehteshamzadeh M, Rafsanjani H H. Investigation of the Microstructure and Corrosion Performance of a Nanostructured Titania-Containing Hybrid Silicate Film on Mild Steel [J].ThinSolidFilms, 2010, 519 (1): 145-150.
[90] Peng S S, Zhao W J, Zeng Z X,etal. Preparation of Anticorrosion Hybrid Silica Sol-Gel Coating Using Ce(No3)(3) as Catalyst [J].JournalofSol-GelScienceandTechnology, 2013, 66 (1): 133-138.
[91] Joncoux-Chabrol K, Bonino J P, Gressier M,etal. Improvement of Barrier Properties of a Hybrid Sol-Gel Coating by Incorporation of Synthetic Talc-Like Phyllosilicates for Corrosion Protection of a Carbon Steel [J].Surface&CoatingsTechnology, 2012, 206 (11-12): 2 884-2 891.
[92] Liu J G, Gong G P, Yan C W. Enhancement of the Erosion-Corrosion Resistance of Dacromet with Hybrid SiO2Sol-Gel [J].Surface&CoatingsTechnology, 2006, 200 (16-17): 4 967-4 975.
[93] Pepe A, Aparicio M, Cere S,etal. Synthesis of Hybrid Silica Sol-Gel Coatings Containing Zn Particles on Carbon Steel and Al/Zn Coated Carbon Steel [J].MaterialsLetters, 2005, 59 (29-30): 3 937-3 940.
[94] Pepe A, Galliano P, Aparicio M,etal. Sol-Gel Coatings on Carbon Steel: Electrochemical Evaluation [J].Surface&CoatingsTechnology, 2006, 200 (11): 3 486-3 491.
[95] Wang H, Akid R. Encapsulated Cerium Nitrate Inhibitors to Provide High-Performance Anti-Corrosion Sol-Gel Coatings on Mild Steel [J].CorrosionScience, 2008, 50 (4): 1 142-1 148.
[96] Chen Y A, Wu C C, Ding X G,etal. Effect of Water Amount and Teos/Mtes Ratio on Anti-Corrosion Property of Organic-Inorganic Film [J].RareMetalMaterialsandEngineering, 2010, 39: 288-291.
[97] Olivier M G, Fedel M, Sciamanna V,etal. Study of the Effect of Nanoclay Incorporation on the Rheological Properties and Corrosion Protection by a Silane Layer [J].ProgressinOrganicCoatings, 2011, 72 (1-2): 15-20.
[98] Conde A, De Damborenea J, Duran A,etal. Protective Properties of a Sol-Gel Coating on Zinc Coated Steel [J].JournalofSol-GelScienceandTechnology, 2006, 37 (1): 79-85.
[99] Chou T P, Chandrasekaran C, Limmer S,etal. Organic-Inorganic Sol-Gel Coating for Corrosion Protection of Stainless Steel [J].JournalofMaterialsScienceLetters, 2002, 21 (3): 251-255.
[100] Chou T P, Chandrasekaran C, Cao G Z. Sol-Gel-Derived Hybrid Coatings for Corrosion Protection [J].JournalofSol-GelScienceandTechnology, 2003, 26 (1-3): 321-327.
[101] Cere S, Pepe A, Aparicio M,etal. Cerium Hybrid Silica Coatings on Stainless Steel Aisi 304 Substrate [J].JournalofSol-GelScienceandTechnology, 2006, 39 (2): 131-138.
[102] Okner R, Domb A J, Mandler D. Electrochemically Deposited Poly(Ethylene Glycol)-Based Sol-Gel Thin Films on Stainless Steel Stents [J].NewJournalofChemistry, 2009, 33 (7): 1 596-1 604.
[103] Ballarre J, Lopez D A, Cavalieri A L. Nano-Indentation of Hybrid Silica Coatings on Surgical Grade Stainless Steel [J].ThinSolidFilms, 2008, 516 (6): 1 082-1 087.
[104] Hosseinalipour S M, Ershad-Langroudi A, Hayati A N,etal. Characterization of Sol-Gel Coated 316l Stainless Steel for Biomedical Applications [J].ProgressinOrganicCoatings, 2010, 67 (4): 371-374.
[105] Sarmento V H V, Schiavetto M G, Hammer P,etal. Corrosion Protection of Stainless Steel by Polysiloxane Hybrid Coatings Prepared Using the Sol-Gel Process [J].Surface&CoatingsTechnology, 2010, 204 (16-17): 2 689-2 701.
[106] Lopez D A, Rosero-Navarro N C, Ballarre J,etal. Multilayer Silica-Methacrylate Hybrid Coatings Prepared by Sol-Gel on Stainless Steel 316l: Electrochemical Evaluation [J].Surface&CoatingsTechnology, 2008, 202 (10): 2 194-2 201.
[107] Ballarre J, Lopez D A, Rosero N C,etal. Electrochemical Evaluation of Multilayer Silica-Metacrylate Hybrid Sol-Gel Coatings Containing Bioactive Particles on Surgical Grade Stainless Steel [J].Surface&CoatingsTechnology, 2008, 203 (1-2): 80-86.
[108] Ghaffari M, Barzegar A, Janghorban K,etal. Investigation of Effective Parameters in Improving Corrosion Resistance of Silica Coated Stainless Steel via Sol-Gel Method [J].CorrosionEngineeringScienceandTechnology, 2011, 46 (5): 605-610.
[109] Gallardo J, Duran A, Garcia I,etal. Effect of Sintering Temperature on the Corrosion and Wear Behavior of Protective SiO2-Based Sol-Gel Coatings [J].JournalofSol-GelScienceandTechnology, 2003, 27 (2): 175-183.
[110] Yang Y Q, Liu L, Hu J M,etal. Improved Barrier Performance of Metal Alkoxide-Modified Methyltrimethoxysilane Films [J].ThinSolidFilms, 2012, 520 (6): 2 052-2 059.
[111] Bescher E, Mackenzie J D. Sol-Gel Coatings for the Protection of Brass and Bronze [J].JournalofSol-GelScienceandTechnology, 2003, 26 (1-3): 1 223-1 226.
[112] Vreugdenhil A J, Woods M E. Continuously Responsive Epoxy-Amine Cross-Linked Silicon Sol-Gel Materials [J].JournalofMaterialsScience, 2006, 41 (22): 7 545-7 554.
[113] Peng S S, Zhao W J, Li H,etal. The Enhancement of Benzotriazole on Epoxy Functionalized Silica Sol-Gel Coating for Copper Protection [J].AppliedSurfaceScience, 2013, 276: 284-290.
[114] Lamaka S V, Montemor M F, Galio A F,etal. Novel Hybrid Sol-Gel Coatings for Corrosion Protection of Az31b Magnesium Alloy [J].ElectrochimicaActa, 2008, 53 (14): 4 773-4 783.
[115] Galio A F, Lamaka S V, Zheludkevich M L,etal. Inhibitor-Doped Sol-Gel Coatings for Corrosion Protection of Magnesium Alloy Az31 [J].Surface&CoatingsTechnology, 2010, 204 (9-10): 1 479-1 486.
[116] Guo X H, An M Z. Experimental Study of Electrochemical Corrosion Behaviour of Bilayer on Az31b Mg Alloy [J].CorrosionScience, 2010, 52 (12): 4 017-4 027.
[117] Wang H M, Akid R, Gobara M. Scratch-Resistant Anticorrosion Sol-Gel Coating for the Protection of Az31 Magnesium Alloy Via a Low Temperature Sol-Gel Route [J].CorrosionScience, 2010, 52 (8): 2 565-2 570.
[118] Hu J Y, Li Q, Zhong X K,etal. Organic Coatings Silane-Based for Az91d Magnesium Alloy [J].ThinSolidFilms, 2010, 519 (4): 1 361-1 366.
[119] Hu J Y, Li Q, Zhong X K,etal. Fluoride Treatment and Sol Film Composite Technology for Az91d Magnesium Alloy [J].TransactionsoftheInstituteofMetalFinishing, 2010, 88 (1): 41-46.
[120] Shi H W, Liu F C, Han E H. Corrosion Protection of Az91d Magnesium Alloy with Sol-Gel Coating Containing 2-Methyl Piperidine [J].ProgressinOrganicCoatings, 2009, 66 (3): 183-191.
[121] Nikrooz B, Zandrahimi M. Optimization of Process Variables and Corrosion Properties of a Multi Layer Silica Sol Gel Coating on Az91d Using the Box-Behnken Design [J].JournalofSol-GelScienceandTechnology, 2011, 59 (3): 640-649.
[122] Tamar Y, Mandler D. Corrosion Inhibition of Magnesium by Combined Zirconia Silica Sol-Gel Films [J].ElectrochimicaActa, 2008, 53 (16): 5 118-5 127.
[123] Hu J, Li Q, Zhong X,etal. Novel Anti-Corrosion Silicon Dioxide Coating Prepared by Sol-Gel Method for Az91d Magnesium Alloy [J].ProgressinOrganicCoatings, 2008, 63 (1): 13-17.
