富营养化湖泊溶解性有机碳生物可利用性研究进展
2014-08-08叶琳琳孔繁翔史小丽闫德智
叶琳琳,孔繁翔,史小丽,阳 振,闫德智,张 民
(1. 南通大学地理科学学院,南通 226000;2. 中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室,南京 210008)
富营养化湖泊溶解性有机碳生物可利用性研究进展
叶琳琳1,孔繁翔2,*,史小丽2,阳 振2,闫德智1,张 民2
(1. 南通大学地理科学学院,南通 226000;2. 中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室,南京 210008)
富营养化湖泊溶解性有机碳(DOC)包括内源和外源性碳源,不同来源碳源在物质化学结构组成和分子量级等方面具有显著差异,进而影响到对细菌的生物可利用性和碳素在食物网中的传递效率。根据国内外文献,综述了内外源DOC在碳稳定同位素值域上的显著差异,建议通过对DOC碳稳定同位素的分析来识别富营养化湖泊中DOC的主要来源;通过对比内外源DOC在碳水化合物、结合态中性糖和腐殖质含量上的差异,并结合细菌生长参数如细菌二级生产力、细菌呼吸作用及细菌生长效率来分析内外源DOC对细菌的生物可利用性。从富营养化湖泊DOC来源的角度探讨其生物可利用性和碳素传递效率,有助于了解富营养化湖泊食物网中碳素循环特征,加强对湖泊生态学的认识,为湖泊环境治理与保护提供科学依据。
富营养化湖泊; 溶解性有机碳; 来源; 生物可利用性; 综述
溶解性有机碳(DOC)在水体中主要以溶解性有机物(DOM)形式存在,是浮游细菌生长的重要碳源。通过细菌的呼吸作用(BR),部分DOC以二氧化碳的形式被释放。另一部分DOC通过细菌吸收利用合成二级生产力(BP),所形成的细胞颗粒通过浮游动物的摄食再进入传统食物链[1- 2]。研究人员用细菌生长效率(BGE=BP/[BP+BR])来表征DOC中碳源通过浮游细菌传递到更高营养级别的效率[3- 4]。但是,DOC的来源、生物化学结构组成及形态存在显著差异,进而影响其降解程度及细菌对其吸收利用途径[5]。
DOC的生物可利用性一直是生态系统研究的重点与热点。Søndergaard 和 Middelboe[6]系统总结了湖泊,河流和海洋中126项DOC的研究表明,DOC中可利用组分与DOC总浓度显著正相关,其中湖泊中DOC可利用组分所占比例为14%。Yokokawa 和 Nagata[7- 8]在日本Otsuchi 湾研究发现,DOC到浮游细菌的碳通量为23μg C L-1d-1,所形成的细菌二级生产力再被浮游动物摄食,产生的碳通量为7μg C L-1d-1。Carpenter等[9]对不同营养级别的湖泊研究发现,在贫营养湖泊中,DOC来源以外源输入为主,外源性DOC在湖泊中的降解常数为0.0001—0.01/d[10]。在亚马逊河Batata湖研究发现,高水位期间,水体DOC以外源性为主,低水位期间,DOC以内源性为主,而低水位期间细菌BGE值要高于高水位期间[11]。但是,外源性DOC对细菌二级生产力的贡献率,DOC分子量级的高低及腐殖质含量对其生物可利用性的影响至今还存在分歧[12- 15]。因此,本文从DOC来源的角度综述了内外源DOC生物可利用性及其在碳素代谢途径和效率方面的显著差异,为今后深入研究富营养化湖泊生态系统中碳素循环机理和湖泊环境保护提供参考依据。
1 溶解性有机碳的来源与区分
利用碳稳定同位素技术可以追溯有机物的来源,但目前的溯源研究主要集中在颗粒态有机物(POM)方面[16- 18]。Gu 等[19]对32个淡水湖泊δ13CPOM进行统计分析,研究结果表明,δ13CPOM年变化值域中最大值与湖泊富营养化参数(总氮、总磷、叶绿素)显著正相关。Heligings等[16]在比利时Scheldt河口研究发现,夏季POM的主要来源为浮游植物,而冬季以陆源输入为主。曾庆飞[20]在太湖研究发现,夏季POM的主要来源为蓝藻,并且δ13C浮游动物与δ13CPOM大量重叠,表明浮游动物摄食了部分蓝藻。但是以碳稳定同位素特征值为工具对富营养化湖泊DOC进行溯源,继而深入研究其生物可利用性的研究还较少。
DOC按其来源可分为内源性和外源性DOC。内源性DOC主要来源于浮游植物光合作用产物的释放以及内源性碎屑物质的分解[21]。研究发现,淡水湖泊藻类的碳稳定同位素δ13C在-35‰—-25‰[22]。外源性DOC主要来源于陆源的流域输入,而多数陆生植物的光合作用主要通过C3途径把大气中CO2(δ13C≈-7‰)合成有机物,其δ13C为-27‰[23]。因此,不同来源有机物δ13C存在差异且值域重叠少,通过碳稳定同位素特征值可以追溯有机物的来源,这为区分内外碳源对有机物的贡献提供了有力保障[24- 25]。
在日本富营养化浅水湖泊霞浦湖研究发现,δ13CDOM变化范围为-25.9‰—-24.2‰,并且春季δ13CDOM值高于秋季,表明DOC来源存在季节性变化规律,春季藻类水华期间DOC主要来源是内源性碳[26]。在美国威斯康辛州Northern Highland湖区的32个湖泊研究发现,δ13CDOM变化范围为-22.9‰—-29.3‰,部分湖泊δ13CDOM与陆源C3植物碳稳定同位素特征值(-27‰)相似,表明其DOC主要来源为外源输入,内源性DOC所占比例低于25%,而在δ13CDOM与δ13CPOM(颗粒态有机物)值域接近的湖泊中,内源性碳对DOC的贡献较高[27- 28]。但是也有研究发现有无藻类水华,法国Rohemel 水库δ13CDOM(-28.1‰—-28.6‰)与外源性δ13CDOM(-28.6‰)相似,DOC来源以外源性碳为主[29]。在苏格兰Loch Lomond湖泊研究发现,内源性DOC的形成不足以改变水体中DOC的碳稳定同位素特征值,虽然δ13CDOM在6—9月期间有显著增长趋势,变化范围为-29.0‰—-28.4‰,但是仍然属于外源性DOC碳稳定同位素值域,表明外源性DOC是水体中DOC的主要来源[30]。因此,通过DOC碳稳定同位素特征值的变化特征,可以区分水体中DOC的主要来源途径。
2 溶解性有机碳的化学结构组成对其生物可利用性的影响
2.1 溶解性有机碳中碳水化合物含量对其生物可利用性的影响
DOC物质结构组成复杂,研究人员对其中部分物质的化学结构还不清楚,但溶解性总碳水化合物(TCHO)在目前可识别的DOC组分中所占比例最高,是细菌生长代谢的重要物质基础,与显色剂2,4,6-反式2-吡啶基三嗪(2,4,6-tripyridyl-s-triazine, TPTZ)可生成紫色络合产物,通过紫外分光光度计在595nm处测定[31- 34]。在匈牙利富营养化湖泊,TCHO在DOC中比例为15%—20%[35]。有研究发现,TCHO/DOC可以表征DOC生物可利用性[36- 37]。在印度Mandovi河口,细菌数量与TCHO/DOC显著负相关,表明细菌通过分解碳水化合物来提供其物质代谢的主要碳源[38]。而有研究发现,在浮游植物水华过程中,溶解性结合态中性糖(DCNS)在碳水化合物中比例高达54%,能为细菌生长提供重要碳源[39- 40]。
2.2 溶解性有机碳的分子量级对其生物可利用性的影响
DOC按其分子量级可分为高分子溶解性有机碳(HMWDOC)和低分子量溶解性有机碳(LMWDOC)。但对于不同量级组分生物利用性的认识,还一直存在争议。Amon 和 Benner[14,41]认为HMWDOC生物可利用性要高于LMWDOC。在墨西哥湾Saint Louis河口,研究发现HMWDOC组分容易被细菌分解利用,TCHO在HMWDOC所占比例(53%—73%)要显著高于在DOC中比例(10%—31%)[42]。但也有研究认为LMWDOC具有较高生物活性,细菌对LMWDOC组分中TCHO利用效率(76%)要显著高于HMWDOC组分(46%)[33,43- 44]。
2.3 溶解性有机碳中腐殖质含量对其生物可利用性的影响
腐殖质(HS)也是DOC中重要的物质组成,其所占比例可高达80%[45]。在匈牙利Balaton湖的入湖河流River Zala和湖区东部,HS在DOC中所占比例分别为75%和50%[46]。一般认为,腐殖质物质不容易被细菌分解利用[15,47]。美国卡罗来纳L湖,HS在DOC所占比例为50%,对细菌生产力的贡献为22%[48]。在挪威Kjelsåsputten湖,细菌对DOC中的HS吸收利用效率低于10%[49]。研究发现在德国富含腐殖质的湖泊(Schwarze kuhle)和清水湖(Schöhsee),DOC中生物活性组分的含量没有显著差异,约为15%—22%[50]。 但近来有研究发现,HS浓度对其生物可利用性具有重要影响[51- 52],在匈牙利Balaton湖,HS浓度与DOC中生物活性组分浓度之间显著正相关[53]。在波兰富营养化湖泊Jeziorak,添加HS浓度为25 mg/L 时细菌数量达到最大值[54]。James[55]也研究发现,在富含HS的湖泊中,HS浓度不超过20 mg/L,其对细菌生长具有促进作用。
3 内源性溶解性有机碳生物可利用性及其对浮游植物水华生消的响应
有研究认为,富营养化湖泊比寡营养和中度富营养化湖泊中DOC含量高[56- 59],其原因是富营养化湖泊中,浮游植物水华过程会导致DOC浓度显著升高。在日本富营养化湖泊Nakanuma春季水华暴发期间,DOC的产生速率是2.8 μmol L-1d-1[60]。在丹麦富营养化湖泊Frederiksborq Slotssø春季硅藻水华消亡期间,DOC的产生速率是9 μ L-1d-1[61]。德国中富营养化湖泊康士坦茨湖硅藻水华暴发时DOC浓度达到峰值,表明有新的DOC形成[62]。有研究发现在超富营养化湖泊太湖的贡湖湾湖区,春夏季蓝藻水华期间,DOC浓度升高,并与叶绿素浓度显著正相关[63]。以上研究结果表明,浮游植物水华生消过程中,DOC浓度会显著增加,并且主要是来源于内源性DOC的形成。
有研究发现,浮游植物细胞内碳水化合物含量为13%—35%,在浮游植物水华过程中,由于藻细胞的胞外释放、被浮游动物摄食和细胞自然裂解,细胞内碳水化合物会释放到水体中从而改变DOC中碳水化合物含量[64- 65]。在富营养化湖泊巢湖夏季蓝藻水华期间,TCHO在DOC中比例为26%[66]。在围格内模拟硅藻水华,新产生的DOC中有16%是由DCNS组成[67]。在美国Delaware河口春季水华过后的4—5月间,DCNS浓度在DOC中所占比例为4%—12%[68]。
此外,浮游植物水华过程会对DOC的分子量级产生影响[63,69- 70]。Gobler和 Saudo-Wilhelmy[71]在美国Pecomic河口研究发现浮游植物暴发期间,HMWDOC含量显著增加,LMWDOC含量基本保持不变,而在水华消亡过程中,LMWDOC成为水体中有机碳主要组分。孙小静等[72]通过室内模拟实验研究发现,蓝藻水华在降解的过程中会释放大量的HMWDOC。Hama等[73]通过碳稳定同位素示踪研究发现,浮游植物经过光合作用后,在黑暗中释放的DOC产物主要以HMWDOC为主。
相对于外源性DOC来说,新产生的内源性DOC富含碳水化合物,因此转化周期短,可以很快被细菌分解利用,从而参与到微食物网中碳素传递过程[74- 77]。TCHO和DCNS都是细菌生长代谢的重要碳源,在德国中富营养化康士坦茨湖研究发现,春季水华暴发和消亡期,细菌对TCHO的利用效率最高[78]。此外,实验模拟的水华产生的DCNS有70%—80%能在35d内被降解[79]。另有研究发现,浮游植物水华产生的DCNS有91%能在15 d内被降解[80]。在罗斯海,浮游植物水华暴发期间,DCNS浓度增加了3倍,在DOC易降解组分中所占比例达到50%[40]。在内源性DOM的降解实验中发现,30%的DOC被细菌分解利用,DCNS在DOC中比例从实验初期的14%降低到实验结束后的5%[81]。此外,有研究发现,浮游植物水华过程中产生的内源性HMWDOC生物可利用性高,比LMWDOC转化速率快[71,73]。综上所述,浮游植物水华生消过程会改变DOC浓度、物质结构组成及分子量级,进而改变其生物可利用性。
在比利时富营养化浅水湖泊Blankaart,浮游细菌生长主要以内源性DOC为主[82]。有研究发现13%的内源性DOC支持了30%—65%的细菌生长代谢活动[79]。在太湖研究发现,浮游植物降解产生的内源性DOC可能是细菌生长的重要碳源[83]。在波兰马祖里湖区,对深水的中度富营养化湖泊Kuc、富营养化湖泊Ryńskie和超富营养化浅水湖泊Szymon进行调查,研究发现DOC主要来源于内源性DOC,细菌生产力和叶绿素浓度显著正相关(表1),结果表明细菌生长的碳源主要来源于内源性DOC[84]。有研究发现,进行营养盐添加增大湖泊初级生产力,有利于提高微生物对内源性DOC的利用份额。在添加了氮磷营养盐的Peter湖,内源性DOC对异养生物呼吸的贡献从60%增大到88%[85]。在芬兰中腐殖质湖泊 Pääjärvi,夏季浮游植物水华过程中释放的内源性DOC含有较高生物可利用性组分,细菌BGE达到26%[86]。综上所述,浮游植物水华过程中产生的内源性DOC富含碳水化合物,结合态中性糖,具有较高生物可利用性,在湖泊微食物网碳素循环中具有重要作用。
但内源性DOC中也有部分组分不易降解[52,87]。在日本富营养化浅水湖泊霞浦湖湖心区域,内源性DOC中不易降解组分浓度从秋季到冬季有所增长[88]。有研究发现,部分内源性DOC能在水里保留1a以上不降解[89]。在添加了营养盐进行的围格实验中,研究发现实验46d后,新产生的内源性DOC中有32%难以降解[80]。实验模拟产生的水华形成的内源性DOC中有25%—30%在2.5a后,仍难以被矿化和利用[87]。研究还发现在淡水围格中,硅藻水华形成阶段,难降解DOC组分含量较高[77]。此外,浮游植物水华在消亡过程中受到磷元素的限制作用,也会产生大量难降解的DOC组分[90]。
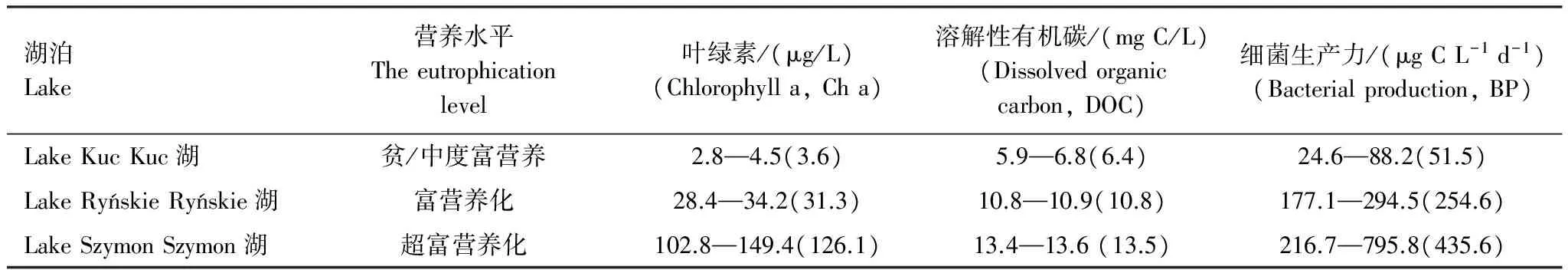
表1 不同营养级别湖泊中叶绿素,溶解性有机碳和细菌生产力的变化
4 外源性溶解性有机碳的生物可利用性
在贫营养的湖泊中,外源性DOC含量要显著高于内源性DOC[9,91]。Carpenter等[9]在2001年和2002年选取威斯康辛州Paul、Peter和Tutesday湖为研究对象,研究结果见表2。Bade[28]也发现没有进行营养盐添加的湖泊,80%—90%的DOC来源于外源性DOC。Cole 等[92]通过C13示踪,发现在富含腐殖质的美国East Long湖泊中,外源性DOC在总有机碳中所占比例高达90%。综上所述,在贫营养和富含腐殖质的湖泊中,外源性DOC所占比例高。
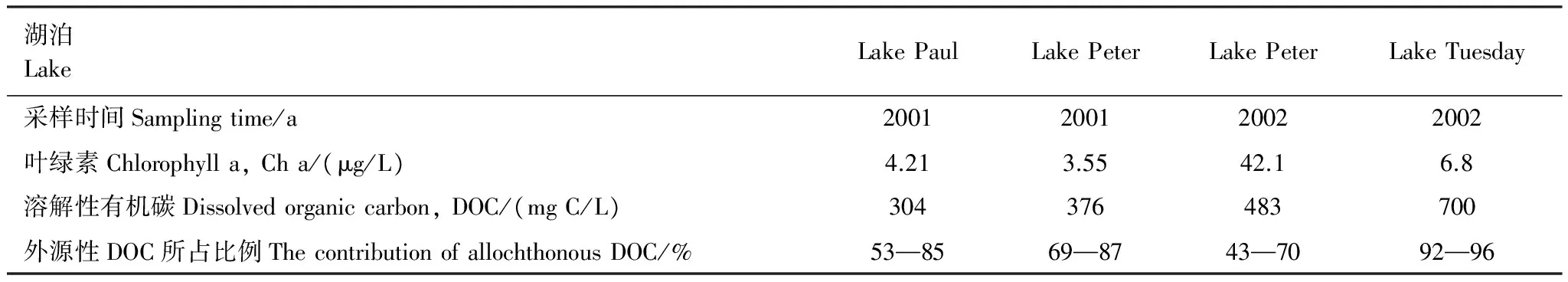
表2 不同营养级别湖泊中外源性DOC贡献率
2001年没有对Paul 和Peter湖添加营养盐;2002年对Peter 湖添加营养盐;Tuesday湖为贫营养湖泊
以往的研究认为外源性DOC主要以腐殖质物质存在,分子量高,氮磷比低,具有芳香性,不易被微生物所利用[93- 94]。有研究发现,在德国贫营养湖泊Groβe Fuchskuhle西部湖区,DOC以外源性为主,其中HS所占比例达到58%[95]。在日本富营养化浅水湖泊霞浦湖湖心,外源性DOC是DOC中不易降解组分的主要来源[88]。在芬兰富含腐殖质的Mekkojärvi湖研究发现95%的外源性DOC不能被细菌分解利用[86]。
但近来有研究发现,很多湖泊属于异养型,整个湖泊生态系统的呼吸作用(R)大于总初级生产力(GPP)。因此,外源性有机碳是湖泊物质代谢过程中的重要补充[96- 98]。有研究发现,在未添加营养盐的湖泊中,外源性有机碳对浮游动物碳源的贡献率为22%—75%[8]。在营养不良的Tuesday湖,外源性DOC对异养生物呼吸的贡献率达到68%[86]。在挪威中腐殖质湖泊Kjelsåsputten[49]和瑞典Örträsket湖[99],90%的细菌生产力来源于外源性DOC。在瑞典12个湖泊中研究发现,细菌二级生产力和呼吸作用均与外源性DOC显著正相关(图1),但其中90%的DOC是用于细菌呼吸作用,因此BGE较低[100]。在East Long湖研究发现DOC以外源性为主,细菌BGE仅为4%[92]。有研究发现,内源性DOC营养价值高,碳氮比值约为12∶1,而外源性DOC碳氮比值约为50∶1[101],不同来源DOC营养价值的差异会影响细菌的生长效率BGE, 有研究表明BGE与生长基质中碳氮比值具有负相关性[102]。因此,以外源性DOC为碳源,细菌生长效率低[11- 12,17]。Kritzberg等[12]研究发现在异养型湖泊中,细菌的二级生产力和内源性DOC具有显著相关性,表明被细菌吸收的外源性DOC不可能被传递到上一层消费者,在食物网中碳素传递效率低。

图1 瑞典12个湖泊中细菌二级生产力BP、呼吸量BR与外源性溶解性有机碳的变化趋势Fig.1 The variation of bacteria production, bacteria respiration and allochthonous dissolved organic carbon in twelve lakes in Sweden
此外有研究发现,外源性DOC分子量级组成对BGE具有显著影响[103- 104]。Berggren等[13]研究发现,外源性DOC中LMWDOC对细菌、原生动物和后生动物二级生产力的贡献分别为80%、54%和23%,通过摄食浮游细菌,这部分碳源可被有效地传递到更高营养级别。在瑞典的溪流和湖泊中,研究发现细菌BGE随着外源性DOC中LMWDOC浓度的升高而增大[105]。新的外源性DOC中,细菌的BGE高达50%,陈年的外源性DOC中BGE只有10%,可能是其中LMWDOC组分耗竭所致[106- 108]。但在芬兰富含腐殖质的Mekkojärvi Lake,细菌吸收利用了外源性DOC中30%的LMWDOC,BGE只有3%,而吸收利用4%的HMWDOC,BGE达到26%,表明外源性DOC中HMWDOC组分比LMWDOC组分营养价值高[86]。 综上所述,外源性DOC也是湖泊食物网中碳循环的重要补充,但其分子量级对其生物可利用性具有重要影响。
5 结论与展望
DOC是湖泊生态系统食物网的重要做成部分。目前,国内就富营养化湖泊中浮游植物水华过程对DOC浓度和形态等方面开展了大量的工作[69,109],张运林等利用三维荧光对太湖溶解性有机碳的来源也进行了分析[110- 111],并取得了丰富的研究成果。但从DOC来源的角度,探索其生物可利用性、碳素代谢途径和效率以及DOC-细菌-浮游植物相互关系的研究较少。
随着人们对湖泊生态系统中碳循环机理的深入研究,有关DOC来源及生物可利用性对浮游植物水华过程的响应急需得到进一步加强。通过碳稳定同位素技术,可以明确富营养化湖泊中DOC的主要来源。通过对不同来源DOC中碳水化合物、溶解性结合态中性糖、分子量级和腐殖质的分析,并结合细菌生长参数,可以明确不同来源DOC生物可利用性的显著差异,及其在湖泊食物网碳循环中作用和碳传递效率,为今后深入研究湖泊生态系统碳素循环机制和生态系统稳定性提供参考依据。
[1] Azam F, Fenchel T, Field J S, Gray J S, Meyer-Reil L A, Thingstad F. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series, 1983, 10: 257- 263.
[2] Jansson M, Persson L, DeRoos A M, Jones R I, Tranvik L J. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends in Ecology and Evolution, 2007, 22(6): 316- 322.
[3] Del Giorgio P A, Cole J J. Bacterial growth efficiency in natural aquatic systems. Annual Review of Ecology and Systematics, 1998, 29(1): 503- 541.
[4] Ram A S P, Nair S, Chandramohan D. Bacterial growth efficiency in a tropical estuary: seasonal variability subsidized by allochthonous carbon. Microbial Ecology, 2007, 53(4): 591- 599.
[5] Wu Q L, Xing P, Li H B, Zeng J. Impacts of regime shift between phytoplankton and macrophyte on the microbial community structure and its carbon cycling in lakes. Microbiology China, 2013, 40(1):87- 97.
[6] Sønderaard M, Middelboe M. A cross-system analysis of labile dissolved organic carbon. Marine Ecology Progress Series, 1995, 118: 283- 294.
[7] Yokokawa T, Nagata T. Growth and grazing mortality rates of phylogenetic groups of bacterioplankton in coastal marine environments. Applied and Environmental Microbiology, 2005, 71(11): 6799- 6807.
[8] Yokokawa T, Nagata T. Linking bacterial community structure to carbon fluxes in marine environments. Journal of Oceanography, 2010, 66(1): 1- 12.
[9] Carpenter S R, Cole J J, Pace M L, Van de Bogert M, Bade D L, Bastviken D, Gille C M, Hodgson J R, Kitchell J F, Kritzberg E S. Ecosystem subsidies: terrestrial support of aquatic food webs from13C addition to contrasting lakes. Ecology, 2005, 86(10): 2737- 2750.
[10] Hanson P C, Hamilton D P, Stanley E H, Preston N, Langman O C, Kara E L. Fate of allochthonous dissolved organic carbon in lakes: a quantitative approach. PloS ONE, 2011, 6(7): e21884.
[11] Farjalla V F, Azevedo D A, Esteves F A, Bozelli R L, Roland F, Enrich-Prast A. Influence of hydrological pulse on bacterial growth and DOC uptake in a clear-water Amazonian Lake. Microbial Ecology, 2006, 52(2): 334- 344.
[12] Kritzberg E S, Cole J J, Pace M M, Granéli W. Does autochthonous primary production drive variability in bacterial metabolism and growth efficiency in lakes dominated by terrestrial C inputs? Aquatic Microbial Ecology, 2005, 38(2):103- 111.
[13] Berggren M, Ström L, Laudon H, Karlsson J, Jonsson A, Giesler R, Bergström A K, Jasson M. Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecology Letters, 2010, 13(7): 870- 880.
[14] Amon R M W, Benner R. Bacterial utilization of different size classes of dissolved organic matter. Limnology and Oceanography, 1996, 41(1): 41- 51.
[15] Geller A. Degradability of dissolved organic lake water compounds in cultures of natural bacterial communities. Archi für Hydrobiology, 1983, 99(1): 60- 79.
[16] Heligings L, Dehairs F, Tackx M, Keppens E, Baeyens W. Origin and fate of organic carbon in the freshwater part of the Scheldt Estuary as traced by stable carbon isotope composition. Biogeochemistry, 1999, 47(2): 167- 186.
[17] de Kluijver A, Yu J L, Houtekamer M, Middelburg J J, Liu Z W. Cyanobacteria as a carbon source for zooplankton in eutrophic lake Taihu, China, measured by13C labeling and fatty acid biomarkers. Limnology and Oceanography, 2012, 57(4): 1245- 1254.
[18] Zeng Q F, Kong F X, Zhang E L, Tan X, Wu X D. Seasonality of stable carbon and nitrogen isotopes within the pelagic food web of Taihu Lake. Annales de Limnologie -International Journal of Limnology, 2008, 44(1): 55- 60.
[19] Gu B H, Schelske C L, Waters M N. Patterns and controls of seasonal variability of carbon stable isotopes of particulate organic matter in Lakes. Oecologia, 2011, 165(4): 1083- 1094.
[20] Zeng Q F. Stable Isotope Compositions of Suspended Particulate Organic Matter and Its Ecological Significance From Lake Taihu [D]. Nanjing: Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, 2008.
[21] Bertilsson S, Jones J B J. Supply of dissolved organic matter to aquatic ecosystems: autochthonous sources // Findlay S E G, Sinsabaugh R L, eds. Aquatic Ecosystems: Interactivity of Dissolved Organic Matter. New York: Academic Press, 2003.
[22] Boutton T W. Stable carbon isotope ratios of natural materials, II. Atmospheric, terrestrial, marine, and freshwater environments // Coleman D C, Fry B, eds. Carbon Isotopes Techniques. New York: Academic Press, 1991.
[23] Amiotte-Suchet P, Linglois N, Leveque J, Andreux F.13C composition of dissolved organic carbon in upland forested catchments of the Morvan Mountains (France): influence of coniferous and deciduous vegetation. Journal of Hydrology, 2007, 335(3/4): 354- 363.
[24] Wang H, Zhang C L, Yang H, Cao J H, Zhang Q, Tang W, Ying Q H, Lin Y. The application of stable carbon isotope to the study of carbon sources in Guijiang Watershed, Guangxi. Acta Geoscientia Sinica, 2011,32(6): 691- 698.
[25] Gandhi H, Wiegner T N, Ostrom P H, Kaplan L A, Ostrom N E. Isotopic (13C) analysis of dissolved organic carbon in stream water using an elemental analyzer coupled to a stable isotope ratio mass spectrometer. Rapid Communications in Mass Spectrometry, 2004, 18(8): 903- 906.
[26] Nara F, Imai A, Yoneda M, Mastushige K, Komatsu K, Nagai T, Shibata Y, Watanabe T. Seasonal variation in sources of dissolved organic carbon in a lacustrine environment revealed by paired isotopic measurements (Δ14C andδ13C). Radiocarbon, 2007, 49(2): 767- 773.
[27] Bade D L, Carpenter S R, Cole J J, Pace M L, Kritzberg E, Van de Bogert M C, Cory R M, McKnight D M. Sources and fates of dissolved organic carbon in lakes as determined by whole-lake carbon isotope additions. Biogeochemistry, 2007, 84(2): 115- 129.
[28] Bade D L. Ecosystem Carbon Cycles: Whole-lake Fluxes Eestimated With Multiple Isotopes [D]. Wisconsin: University of Wisconsin, 2004.
[29] Pierson-Wickmann A C, Gruau G, Jardé E, Gaury N, Brient L, Lengronne M, Crocq A, Helle D, Lambert T. Development of a combined isotopic and mass-balance approach to determine dissolved organic carbon sources in eutrophic reservoirs. Chemosphere, 2011, 83(3):356- 366.
[30] Bass A M. Stable Isotope Insight into Pelagic Carbon Cycling in Loch Lomond: A large, Temperate Latitude Lake [D]. Glasgow: University of Glasgow, 2007.
[31] Pakulski J D, Benner R. Abundance and distribution of carbohydrates in the ocean. Limnology and Oceanography, 1994, 39(4):930- 940.
[32] Hayakawa K. Seasonal variations and dynamics of dissolved carbohydrates in Lake Biwa. Organic Geochemistry, 2004, 35(2):169- 147.
[33] Khodse V B, Bhosle N B. Bacterial utilization of size-fractionated dissolved organic matter. Aquatic Microbial Ecology, 2011, 64(3): 299- 309.
[34] Myklestad S M, Skånøy E, Hestamann S. A sensitive and rapid method for analysis of dissolved monoand polysaccharides in seawater. Marine Chemistry, 1997, 56(3/4): 279- 286.
[35] Grigorszky I, Borics G, Kiss K T, Schnitchen C, Béres V, Gligora M, Padisák J, Borbely G. Seasonal variation of organic compounds in a eutrophic oxbow lake. Verhandlungen des Internationalen Verein Limnologie, 2005, 29: 650- 653.
[36] Goldberg S J, Carlson C A, Hansell D A, Nelson N B, Siegel D A. Temporal dynamics of dissolved combined neutral sugars and the quality of dissolved organic matter in the Northwestern Sargasso Sea. Deep-Sea Research I, Oceanographic Research Papers, 2009, 56(5): 672- 685.
[37] Hung C-C, Warnken K W, Santschi P H. A seasonal survey of carbohydrates and uronic acids in the Trinity River, Texas. Organic Geochemistry, 2005, 36(3): 463- 474.
[38] Khodse V B, Bhosle N B, Matondkar S G P. Distribution of dissolved carbohydrates and uronic acids in a tropical estuary, India. Journal of Earth System Science, 2010, 119(4): 519- 530.
[39] Biersmith A, Benner R. Carbohydrates in phytoplankton and freshly produced dissolved organic matter. Marine Chemistry, 1998, 63(1/2):131- 144.
[40] Kirchman D, Meon B, Ducklow H W, Carlson C A, Hansell D A, Steward G F. Glucose fluxes and concentrations of dissolved combined neutral sugars (polysaccharides) in the Ross Sea and Polar Front Zone, Antarctica. Deap-Sea Research II: Topical Studies in Oceanography, 2001, 48(19/20):4179- 4197.
[41] Amon R M W, Benner R. Rapid cycling of high-molecular weight dissolved organic matter in the ocean. Nature, 1994, 369(6481): 549- 552.
[42] Wang X R, Cai Y H, Guo L D. Preferential removal of dissolved carbohydrates during estuarine mixing in the Bay of Saint Louis in the northern Gulf of Mexico. Marine Chemistry, 2010, 119(1/4):130- 138.
[43] Rosenstock B, Zwisler W, Simon M. Bacterial consumption of humic and non-humic low and high molecular weight Dom and the effect of solar irradiation on the turnover of labile DOM in the southern ocean. Microbial Ecology, 2005, 50(1): 90- 101.
[44] Covert J S, Moran M A. Molecular characterization of estuarine bacterial communities that use high- and low- molecular weight fractions of dissolved organic carbon. Aquatic Microbial Ecology, 2001, 25(2): 127- 139.
[45] Thurman E M. Organic Geochemistry of Natural Waters. Martinus Nijhoff/Dr W. Junk Publishrs, Boston, 1985.
[46] V.-Balogh K, Vörös L, Tóth N, Bokros M. Changes of organic matter quality along the longitudinal axis of a large shallow lake (Lake Balaton). Hydrobiologia, 2003, 506- 509(1/3): 67- 74.
[47] Imai A, Fukushima T, Matsushige K, Kim Y H. Fractionation and characterization of dissolved organic matter in a shallow eutrophic lake, its inflowing rivers, and other organic matter sources. Water Research, 2001, 35(17): 4019- 4028.
[48] Moran M A, Hodson R E. Bacterial production on humic and nonhumic components of dissolved organic carbon. Limnology and Oceanography, 1990, 35(8): 1744- 1756.
[49] Hessen D O. Dissolved organic carbon in a humic lake: effects on bacterial production and respiration. Hydrobiologia, 1992, 229(1): 115- 123.
[50] Tranvik L J, Höfle M G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Applied and Environmental Microbiology, 1987, 53(3): 482- 488.
[51] Hessen D O. The relation between bacterial carbon and dissolved humic compounds in oligotrophic lakes. FEMS Microbiology Letters, 1985, 31(4): 215- 223.
[52] Tranvik L J. Degradation of dissolved organic matter in humic waters by bacteria // Hessen D O, Tranvik L, eds. Aquatic Humic Substances: Ecology and Biogeochemistry. Berlin: Springer-Verlag, 1998: 259- 283.
[53] Tóth N, Vörös L, Mózes A, V.-Balogh K. Biological availability and humic properties of dissolved organic carbon in Lake Balaton (Hungary). Hydrobiologia, 2007, 592(1): 281- 290.
[54] Burkowska A, Donderski W. Impact of humic substances on bacterioplankton in europhic lake. Polish Journal of Ecology, 2007, 55(1): 155- 160.
[55] James R T. Microbiology and chemistry of acid lakes in Florida: I. Effect of drought and post-drought conditions. Hydrobiologia, 1991, 213(3): 205- 225.
[56] Sugiyama Y, Anagawa A, Kumagai T, Harita Y, Hori T, Sugiyama M. Distribution of dissolved organic carbon in lakes of different trophic types. Limnology, 2004, 5(3): 165- 176.
[57] Yoshioka T, Ueda S, Khodzher T, Bashenkhaeva N, Korovykova I, Sorokovikova L, Gorbunova L. Distribution of dissolved organic carbon in Lake Baikal and its watershed. Limnology, 2002, 3(3):159- 168.
[58] Robarts R D, Wicks R J, Gehr R. Seasonal changes in the dissolved free amino acid and DOC concentrations in a hypertrophic African reservoir and its inflowing rivers. Hydrobiologia, 1990, 199(3): 201- 216.
[59] Kim B, Choi K, Kim C, Lee U H, Kim Y H. Effects of the summer monsoon on the distribution and loading of organic carbon in a deep reservoir, Lake Soyang, Korea. Water Research, 2000, 34(14): 3495- 3504.
[60] Ochiai M, Nakajima T, Hanya T. Seasonal fluctuation of dissolved organic matter in Lake Nakanuma. Japanese Journal of Limnology (Rikusuigaku Zasshi), 1979, 40(4): 185- 190.
[61] Søndergaard M, Hansen B, Markagr S. Dynamics of dissolved organic carbon lability in a eutrophic lake. Limnology and Oceanography, 1995, 40(1): 46- 54.
[62] Weiss M, Simon M. Consumption of labile dissolved organic matter by limnetic bacterioplankton: the relative significance of amino acids and carbohydrates. Aquatic Microbial Ecology, 1999, 17(1): 1- 12.
[63] Ye L L, Shi X L, Wu X D, Kong F X. Nitrate limitation and accumulation of dissolved organic carbon during a spring-summer cyanobacterial bloom in Lake Taihu (China). Journal of Limnology, 2012, 71(1): 67- 71.
[64] Biddanda B, Benner R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnology and Oceanography, 1997, 42(3): 506- 518.
[65] Ye L L, Wu X D, Tan X, Shi X L, Li D M, Yu Y, Zhang M, Kong F X. Cell lysis of cyanobacteria and its implications for nutrient dynamics. International Review of Hydrobiology, 2010, 95(3): 235- 245.
[66] Ye L L, Shi X L, Zhang M, Wu X D, Kong F X. Distribution of carbohydrates species during summer bloom in Lake Chaohu. China Environmental Science, 2012, 32(2): 318- 323.
[67] Søndergaard M, Williams P J L E B, Cauwet G, Riemann B, Robinson C, Terzic S, Woodward EMS, Worm J. Net accumulation and flux of dissolved organic carbon and dissolved organic nitrogen in marine plankton communities. Limnology and Oceanography, 2000, 45(5): 1097- 1111.
[68] Kirchman D L, Borch N H. Fluxes of dissolved combined neutral sugars (polysaccharides) in the Delaware Estuary. Estuaries, 2003, 26(4):894- 904.
[69] Zhang Z P, Zhu G W, Sun X J, Chi Q Q. Temporal and spatial changes of the content of colloidal organic carbon in Taihu Lake, China. Acta Scientiae Circumstantiae, 2008, 28(8): 1668- 1673.
[70] Kepkay P E, Niven S E H, Jellett J F. Colloidal organic carbon and phytoplankton speciation during a coastal bloom. Journal of Plankton Research, 1997, 19(3): 369- 389.
[71] Gobler C J, Saudo-Wilhelmy S A. Cycling of colloidal organic carbon and nitrogen during an estuarine phytoplankton bloom. Limnology and Oceanography, 2003, 48(6): 2314- 2320.
[72] Sun X J, Qin B Q, Zhu G W. Release of colloidal phosphorus, nitrogen and organic carbon in the course of dying and decomposing of cyanobacteria. China Environmental Science, 2007, 27(3): 341- 345.
[73] Hama T, Yanagi K, Hama J. Decrease in molecular weight of photosynthetic products of marine phytoplankton during early diagenesis. Limnology and Oceanography, 2004, 49(2): 471- 481.
[74] Coffin R B, Connolly J P, Harris P S. Availability of dissolved organic carbon to bacterioplankton examined by oxygen utilization. Marine Ecology Progress Series, 1993, 101(4): 9- 22.
[75] Kritzberg E S, Cole J, Pace M L, Granéli W, Bade D L. Autochthonous versus allochthonous carbon sources to bacteria: Results from whole-lake13C addition experiments. Limnology and Oceanography, 2004, 49(2): 588- 596.
[76] Maki K, Kim C, Yoshimizu C, Tayasu I, Miyajima T, Nagata T. Autochthonous origin of semi-labile dissolved organic carbon in a large monomictic lake (Lake Biwa): carbon stable isotopic evidence. Limnology, 2010, 11(2): 143- 153.
[77] Søndergaard M, Borch N H, Riemann B. Dynamics of biodegradable DOC produced by freshwater plankton communities. Aquatic Microbial Ecology, 2000, 23(1): 73- 83.
[78] Hanisch K, Schweitzer B, Simon M. Use of dissolved carbohydrates by planktonic bacteria in a Mesotrophic Lake. Microbial Ecology, 1996, 31(1): 41- 55.
[79] Kragh T, Søndergaard M, Borch N H. The effect of zooplankton on the dynamics and molecular composition of carbohydrates during an experimental algal bloom. Journal of Limnology, 2006, 65(1): 52- 58.
[80] Meon B, Kirchman D L. Dynamics and molecular composition of dissolved organic material during experimental phytoplankton blooms. Marine Chemistry, 2001, 75(3): 185- 199.
[81] Amon R M W, Fitznar H P, Benner R. Linkage among the bioreactivity, chemical composition, and diagenetic state of marine dissolved organic matter. Limnology and Oceanography, 2001, 46(2): 287- 297.
[82] Muylaert K, Van der Gutht K, Vloemans N, Meester L D, Gillis M, Vyverman W. Relationship between bacterial community composition and bottom-up versus top-down variables in four eutrophic shallow lakes. Applied and environmental microbiology, 2002, 68(10): 4740- 4750.
[83] Feng S, Gao guang, Qin B Q, Chen M. Variability of bacterioplankton in the north zone of Lake Taihu. Journal of Lake Sciences, 2006, 18(6): 636- 642.
[84] Chróst R J, Koton M, Siuda W. Bacterial secondary production and bacterial biomass in four Mazurian Lakes of differing trophic status. Polish Journal of Environmental Studies, 2000, 9(4): 255- 266.
[85] Cole J J, Carpenter S R, Pace M L, Van de Bogert M C, Kithcell J L, Hodgson J R. Differential support of lake food webs by three types of terrestrial organic carbon. Ecology Letters, 2006, 9(5): 558- 568.
[86] Tulonen T. Role of Allochthonous and Autochthonous Dissolved Organic Matter (DOM) as a Carbon Source for Bacterioplankton in Boreal Humic Lakes [D]. Finland: University of Helsinki, 2004.
[87] Fry B, Hopkinson C S, Nolin A, Norrman B, Zweifel U L. Long-term decomposition of DOC from experimental diatom blooms. Limnology and Oceanography, 1996, 41(6): 1344- 1347.
[88] Fukushima T, Park J C, Imai A, Matsushige K. Dissolved organic carbon in a eutrophic lake; dynamics, biodegradability and origin. Aquatic Sciences, 1996, 58(2): 139- 157.
[89] Schindler D W, Bayley S E, Curti P J, Parker B R, Stainton M P, Kelley C A. Natural and man-caused factors affecting the abundance and cycling of dissolved organic substances in precambrian shield lakes. Hydrobiologia, 1992, 229(1): 1- 21.
[90] Kragh T, Søndergaard M. Production and decomposition of new DOC by marine plankton communities: carbohydrates, refractory components and nutrient limitation. Biogeochemistry, 2009, 96(1/3): 177- 187.
[91] Kalinowska K. Bacteria, nanoflagellates and ciliates as components of the microbial loop in three lakes of different trophic status. Polish Journal of Ecology, 2004, 52(1): 19- 34.
[92] Cole J J, Carpenter S R, Kitchell J F, Pace ML. Pathways of organic carbon utilization in small lakes: results from a whole-lake13C addition and coupled model. Limnology and Oceanography, 2002, 47(6): 1664- 1675.
[93] Moran M A, Hodson R E. Support of bacterioplankton production by dissolved humic substances from three marine environments. Marine Ecology Progress Series, 1994, 110:241- 247.
[94] Schiff S L, Aravena R, Trumbore S E, Hinton M J, Elgood R, Dillon P J. Export of DOC from forested catchments on the Precambrian Shield of Central Ontario: Clues from13C and14C. Biogeochemistry, 1997, 36(1): 43- 65.
[95] Sachse A, Babernzien D, Ginzel G, Gelbrecht J, Steinberg C E W. Characterization of dissolved organic carbon (DOC) in a dystrophic lake and an adjacent fen. Biogeochemistry, 2001, 54(3): 279- 296.
[96] Del Giorgio P A, Cole J J, Cembleris A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature, 1997, 385(6612):148- 151.
[97] Jansson M, Karlsson J, Blomqvist P. Allochthonous organic carbon decreases pelagic energy mobilization in lakes. Limnology and Oceanography, 2003, 48(4): 1711- 1716.
[98] Karlsson J, Jansson M, Jonsson A. Similar relationships between pelagic primary and bacterial production in clearwater and humic lakes. Ecology, 2002, 83(10): 2902- 2910.
[99] Jasson M, Bergström A K, Blomkvist P, Isaksson A, Jonsson A. Impact of allochthonous organic carbon on microbial food web carbon dynamics and structure in Lake Örträsket. Archiv für Hydrobiologie, 1999, 144(4): 409- 428.
[100] Jansson M, Hickler T, Jonsson A, Karlsson J. Links between terrestrial primary production and bacterial production and respiration in lakes in a climate gradient in subarctic Sweden. Ecosystems, 2008, 11(3): 367- 376.
[101] Wetzel R G. Limnology: Lake and River Ecosystems. 3rd ed. San Diego: Academic Press, 2001: 10- 20.
[102] Cimberlis A C P, Kalff J. Planktonic bacterial respiration as a function of C:N:P ratios across temperate lakes. Hydrobiologia, 1998, 384(1/3): 89- 100.
[103] Ågren A, Berggren M, Laudon H, Jansson M. Terrestrial export of highly bioavailable carbon from small boreal catchments in spring floods. Freshwater Biology, 2008, 53(5): 964- 972.
[104] Jonsson A, Ström L, Åberg J. Composition and variations in the occurrence of dissolved free simple organic compounds of an unproductive lake ecosystem in northern Sweden. Biogeochemistry, 2007, 82(2): 153- 163.
[105] Berggren M. Bacterial Use of Allochthonous Organic Carbon for Respiration and Growth in Boreal Freshwater Systems [D]. Virginia : University of Virginia, 2009.
[106] Lennon J T, Pfaff L E. Source and supply of terrestrial organic matter affects aquatic microbial metabolism. Aquatic Microbial Ecology, 2005, 39(2): 107- 119.
[107] Eiler A S, Langenheder S, Bertilsson S, Tranvik L J. Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Applied and Environmental Microbiology, 2003, 69(7): 3701- 3709.
[108] McArthur M D, Richardson J S. Microbial utilization of dissolved organic carbon leached from riparian literfall. Candian Journal of Fisheries and Aquatic Sciences, 2002, 59(10): 1668- 1676.
[109] Qian K M, Wang L P, Chen YW. The production of organic carbon by phytoplankton in Lake Taihu and its influence factors. Journal of Lake Sciences, 2009, 21(6): 834- 838.
[110] Zhang Y L, Qin B Q. Feature of CDOM and its possible source in Meiliang bay and Da Taihu Lake in Taihu Lake in summer and winter. Advances in Water Science, 2007, 18(3): 415- 423.
[111] Zhang Y L, Van Dijk M A, Liu M L, Zhu G W, Qin B Q. The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes: Field and experimental evidence. Water Research, 2009, 43(18): 4685- 4697.
参考文献:
[5] 吴庆龙,刑鹏,李化炳,曾巾. 草藻型稳态转换对湖泊微生物结构及其碳循环功能的影响. 微生物学通报, 2013, 40(1):87- 97.
[20] 曾庆飞. 太湖悬浮颗粒稳定同位素特征及生态学意义研究 [D]. 南京:中国科学院南京地理与湖泊研究所,2008.
[24] 王华,张春来,杨会,曹建华,张强,唐伟,应启和,林宇. 利用稳定同位素技术研究广西桂江流域水体中碳的来源. 地球学报,2011, 32(6): 691- 698.
[66] 叶琳琳,史小丽,张民,吴晓东,孔繁翔. 巢湖夏季水华期间水体中溶解性碳水化合物的研究. 中国环境科学,2012,32(2): 318- 323.
[69] 张战平, 朱广伟, 孙小静, 池俏俏. 太湖典型湖区中胶体有机碳浓度的时空变化. 环境科学学报, 2008, 28(8): 1668- 1673.
[72] 孙小静,秦伯强,朱广伟. 蓝藻死亡分解过程中胶体态磷、氮、有机碳的释放.中国环境科学, 2007, 27(3): 341- 345.
[83] 冯胜,高光,秦伯强,陈默. 太湖北部湖区水体中浮游细菌的动态变化. 湖泊科学,2006,18(6): 636- 642.
[109] 钱奎梅,王丽萍,陈宇炜. 太湖浮游植物群落的有机碳生产及其影响因子分析. 湖泊科学, 2009, 21(6): 834- 838.
[110] 张运林, 秦伯强. 梅梁湾、大太湖夏季和冬季CDOM特征及可能来源分析. 水科学进展, 2007, 18(3):415- 423.
The bioavailability of dissolved organic carbon in the eutrophic lakes
YE Linlin1, KONG Fanxiang2,*, SHI Xiaoli2, YANG Zhen2, YAN Dezhi1, ZHANG Min2
1GeographicalSciencesCollege,NantongUniversity,Nantong226000 2StateKeyLaboratoryofLake&EnvironmentalScience,NanjingInstituteofGeographyandLimnology,ChineseAcademyofSciences,Nanjing210008,China
The dissolved organic carbon (DOC) pool is composed of both autochthonous and allochthonous DOC, and its concentration in lakes generally increases with the trophic status. Accumulation of the autochthonous DOC was observed in the eutrophic lake, and the allochthonous DOC was highest in the dystrophic lake. Carbohydrates constitute a large component of the DOC, the consumption of DOC by heterotrophic bacteria is one of the largest fluxes of carbon in most aquatic ecosystems, but the bioavailability and the efficiency of carbon transfer in lakes food web is affected by the distribution of molecular weight and chemical composition. The DOC can be separated into high and low molecular weight DOC fractions by cross-flow ultrafiltration, but which fraction is more bioreactive is still in dispute.
Stable carbon isotope can be used to trace the origins of organic carbon, and the approach depends on the fact that DOC from different origins has different stable isotopic compositions. The riverine DOC has a δ13C value of -27‰, which is different from freshwater phytoplankton, with a range from -35‰ to -25‰. This paper reviewed the researches on the stable carbon isotope ratio of the autochthonous and allochthonous DOC, suggesting that the main sources of DOC in eutrophic lakes can be identified by using natural stable carbon isotope ratio of DOC; the difference on the total dissolved carbohydrates (TCHO) and dissolved combined neutral sugar (DCNS) concentrations, as well as the humic substances (HS) was compared between the autochthonous and allochthonous DOC. Net increases in TCHO and DCNS were observed in the autochthonous DOC during phytoplankton blooms, whereas the HS fraction was quantitatively important in the allochthonous DOC. Many studies have reported the bacterial availability of TCHO and DCNS, and the ratio of TCHO/DOC is used to characterize the bioavailability of DOC, whereas HS can also increase bacterial secondary production and support bacterial growth if labile substrates are abundant.
Furthermore, to elucidate the bioavailability of the two sources of DOC, the bacterial secondary production, bacterial respiration and bacteria growth efficiency (BGE) was analyzed together. The DOC can be either transformed to bacterial secondary production or respired to inorganic carbon. BGE is the fraction of assimilated organic carbon that supports growth. The source of DOC and its chemical composition could be a key regulator of BGE. Traditionally, the autochthonous DOC has been considered to be the main source for bacterial as well as other secondary production, the allochthonous DOC was long considered relatively recalcitrant to bacterial degradation. However, in lakes which are both humic-rich and oligotrophic, the ecosystem respiration exceeds gross primary production, suggesting that the allochthonous DOC can be incorporated into the bacteria biomass and makes a significant carbon and energy subsidy for lakes food web, but little of the allochthonous carbon assimilated by bacteria is likely to reach higher consumers. Recent studies suggest that bacterial BGE increases with the concentration of the low molecular weight DOC in allochthonous DOC.
Discussing the bioavailability and efficiency of carbon transfer in the food web from the sources of DOC, will be helpful to investigate the characterization of carbon cycling in the eutrophic lakes, enhance our understanding of the lake ecology and to provide scientific references for the lake management and protection.
eutrophic lake; dissolved organic carbon; source; bioavailability; review
国家自然科学基金资助项目(41201076, 31270507, 31070420);湖泊与环境国家重点实验室开放基金资助项目(2012SKL006)
2012- 09- 24;
2013- 03- 21
10.5846/stxb201209241349
*通讯作者Corresponding author.E-mail: fxkong@niglas.ac.cn
叶琳琳,孔繁翔,史小丽,阳振,闫德智,张民.富营养化湖泊溶解性有机碳生物可利用性研究进展.生态学报,2014,34(4):779- 788.
Ye L L, Kong F X, Shi X L, Yang Z, Yan D Z, Zhang M.The bioavailability of dissolved organic carbon in the eutrophic lakes.Acta Ecologica Sinica,2014,34(4):779- 788.
