石墨化温度对多孔碳微球/石蜡复合相变热界面材料性能的影响
2014-07-24曹志华徐益涛史剑吴晓琳符显珠孙蓉袁铭辉汪正平
曹志华徐益涛史 剑吴晓琳符显珠孙 蓉袁铭辉汪正平
1(中国科学院深圳先进技术研究院深圳电子封装材料工程实验室 深圳 518055)2(香港科技大学机械与航空系 香港 999077)3(香港中文大学 香港 999077)
石墨化温度对多孔碳微球/石蜡复合相变热界面材料性能的影响
曹志华1徐益涛1史 剑1吴晓琳1符显珠1孙 蓉1袁铭辉2汪正平3
1(中国科学院深圳先进技术研究院深圳电子封装材料工程实验室 深圳 518055)2(香港科技大学机械与航空系 香港 999077)3(香港中文大学 香港 999077)
文章利用葡萄糖水热法合成炭微球,并用氢氧化钾进行烧结处理,得到多孔结构的炭微球。在以硝酸铁为催化剂的条件下,对多孔炭微球进行不同温度下的石墨化处理,利用 SEM、XRD、FTIR 和 BET 对材料进行了表征。结果表明,炭微球表现出了良好的球形形貌、丰富的孔结构及大的比表面积。炭微球在经过 1500℃ 处理后,其石墨化程度达 90%。通过对石蜡进行物理吸附,制备了多孔石墨化炭微球/石蜡相变复合材料并用作热界面材料,其热导率随石墨化温度的增加而增加。
多孔炭微球;石墨化;相变;热界面材料;石蜡
1 Introduction
Phase change thermal interface materials (TIMs) might be more effectively used in the electronics compared with conventional TIMs since the large latent heat absorption during the phase change process can delay or modify the temperature increase. Paraffin waxes are usually used as the phase change materials (PCMs) due to their advantages such as high latent heat, chemically stable, and commercially available at low cost[1]. However, paraffin wax has low thermal conductivity and might overflow when it melts, which has impeded the absorption and release of heat for the phase change materials.
Various techniques have been used to improve the performance of thermal conductivity of phase change materials, such as filling high thermal fillers into organic PCMs and providing an effective thermal conductive way for the PCMs, choosing high thermal conductive porous materials as substrate or encapsulating phase change materials with high thermal conductive shell[2-6].
Carbonaceous materials such as expanded graphite[7]and carbon fiber[8]would be good filler to enhance thermal conductivity of PCMs. However, many carbonaceous materials could not provide three-dimensional thermal conduction path because of their low dimension structure. Carbon microsphere could be a better choice due to its 3D structure. In order to obtain a better coordination with paraffin, it is essential to increase the specific surface area and graphitization.
In this paper, we report the porous graphitized carbon microspheres-paraffin composite as phase change thermal interface materials. The effect of graphitization of porous carbon microspheres on the thermal performance was also investigated.
2 Experimental
2.1 Preparation of Carbon Microspheres
A certain quality of glucose and Fe(NO3)3.9H2O were dissolved in aqueous solution respectively and mixed into 60 mL solution. After ultrasonic dispersing, the mixed solution was put into PTFE lining and the hydrothermal reaction happened when the hydrothermal synthesis reactor was put into a drying oven. After hydrothermal reaction, the dark brown colloid was washed with anhydrous ethanol and deionized water. At last, carbon microspheres were obtained after drying and grinding.
2.2 Preparation of Porous and Graphitic Carbon Microspheres
A certain quality of carbon microspheres and KOH were dissolved in aqueous solution respectively and mixed. After ultrasonic dispersing, the mixed solution was dried in the drying oven and the drying powder was put into ceramic crucible. Then
3 Results and Discussion
the drying powder was calcined in tube furnace at nitrogen atmosphere. The heating rate was 5℃/min and the calcined temperature was 700℃. The porous carbon microspheres were obtained after washing the calcined product with HCl and deionized water. The preparation of porous graphitized carbon microspheres was similar to porous carbon microspheres except that original materials were porous carbon microspheres and Fe(NO3)3.9H2O and the calcined temperature was higher than 1000℃. At last, porous and graphitized carbon microspheres were obtained after drying and grinding.
2.3 Characterization and Measurement
The morphology of samples was characterized by field emission scanning electron microscopy (FE-SEM, FEI Nova Nano SEM 450). The X-ray diffraction (XRD, Rigaku D/Max 2500, Japan) with Cu-Kα radiation was taken to measure the crystallographic structure of the products. Adsorption-desorption measurements were conducted on a micromeritics ASAP 2020 BET apparatus with liquid nitrogen at 77 K. The thermal conductivity was investigated by TIM thermal resistance & conductivity measurement apparatus (LW-9389), as is shown in Fig. 1.
As is shown in Fig. 2 (a, b), the particle size of initial carbon microspheres prepared by hydrothermal method were about 5—10 μm with good sphericity. The dehydration reaction take place between glucose molecules with long chains gave rise to the bonding between carbon microspheres. The dispersion of the carbon microspheres was improved by ultrasonic and more homogeneous carbon microspheres could be prepared. As is shown in Fig. 2 (c, d), the highly graphitized porous carbon microspheres remain good sphericity. The specific surface area was expected to have a substantial increase after porous-forming treating at 700℃ and graphitization at 1500℃.

Fig. 1. The core part of the TIM thermal resistance and thermal conductivity measurement apparatus (LW-9389)

Fig. 2. SEM of carbon microspheres
The principle of porous treatment was shown in equation (1), (2), (3) and (4). At high temperature (700℃), KOH had been decomposed into K2O andH2O, and the K2O and H2O reacted with the carbon microspheres. After the reaction between KOH and carbon microspheres, more and more holes had been obtained and the surface area improved substantially.
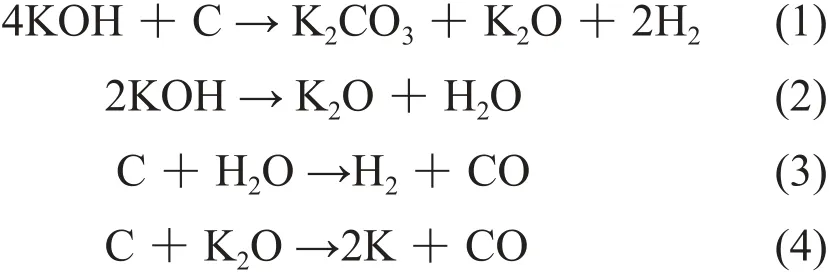
Graphitized carbon microspheres were obtained by adding Fe(NO3)3in the carbon microspheres and being calcined together to reorder carbon atoms of carbon microspheres. As is shown in Fig. 3, the XRD curves of carbon microspheres which were prepared at different temperature showed different shape. The curve of initial carbon microspheres displayed a broad and weak peak. It suggested that initial carbon microspheres were amorphous carbon and the degree of graphitization was very low. After treated with KOH and calcined at 700℃, the peak of curve changed sharply but the degree of crystallinity and the graphitization of carbon microspheres were still very low. As the temperature increased, the degree of crystallinity and the graphitization of carbon microspheres increased and were closer to graphite. The degree of graphitization was calculated by equation (5) and the corresponding values of 1200℃, 1300℃, 1400℃ and 1500℃ were 68%, 75%, 79% and 90%, respectively. High temperature made the carbon atoms react with iron atoms to generate compounds and higher temperature made the reaction be completed more fully. As the temperature fell, the carbon atoms were separated out from compounds and arranged into structure of graphite[9-11].
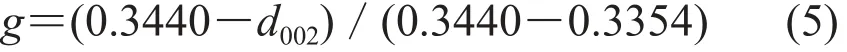

Fig. 3. XRD curves of porous carbon microspheres treated by porous-forming at 700℃ and graphitization at different temperatures
Here, g means the degree of graphitization, d002=λ/(2sinθ) corresponds to the interlayer distance, λ is X-ray wavelength, and θ is (002) plane diffraction angle.
As is shown in Fig. 4, the carbon microspheres prepared by the hydrothermal method contained more functional groups, suggesting that the graphitization of initial carbon microspheres was very low which was consistent with the XRD results. The graphitization of carbon microspheres was improved at higher temperatures. After treatment at 700℃, some infrared absorption peaks disappeared or the intensity was reduced, for example, the absorption peaks at 1016.6 cm—1and 1693.5 cm—1disappeared and the one at 1372.8 cm—1was reduced. When the initial carbon microspheres were treated at a high temperature, the process of dehydration and carbonization made initial carbon microspheres lose H and O atoms, therefore the amount of C-H and C-O functional groups was decreased and the absorption peaks disappeared or became weakened. Although many functional groups were decomposed during porous progress, the carbon microspheres were amorphous and the graphitization of porous carbon microspheres were low. As is shown in Fig. 4(c), after being graphitized at 1500℃, the functional groups were further reduced or disappeared. The hydrogen and oxygen contained in carbon microspheres have been removed after calcination at 1500℃.
In order to increase the surface area and improve the adsorptivity of carbon microspheres, the porous carbon microspheres were prepared by calcination with KOH at 700℃. After calcination, the pore size in the surface of carbon microspheres and surface area of carbon microspheres were improved obviously as is shown in Fig. 5. The BET surface

Fig. 4. Infrared absorption spectrum of carbon microspheres

Fig. 5. BET adsorption curves and pore size distribution
area of initial carbon microspheres was 5.7 m2/g and the average size of carbon microspheres was less than 5 nm. After porous-forming at 700℃ and graphitized at 1500℃, the BET surface area of carbon microspheres was 1307.5 m2/g and the average size of carbon microspheres was greater than 10 nm. It is expected that the high surface area could improve the absorption of paraffin.

Fig. 6. Thermal conductivity of paraffin composite phase change thermal interface materials with porous carbon microspheres graphitized at different temperature
As is shown in Fig. 6, the thermal conductivity of porous graphitic carbon microspheres (20 wt%)-paraffin (80 wt%) composite thermal interface materials gradually increased along with the graphitized temperature. When the temperature was higher than 1000℃, Fe atoms reacted with C and generated iron-carbon compounds. As thetemperature was higher, the reaction was more intensive. When the temperature dropped, Fe of ironcarbon compounds was separated and carbon atoms were recombined into graphite. And the higher temperature resulted in higher graphitization and better thermal conductivity. Compared with the pure paraffin wax, the thermal conductivity of composite thermal interface material was improved 300% and reached 1.6 W/(m·K) when the porous carbon microspheres were graphitized at 1400℃.
4 Conclusions
Highly graphitized porous carbon microspheres were obtained and used as substrate for phase change thermal interface materials. After porousforming and graphitization at high temperature, the surface area of carbon microspheres was improved 260 times to achieve 1307.5 m2/g and the degree of graphitization of carbon microspheres reached 90%. With the increase of graphitized temperature, the thermal conductivity of graphitized porous carbon microspheres-paraffin composite phase change thermal interface materials was also enhanced. Compared with the two-dimensional graphite, carbon microspheres with high graphitization and large specific surface area have the advantages of 3D thermal conductive path, which might be a more attractive filler for thermal management.
[1] Zegers P. Overview of energy storage work carried out in the framework of the European Community’s energy storage [C] // The 2nd BHRA Fluid Engineering International Conference on Energy Storage and Energy Management, Cranfied, 1983∶19-28.
[2] Bugaje IM. Enhancing the thermal response of latent heat storage systems [J]. International Journal of Energy Research, 1997, 21∶ 759-766.
[3] Zhou D, Zhao CY. Experimental investigations on heat transfer in phase change materials (PCMs) embedded in porous materials [J]. Applied Thermal Engineering, 2011, 31∶ 970-977.
[4] Tian Y, Zhao CY. Heat transfer analysis for phase change material (PCMs) [C] // The 11th International Conference on Energy Storage (Effstock 2009), 2009∶ 1-8.
[5] Zhao CY, Lu W, Tian Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs) [J]. Solar Energy, 2010, 84∶ 1402-1412.
[6] Boomsma K, Poulikakos D, Zwick F. Metal foams as compact high performance heat exchangers [J]. Mechanics of Materials, 2003, 35(12)∶ 1161-1176.
[7] Zhang ZG, Fang XM. Study on paraffin/expanded graphite composite phase change thermal energy storage material [J]. Energy Conversion and Management, 2006, 47∶ 303-310.
[8] Jun FK, Makoto K, Kodama Y, et al. Thermal conductivity enhancement of energy storage media using carbon fibers [J]. Energy Conversion Management, 2000, 41(14)∶ 1543-1556.
[9] Dhakate SR, Mathur RB, Bahl OP. Catalytic effect of iron oxide on carbon/carbon composites during graphitization [J]. Carbon, 1997, 35(12)∶ 1753-1756.
[10] Oya A, Otani S, Tomizuka I. Electron microscopic study on the turbostratic carbon formed in phenolic resin carbon by catalytic action of finely dispersed nickel [J]. Carbon, 1979, 17(1)∶ 71-76.
[11] Oya A, Otani S. Influence of particle size of metal on catalytic graphitization on non-graphitizing carbons [J]. Carbon, 1981, 19(5)∶ 391-394.
Effect of Graphitization Temperature on the Performance of Porous Carbon Microspheres/Paraffin Composite Phase Change Thermal Interface Materials
CAO Zhihua1XU Yitao1SHI Jian1WU Xiaolin1FU Xianzhu1SUN Rong1YUEN Matthew2WONG Chingping31
( Shenzhen Electronic Packaging Materials Engineering Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China )2( Department of Mechanical and Aerospace Engineering, The Hong Kong University of Science and Technology, Hong Kong 999077, China )3( The Chinese University of Hong Kong, Hong Kong 999077,China )
Graphitized porous carbon microspheres/paraffin composite phase change materials were prepared whichcould be used for the thermal management. KOH was introduced to help carbon microspheres to form the porous structure. The porous carbon microspheres exhibited good sphericity, abundant pores and large surface area, which would be more convenient to absorb the paraffin. The porous carbon microspheres were treated under different temperatures using Fe(NO3)3as catalyst to investigate the degree of graphitization. The graphitization temperature played an important role in improving the thermal conductivity of the phase change composite.
porous carbon microspheres; graphitization; phase change; thermal interface materials; paraffin
2014-07-31
TK 124
A
Foundation:Guangdong Innovative Research Team Program(2011D052);Shenzhen Peacock Pragram(KYPT20121228160843692);Shenzhen Electronic Packaging Materials (深发改【2012】372 号)
Author:Cao Zhihua, Master’s degree candidate. His research interests are the synthesis and application of carbon-based composite materials; Xu Yitao, Master’s degree candidate. His research interests are the synthesis and application of micro/nano-composite materials; Shi Jian, Master’s degree candidate. His research interests are the phase change thermal interface materials; Wu Xiaolin, Ph.D., Research Assistant. Her research interest is materials science; Fu Xianzhu, Ph. D., Associate Professor. His research interests include electronic & packaging materials and applied electrochemistry; Sun Rong(corresponding author), Ph. D., Professor. Her research interest is electronic packaging materials, E-mail:rong.sun@siat.ac.cn; Yuen Matthew, Ph.D., Professor. His research interests are advanced materials & technology, design & manufacturing, CAD/CAM, electronic packaging, energy, microsystems and technology; Wong Chingping, Ph. D., Professor. His research interests are polymer nanocomposites and high-density electric packaging materials.
