分子筛膜反应器中环己酮与乙二醇缩合反应的合成优化与反应动力学
2014-07-19安顺永邱灵芳周荣飞陈祥树
安顺永,张 飞,桂 田,邱灵芳,周荣飞,陈祥树
(江西师范大学 化学化工学院 江西省无机膜材料工程技术研究中心, 江西 南昌330022)
Membrane reactors which coupled membrane process with equilibrium-limited reactions were increasingly attracting much attention due to their selective removal of a reaction product[1-7].Some investigations focused on water-permeable pervaporation(PV)membrane reactors to promote the reactions such as esterifications[3-7].The PV process coupling with reactions allows the coverage of permeation components adsorbed in the membrane being easily close to saturation at lower temperatures. However, the PV-aid reaction requires that the membrane can hold against the chemicals when it contacts directly the liquids in reaction.Zeolite NaA membrane is unstable in acidic solutions[3].Zeolite T membrane with the crystal Si/Al molar ratios higher than that of zeolite NaA membrane showed good stability in acidic conditions[6].Zeolite T membranes have been applied to PV membrane reactor to improve the esterifications of acetic acid with ethanol andn-butyl alcohol[3,8].
Ketals are a family of important perfume materials and industrial row materials.Ethylene glycol cyclohexanone ketal is normally synthesized by the condensation of cyclohexanone with ethylene glycol by using traditional liquid acid catalyst such as sulphuric acid,hydrochloric acid andp-toulenesulfonic acid.An azeotropic distillation coupled with the ketalization could enhance the conversion of cyclohexanone[9-12]. However,the added azeotropic agents increased the impurity to the ketals,and the high temperature for azeotropic distillation resulted in the side reaction of self-condensation of ethylene glycol, when homogeneous catalysts were used.The PV process is believed to be a good candidate for replacing azeotropic distillation to couple with the condensation reaction.There is yet no report involving the synthesis of ketals with the assistance of PV process.
The permeation flux and selectivity of the membranes together with membrane reusability are important factors to their industrial applications,among which the permeation flux is the primary one for the economics and flexibility of membrane reactors.Zeolite NaA membrane showed an approximately flux of 2.0kg/(m2·h)for the mixture of water and ethanol with mass ratio of 10/90 at 75℃[13-14].Similarly,zeolite T membranes had the flux ranged from 0.6to 1.2kg/(m2·h)[7-8,15-16]in the same test condition.Recently,it is reported that a zeolite T membrane prepared in fluoride media had a high flux of 3.5kg/(m2·h)together with good separation factor(2900)under the same test system[17].This flux is two times higher than that of reported zeolite T membranes[7-8,15-16].In general,an improved separation performance in the system of water and ethanol can also be obtained for dehydration of other organics by a certain kind of zeolite membrane[8,13-14].A high flux has a great advantage to build up a compact membrane reactor[18].
The author reported a PV-aided ketalization of cyclohexanone with ethylene glycol for the first time.A kinetic model was set to describe the relaxation of membrane dehydration and the conversion.The stability and reusability of the membrane and catalyst were also studied.
1 Experimental
1.1 Materials and reagents
The cyclohexanone and ethylene glycol were purchased from Tianjin Fuchen chemical reagent Co.Ludox TM-40,NaOH,KOH,NaF,KF,aluminum powder were purchased from Aldrich Co.Al(OH)3was purchased from Wako Co.Zeolite H-βwas purchased from Catalysis Institute of Tianjin University.Tubular porous mullite support with average pore size of 1.0μm was purchased from Nikkato Co.The deionized(DI)water was prepared by using a Millipore membrane system with a resistivity of more than18MΩ.
1.2 Membrane preparation
Zeolite T membranes were synthesized on the outer surface of porous mullite supports by secondary hydrothermal method as described in previous work[17].The outer surface of the support was rub-coated with water slurry of zeolite T seeds.The slurry was prepared by mixing 5.0g seed powders and 20g water and sonicated for 30min.All the outer surface of the tubes was rubbed back and forth for a total time of approximately 1.0min.The seeded tubes were dried at 80℃for 1.0h.The excessive seeds on the surface were then removed by a cotton swab slowly.A fluoride-containing gel was obtained by mixing colloidal silica,Al(OH)3,NaOH, NaF, KF and deionized water. The resultant gel had a mole ratio ofn(SiO2)∶n(Al2O3)∶n(Na2O)∶n(K2O)∶n(NaF)∶n(KF)∶n(H2O)=1∶0.05∶0.26∶0.09∶0.375∶0.125∶35,and was aged for 24hwith stirring.The seeded supports were vertically immersed in the gel.The autoclave was sealed and moved to a preheated electronic oven.After hydrothermal synthesis at 150℃for 6.0h,the membrane was withdrawn,washed with distilled(DI)water to neutral and dried at 100℃for 3.0h.
1.3 Ketalization reactions
The ketalization of cyclohexanone with ethylene glycol was carried out in a batch PV membrane reactor,and the experiment schematic was shown in Fig.1.Typically,40g cyclohexanone and 30.4g ethylene glycol were added in the reactor.The commercial zeolite H-βcatalyst was calcined at 550℃for 6hfor activation.The catalyst loading ranged from 0to 2%of the total reaction mixture mass.The reaction was carried out at 100℃ with the assistance of PV process.The effective membrane areas were approximately 19.0cm2.The permeate was collected in a cold trap by liquid N2under vacuum,and 0.1g reaction mixture was withdrawn from the reactor at regular intervals.For comparison,the reaction was also carried out in traditional reactors in the absence of membrane.The samples were analyzed by using a Shimadzu GC-14Cgas chromatograph equipped with a thermal conductivity detector (TCD)and 3m column packed with Polarpark Q poly(ethylene glycol)-1000,and a flame ionization detector and a 30mAgilent HP-1capillary column,respectively.The permeate sample was directly injected into the TCD channel with H2carrier gas,and the sample from the reaction was however filtered through a syringe filter to separate the catalyst before being detected by FID,in which N2was as carrier gas.
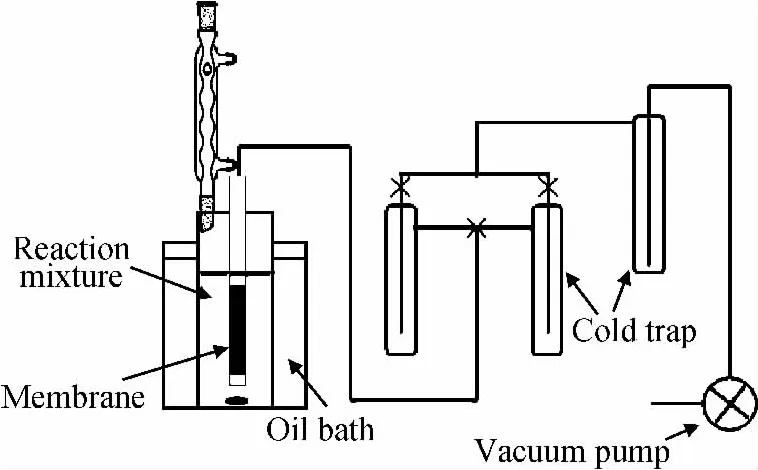
Fig.1 Set-up schematic of PV-aid ketalization
1.4 Characterization of zeolite T membranes and catalyst
Powder X-ray diffraction (XRD)patterns of the synthesized zeolite T membranes and the commercial catalyst were collected on the Rigaku company Ultima IV X-ray powder diffractometer with CuKαradiation of 40kV and 30mA.The step size of 2θwas 0.02°with a count time of 0.3sand a scan rate of 4.0°/min between 2θrange of 5°-45°.The morphologies of zeolite T membranes were observed by FEI Qunta 200scanning electron microscope(SEM).
PV experiments for water/ethanol mixtures were carried out in a batch system[19].The inside of the membrane tube was evacuated through a vacuum line.The permeation vapor was collected by a cold trap.The downstream pressure was maintained below 13.3Pa.The compositions of the feed and permeation were analyzed by a Shimadzu GC-14C gas chromatograph.The flux(J)was calculated by weighing the condensed permeation.The separation factor(α)was determined as Eq.(1).
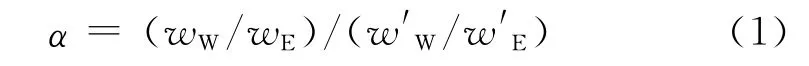
In Eq.(1),wW,wE,w′W,wEdenote the mass fractions of components W (water)and E (ethanol)in feed and permeate sides,respectively,%.
2 Results and discussions
2.1 Characterization results of zeolite T membranes and catalyst
2.1.1 XRD analysis
Fig.2showed the XRD patterns of fresh zeolite T membrane and the one after 15-time reuses.From Fig.2,it is seen that zeolite T membrane after 15-time reuses still had obvious characteristic XRD peaks of zeolite T membrane similar to the fresh membrane,indicating that zeolite layer was not destroyed during the reaction.
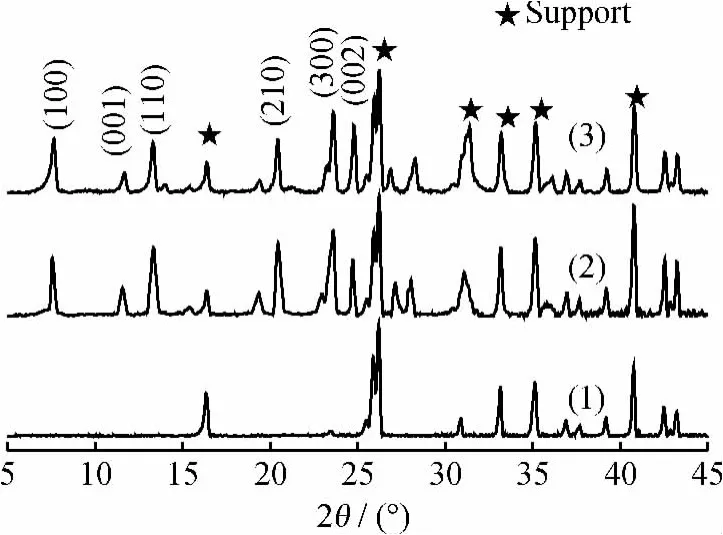
Fig.2 XRD patterns of mullite support,fresh zeolite T membrane and the one after 15-time reuses
The XRD patterns of the solid catalyst zeolite H-βafter 10-time reuses was shown in Fig.3.The fresh and used catalysts exhibited the same characteristic XRD peaks as the simulated zeolite H-βat 2θof 7.79°and 22.57°,indicating that the commercial zeolite H-βcrystals were free of impurity and stable in the synthesis solution.
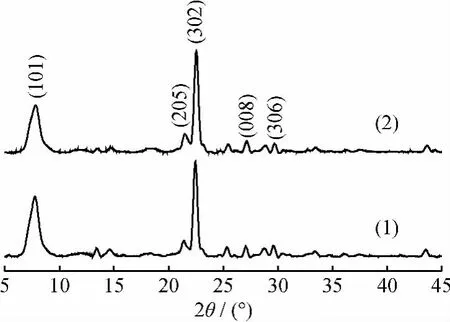
Fig.3 XRD patterns of fresh zeolite H-βcatalyst and that after 10-time reuses
2.1.2 SEM analysis
The SEM images of zeolite T membrane before and after 15-time reuses of the reaction were shown in Fig.4.The surface and cross-sectional SEM images of fresh membrane (Fig.4(a)and 4(b))revealed that zeolite T membrane contained an oriented top layer with agglomerative needle-like particles.After 15-time reuses,continuous zeolite T layers could still be observed (Fig.4(c)and 4(d)).The SEM observation in Fig.4further verified that zeolite T membrane was stable in the reaction.
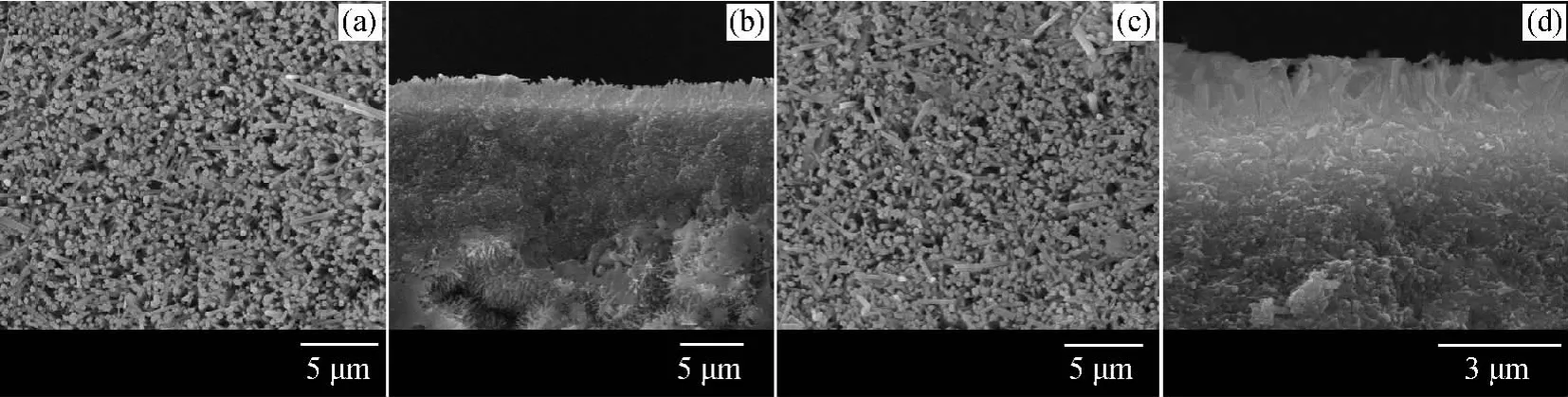
Fig.4 SEM images of fresh membrane and membrane after 15-time reuses
2.2 Effect of reaction conditions on cyclohexanone(CH)ketalization with ethylene glycol(EG)
2.2.1 Effect of cyclohexanone-to-ethylene glycol molar ratio(n(CH)/n(EG))
At the reaction conditions ofn(CH)/n(EG)varying from 1to 1/2,reaction temperature 100℃,zeolite H-βcatalyst of 1%mass fraction of total reaction mixture the cyclohexanone conversion of cyclohexanone ketalization with ethylene glycol versusn(CH)/n(EG)at different reaction time was illustrated in Fig.5,from which it can be seen that final cyclohexanone conversion after 6hreaction was 89.0%undern(CH)/n(EG)of 1and increased to 98.4%undern(CH)/n(EG)of 1/1.2.The fact that the increase of ethylene glycol amount would drive the reaction equilibrium toward the product side in traditional ketalization without PV was confirmed in some studies[11-12].An(CH)/n(EG)of 1/4was used in the traditional reaction catalyzed by Al-MCM-41to obtain higher cyclohexanone conversion(75.1%)[12].However,further increase ofn(CH)/n(EG)from 1.2to 2in the PV-aided reaction made the final cyclohexanone conversion decreased,which attributed to the low water removing rate because the water content in reaction mixture was decreased.The highest conversion for esterification was also obtained at moderate acid-toalcohol molar ratio of around 1/2other than higher values[3].In addition,the optimizedn(CH)/n(EG)ratio was 1/1.2which was much close to ideal value of 1according to the reaction formula.The cyclohexanone conversion arrived at almost complete value of 98.4%by the aid of PV process at a lown(CH)/n(EG),as compared to the low equilibrium conversion of 64.3%.
2.2.2 Effect of catalyst mass fraction
Fig.6showed the evolution of the cyclohexanone conversion in cyclohexanone ketalization with ethylene glycol by using different catalyst mass fraction from 0to 2%of total reaction mixture undern(CH)/n(EG)of 1/1.2.The reaction was processed very slowly even with the assistance of PV process when no catalyst was used.The velocity of the reaction obviously increased with the increase of the catalyst mass fraction from 0to 0.5%.The cyclohexanone conversion was 90.0%with the catalyst mass fraction of 0.5%and reaction time of 6h,but increased more than 98.0%when the catalyst mass fraction was enhanced to 1%and 2%.
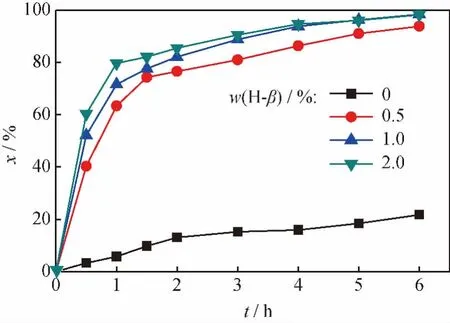
Fig.6 Cyclohexanone conversion(x)vs catalyst mass fraction in cyclohexanone ketalization with ethylene glycol
2.3 Membrane reusability in cyclohexanone ketalization with ethylene glycol
Membrane stability and reusability are known as important issues for membrane reactor.15-time reuses of a zeolite T membrane were investigated under optimized reaction conditions(n(CH)/n(EG)of 1/1.2,reaction temperature of 100℃ and fresh catalyst of 1%of the total reactant mass fraction).A simple washing with hot DI water(at 60-70℃)under vacuum was performed for 4hto treat the membrane after each cycle.The cyclohexanone conversion was almost independent of recycled times of the membrane as shown in Fig.7,which was still as high as above 95.0%,only a little lower than that in the first cycle (98.4%). The results revealed that zeolite T membrane had a bright prospect in the application of cyclohexanone ketalization with good stability and reusability.XRD (See Fig.2) and SEM (See Fig.3)characterizations also supported that the zeolite T layers were stable after 15-time reuses.
Table 1presents the PV performance of zeolite T membrane before and after 15-time reuses with the mixture of water and ethanol of 10/90mass ratio at 75℃. The used membranes displayed better selectivity and lower flux than the fresh membrane,indicating that as-synthesized zeolite T membrane was stable in the reaction.The small changes in flux and selectivity for the used membrane could be contributed to the adsorbed organics,which hindered the permeation of both water and ethanol molecules,and may be hardly totally removed by the simple washing process.
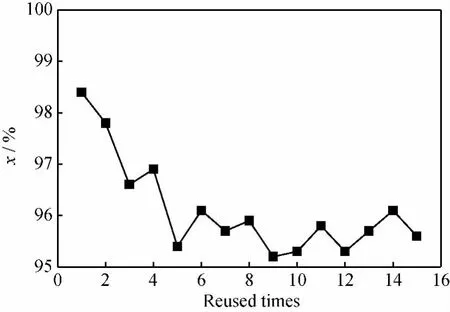
n(CH)/n(EG)=1/1.2;T=100℃;w(H-β)=1%
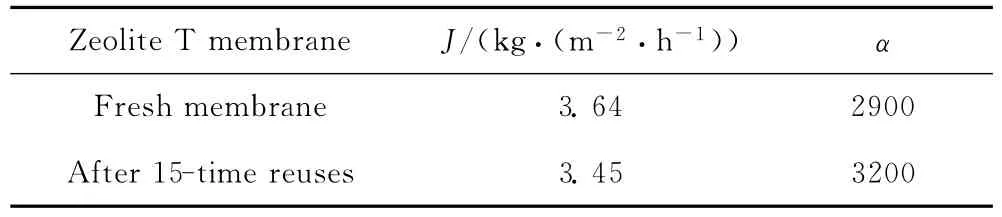
Table 1 PV performance of zeolite T membrane before and after 15-time reuses in cyclohexanone ketalization with ethylene glycol at 75℃
2.4 Catalyst reusability in cyclohexanone ketalization with ethylene glycol
The reusability of zeolite H-βcatalyst for cyclohexanone ketalization with ethylene glycol was investigated in a traditional reactor in the absence of membrane.The reaction volume was reduced by approximately 5%for each repeated cycle due to approximately 5%mass loss of catalyst for each recovery. Fig.8showed the cyclohexanone conversion of cyclohexanone ketalization with ethylene glycol catalyzed by the reused catalyst as well as fresh catalyst.The catalytic performance of the reused but uncalcined catalyst dropped right after the second cycle and then kept constant.However,the recalcination progress for the catalyst after each cycle could recover its catalytic performance.The drop in catalytic performance for the uncalcined catalyst could attribute to the adsorbed organics other than the damage of crystal framework,which was evidenced by XRD (See Fig.4). The declined trend in the equilibrium conversion was not found in cyclohexanone ketalization with ethylene glycol catalyzed by the catalyst after 10-time reuses and recalcined,suggesting that there was little loss of catalytic sites of zeolite H-βduring the tested period.
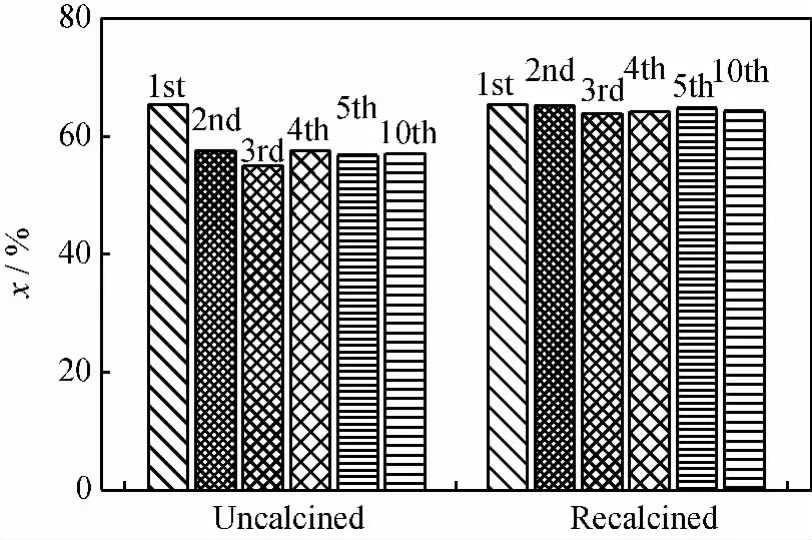
Fig.8 Cyclohexanone conversion(x)of cyclohexanone ketalization with ethylene glycol catalyzed by uncalcined and recalcined catalyst of different reused times
2.5 Kinetics studies of cyclohexanone ketalization with ethylene glycol
The cyclohexanone ketalization with ethylene glycol catalyzed by zeolite H-βis one-step reaction shown in Eq.(2),and which can be represented schematically by Eq.(3).
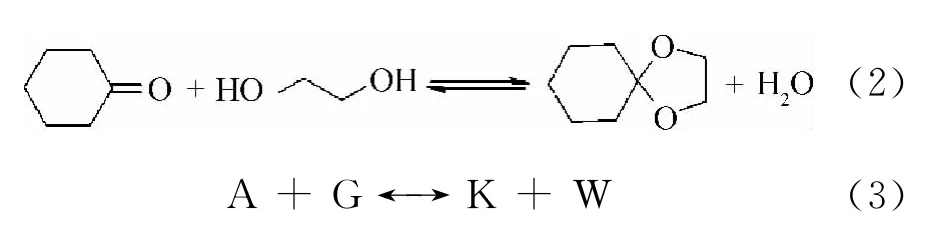
In Eq.(3),A,G,K and W represented cyclohexanone,ethylene glycol,ethylene glycolcyclohexanone ketal and water,respectively.
In order to simulate the reaction process of the PV-aided cyclohexanone ketalization with ethylene glycol,a simply model was made based on the three assumptions as follows.
(1) The ketalization is a second-order reversible reaction.
(2)The membrane is perfect,namely the permeation is mainly water and other species could be neglected.
(3)The flux through membranes is liner relationship to the water mass fraction due to the low water mass fraction in the reaction mixture.
For the traditional ketalization without PV,dilute solutions can be obtained when a large excess of alcohols (300%excess[12])is used and the changes in activity coefficient are normally omitted in kinetic model.However,if high yield per volume is to be achieved in PV-aided ketalization,the initial excess of alcohols will be relatively small(20%excess in this system),which implies that the mass fractions of the different components will vary significantly. Therefore, it is expected that variations in the activity coefficients will have to be taken into account,since these variations cause variations in reactivity.The activity of every species in reaction mixture can be written as Eq.(4).

In Eq.(4),aiis the activity of componenti,mol/L.Ciis the concentration of componenti,mol/L.γiis the activity coefficient of componenti.According to the kinetic expression for second-order reversible reaction,the reaction rate of each component is written as Eq.(5)-(8).And the reaction raterof ketalization is described as Eq.(9).
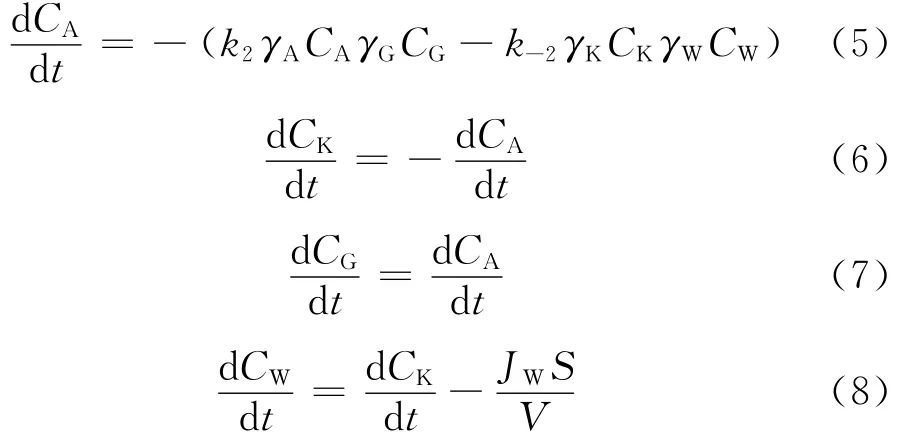
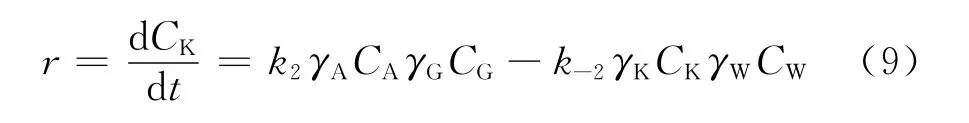
In Eq.(5)-(9),k2andk-2are the reaction rate constants for forward and reverse reactions,L/(mol·min).JWis the water flux,mol/(m2·h).VandSare the volume of reaction mixture and the effective membrane area,respectively,L and m2.
The change of reaction volume dependent of synthesis time was described as Eq.(10).
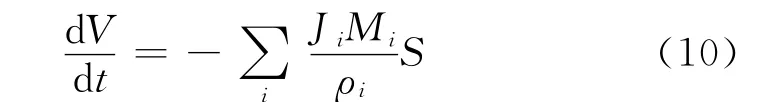
In Eq.(10),Jiis the flux of each permeation component,andMiandρiare the molecular mass and density of speciesi,respectively,mol/(m2·h),kg/mol and kg/L.Fig.9showed the mass fraction of each component in the permeation dependence of reaction time in PV-aided ketalization.From Fig.9,it was found that the mass fraction of water in permeate was above 97%at 100℃.Therefore,it is considered that the second assumption is resonable in the system,namely,JA=JG=JK=0,Eq.(10)also can be written as Eq.(11).
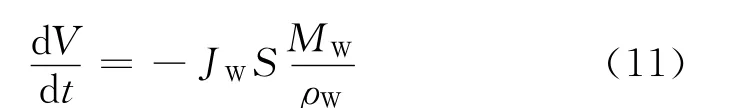
It is assumed that the water flux is proportional to the water content,as described in Eq.(12).

In Eq.(12),qis the coefficient of membrane permeability measured from ketalization reaction mixture in which the mass fraction of water was 10%at 100℃.
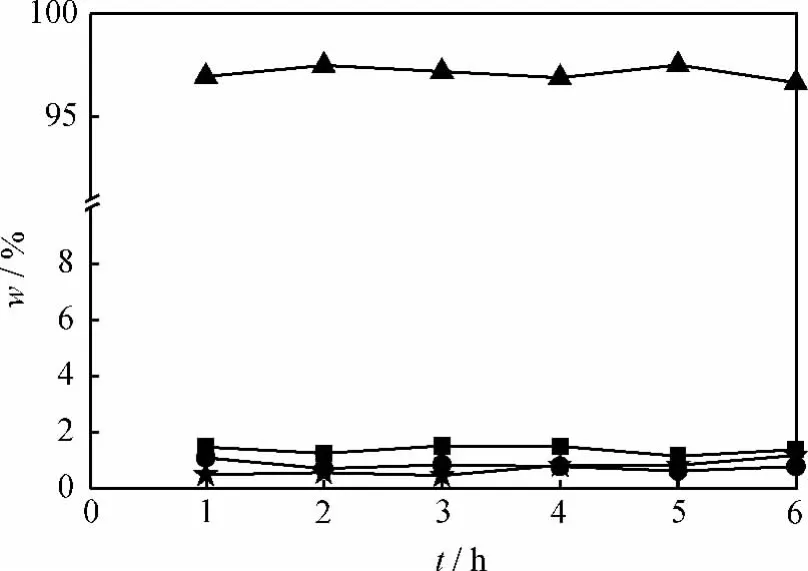
Fig.9 The mass fraction of each component vs reaction time in PV-aided cyclohexanone ketalization with ethylene glycol
The activity coefficientγidepends on the composition of the reaction mixture,reaction temperature and pressure,which can be calculated by using UNIQUAC group contribution method[20].
The reaction rate constants for the PV-aided reactions were the same as those in the reaction without PV since the membrane was inert(not catalytic). By plotting lnkversus 1/T,an Arrhenius plot was obtained which was shown in Fig.10.From this plot the activation energy of 67.7kJ/mol could be calculated according to Eq.(13).
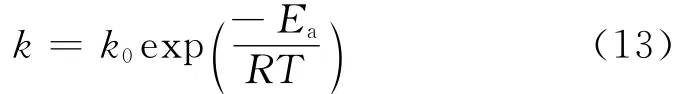
In Eq.(13),Eaandk0represent activity energy(kJ/mol)and frequent factor,respectively.
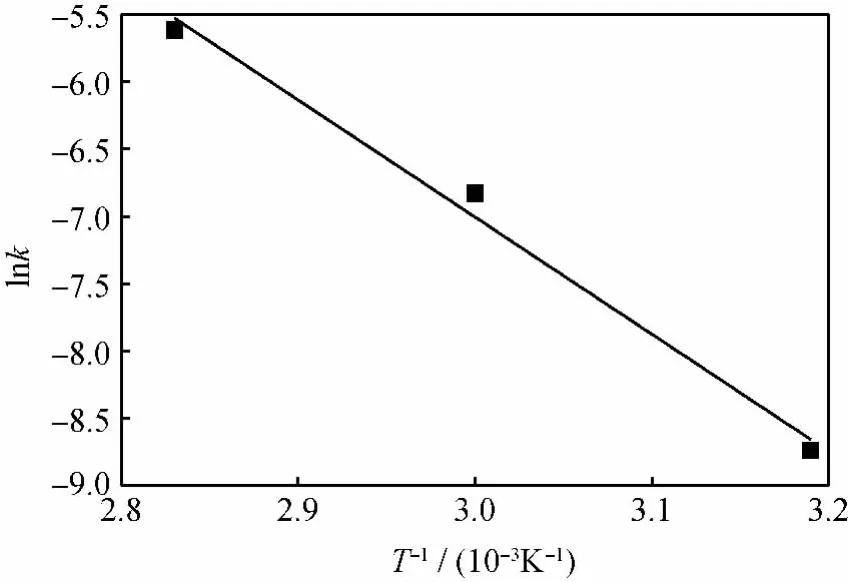
Fig.10 The apparent reaction rate(k)vs reaction temperature(T)in cyclohexanone ketalization with ethylene glycol
Fig.11showed the variation of cyclohexanone conversion with reaction time in cyclohexanoneketali-zation with or without PV obtained from the kinetic model and experiment both.The lines in Fig.11was calculated from these equations[Eq.(5)-(12)]by using the parameter values.From Fig.11 it is seen that the calculated values were in good agreement with the experimental data for the ketalization with and without PV process.
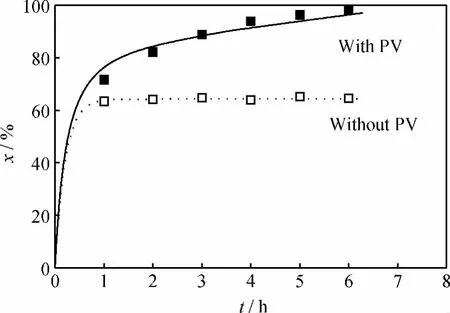
Fig.11 Cyclohexanone conversion(x)vs reaction time(t)in cyclohexanone ketalization with ethylene glycol with or without PV-aid obtained from the kinetic model and experiments
3 Conclusions
Zeolite T membrane was applied to the zeolite-H-βcatalyzed ketalization of cyclohexanone with ethylene glycol.The assistance of PV process broke down the equilibrium and increased cyclohexanone conversion from 64.3%to 98.4%within 6hof reaction time.All the cyclohexanone conversions for the tested 15-time reuses of the membrane were above 95.0%since the used membrane displayed high PV performance similar to the fresh one.The catalytic activity of commercial zeolite H-βcatalyst did not obviously decrease after 10-time reuses and recalcined.The PV-aided ketalization process was well simulated by a kinetic model.
[1]ZHANG Y,WU Z,HONG Z,et al.Hydrogen-selective zeolite membrane reactor for low temperature water gas shift reaction[J].Chem Eng J,2012,197(1):314-321.
[2]WANG X B,ZHANG X F,LIU H O,et al.Preparation of titanium silicalite-1catalytic films and application as catalytic membrane reactors[J].Chem Eng J,2010,156(3):562-570.
[3]TANAKA K, YOSHIKAWA R,CUI Y,et al.Application of zeolite membranes to esterification reactions[J].Catal Today,2001,67(1-3):121-125.
[4]KAZUHIRO T, RYUUHEI Y, CUI Y,et al.Application of zeolite T membrane to vapor-permeationaided esterification of lactic acid with ethanol[J].Chem Eng Sci,2002,57(9):1577-1584.
[5]李雪辉,王乐夫.催化剂对酯化反应渗透汽化膜稳定性的影响[J].高校化学工程学报,2000,14(4):387-389.(LI Xuehui,WANG Lefu.Effects of catalyst on the stability of membranes in esterification-pervaporation process[J].J Chem Eng Chin Univ,2000,14(4):387-389.)
[6]阎建民,陈观文.渗透汽化-蒸馏-酯化反应耦合过程动力学[J].膜科学与技术,2002,22(5):6-11.(YAN Jianmin, CHEN Guanwen. Kinetic model of pervaporation-distillation-esterification coupling process[J].Membr Sci Technol,2002,22(5):6-11.)
[7]CUI Y, KITA H, OKAMOTO K I.Zeolite T membrane:Preparation,characterization,pervaporation of water/organic liquid mixtures and acid stability[J].J Membr Sci,2004,236(1-2):17-27.
[8]周汉,李砚硕,朱广奇,等.微波合成a&b取向的T型分子筛膜及其在渗透汽化耦合酯化反应中的应用[J].催化学报,2008,29(7):592-594.(ZHOU Han,LI Yanshuo,ZHU Guangqi,et al.Microwave synthesis of a&b-oriented zeolite T membranes and their application in pervaporation-assisted esterification[J].Chin J Catal,2008,29(7):592-594.)
[9]WU S,DAI W,YIN S,et al.Bismuth subnitrate as an efficient heterogeneous catalyst for acetalization and ketalization of carbonyl compounds with diols[J].Catal Lett,2008,124(1-2):127-132.
[10]BAAR M R,RUSSELL C E,WUSTHOLZ K L,et al.The ethylene ketal protecting group revisited:The synthesis of 4-hydroxy-4,4-diphenyl-2-butanone[J].J Chem Educ,2005,82(7):1057-1064.
[11]RANU B C,JANA R,SAMANTA S.A simple,efficient and general procedure for acetalization of carbonyl compounds and deprotection of acetals under the catalysis of indium(Ⅲ)chloride[J].Adv Synth Catal,2004,346(4):446-450.
[12]RABINDRAN J B,PANDURANGAN A.Al-MCM-41 as an efficient heterogeneous catalyst in the acetalization of cyclohexanone with methanol,ethylene glycol and pentaerythritol[J].J Mo Catal A:Chem,2006,256(1-2):184-192.
[13]KITA H,HORII K,OHTOSHI Y,et al.Synthesis of a zeolite NaA membrane for pervaporation of water/organic liquid mixtures[J].J Mater Sci Lett,1995,14(3):206-208.
[14]OKAMOTO K I,KITA H,HORII K.Zeolite NaA membrane:Preparation,single-gas permeation,and pervaporation and vapor permeation of water/organic liquid mixtures[J].Ind Eng Chem Res,2001,40(1):163-175.
[15]ZHOU H,LI Y,ZHU G,et al.Preparation of zeolite T membranes by microwave-assisted in situ nucleation and secondary growth[J].Mater Lett,2009,63(2):255-257.
[16]CHEN X X,WANG J Q,YIN D H,et al.Highperformance zeolite T membrane for dehydration of organics by a new varying temperature hot-dip coating method[J].AIChE J,2013,59(3):936-947.
[17]ZHOU R F,ZHANG F,HU N,et al.Fast preparation of high-performance zeolite T membranes in fluoride media[J].Chem Lett,2011,40(12):1383-1385.
[18]HASEGAWA Y,ABE C, MIZUKAMI F,et al.Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst[J].J Membr Sci,2012,415-416(1):368-374.
[19]ZHOU R F,HU Z L,HU N,et al.Preparation and microstructural analysis of high-performance mordenite membranes in fluoride media[J].Micropor Mesopor Mater,2012,156(1):166-170.
[20]ABRAMS D S, PRAUSNITZ J M. Statistical thermodynamics of liquid-mixtures-new expression for excess Gibbs energy of partly or completely miscible systems[J].AIChE J,1975,21(1):116-128.
