Synthesis and Antimicrobial Activity of Boron-doped Titania Nano-materials*
2014-07-18王昱征,薛向欣,杨合
Synthesis and Antimicrobial Activity of Boron-doped Titania Nano-materials*
WANG Yuzheng (王昱征)1,2,3,4, XUE Xiangxin (薛向欣)1,2,3,4,**and YANG He (杨合)1,2,3,44
1Institute of Metallurgical Resources and Environmental Engineering, Northeastern University, Shenyang 110819, China
2Liaoning Key Laboratory of Metallurgical Resources Recycling Science, Shenyang 110819, China
3Liaoning Engineering and Technology Research Center of Boron Resources Comprehensive Utilization, Shenyang 110819, China
4Liaoning Provincial Universities Key Laboratory of Boron Resources Ecological Utilization Technology and Boron Materials, Shenyang 110819, China
Antibacterial activity of boron-doped TiO2(B/TiO2) nano-materials under visible light irradiation and in the dark was investigated. A simple sol-gel method was used to synthesize TiO2nano-materials. X-ray diffraction pattern of B/TiO2nano-materials represents the diffraction peaks relating to the crystal planes of TiO2(anatase and rutile). X-ray photoelectron spectroscopy result shows that part of boron ions incorporates into TiO2lattice to form a possible chemical environment like Ti O B and the rest exist in the form of B2O3. The study on antibacterial effect of B/TiO2nano-materials on fungal Candida albicans (ATCC10231), Gram-negative Escherichia coli (ATCC25922) and Gram-positive Staphylococcus aureus (ATCC6538) shows that the antibacterial action is more significant on Candida albicans than on Escherichia coli and Staphylococcus aureus. Under visible light irradiation, the antibacterial activity is superior to that in the dark.
boron doping, titania, antimicrobial activity
1 INTRODUCTION
Contamination by microorganisms is of great concern in a variety of areas, such as medical devices, healthcare products, water purification systems, hospitals, dental office equipment, food packaging, food storage, and household sanitation [1-3]. It is essential to have appropriate antibacterial method. Photocatalysis is a promising technology based on the interaction between light and solid semiconductor particles and is able to produce highly oxidative species that destroy bacteria and a large variety of chemical contaminants. Among the photoactive semiconductors are TiO2, ZnO, Fe2O3, WO3and CdSe. TiO2is most widely used in different media as photocatalyst because of its high stability, low cost and wide availability [4-6]. Matsunaga et al. [7] reported for the first time the microbiocidal effect of TiO2photocatalytic reactions. Since then, the research on photocatalytic killing of TiO2has been intensively conducted on a wide spectrum of organisms including viruses, bacteria, fungi, algae and cancer cells. From these experiments it is unclear as to the primary mechanism for toxicity, with three possible candidates: (i) oxidation of phospholipid membranes, (ii) oxidation of Coenzyme A or (iii) direct cleavage of DNA. The latter two mechanisms are reliant upon particulate TiO2entering the cell structure [8].
However, the wide band gap of TiO2greatly limits its applications. Photocatalysts exhibiting reactivity under visible light (λ>400 nm) could be obtained by non-metal doping such as nitrogen [9], sulfur [10], carbon [11], fluorine [12] and boron [13, 14]. Among these anion doped TiO2, boron doping TiO2has attracted more attentions in the application studies of functional materials due to its prompting creation of electron acceptor level. Zaleska et al. [15] synthesized boron modified TiO2using boric acid and boric acid triethyl ester by the sol-gel method and by grinding anatase powder with boron dopant. They found that boron doping could result in absorption of the visible light and these B-TiO2samples had higher activity for photo-oxidation of phenol under the visible light irradiation than pure TiO2.
Boric acid is a well-known antibacterial and anti-fungal agent, inhibiting a variety of bacteria and fungi. However, boric acid is low melting and boiling, so it cannot be directly applied in high temperature products. This greatly limits its applications in antimicrobial material. No work has been reported on boron-doped TiO2in antimicrobial field.
In this study, boron-doped TiO2nano-materials are prepared by a sol-gel method. Their antibacterial effect is examined against Candida albicans, Escherichia coli and Staphylococcus aureus. These microorganisms are selected because of their relatively diverse make up in terms of biological structure, in particular the cell walls of the organisms, with high resistance to conventional antibacterial techniques. Our aim is to better understand the interaction of boron with anatase and potential effects in the antimicrobial ability.
2 EXPERIMENTAL
2.1 Preparation of boron-doped TiO2nano-materials The boron doped TiO2was synthesized by sol-gel method. 10 ml tetrabutyl titanate was dissolved into
* Supported by the National Natural Science Foundation of China (51090384).
** To whom correspondence should be addressed. E-mail: xuexx@mail.neu.edu.cn35 ml anhydrous ethanol (solution A), and solution B consisted of 35 ml anhydrous ethanol, 4 ml acetic acid, 10 ml water, and 0.61 g boric acid. Then solution A was added drop-wise to solution B under magnetic stirring. The resultant mixture was stirred at room temperature for 3 h until the transparent sol was obtained. The sol was then aged for 1 day and the gel was obtained. The gel was dried at 80 °C for 24 h, and the dried powder was calcinated at various temperatures (600, 700, 800 and 900 °C) at a heating rate of 5 °C per minute and held at these temperatures for 1 h, then cooled down naturally. The resulting materials were labeled as B/TiO2-x, where x denotes the calcination temperature (°C).
2.2 Characterization
X-ray diffractometry (XRD; Shimadzu) equipped with a copper target (1Kαλ=0.1541874 nm) was used to identify the formation phase of the powder samples. The morphology and crystallite size of the powder were taken with TECNAI G2 F20 transmission electron microscope (TEM). The binding energy was identified by X-ray photoelectron spectroscopy (XPS) with Mg Kαradiation (ESCALAB250). The BET surface area was determined by N2adsorption on a Quantachrome NOVA 1200e apparatus at 77 K.
2.3 Determination of antimicrobial effect
Candida albicans (ATCC 10231), Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 6538) were chosen as the bacteria in the antibacterial examinations. Antibacterial performance was evaluated by inhibition ring method. All glassware and materials were autoclaved at 120 °C for 15 min to ensure the sterility for testing. Microorganisms were inoculated and grew aerobically in 25 ml liquid nutrient broth at 37 °C on a rotary shaker (120 r·min−1) for 18 h. They were adjusted to a concentration of 104-105CFU·ml−1in the antibacterial assay.
The experiments were carried out under two irradiation conditions: visible light and dark. The visible light source was 15 common fluorescent tubes, mounted on the inside of an artificial climate box (with total power of 15×18 W). The experimental procedures were as follows. Inhibition ring method was carried out by pouring agar into Petri dishes to form 4 mm thick layers and the Petri dishes were left for 10 min to dry in the air. Dense inoculum of the tested microorganisms was added to Petri dishes, and then the compacted powder (0.70 g of samples, 14 mm in diameter) was arranged on the agar surface and incubated at 37 °C for 24 h in the artificial climate box under the visible light or in the dark. A vernier caliper was adopted to measure the diameter of the width of inhibition zone (mm).
3 RESULTS AND DISCUSSION
3.1 XRD analysis
Figure 1 shows the XRD patterns of boron-doped TiO2nano-materials with different calcination temperatures. As the calcination temperature increases from 600 to 800 °C, a diffraction peak corresponding to the (101) plane (peak 25.3°) of anatase phase appears. The anatase is predominant in crystalline phases of all calcinated samples. A small amount of rutile (peak 27.4°) appears in B/TiO2at 700 °C. It demonstrates that the phase transformation from anatase to rutile occurs at 700 °C, which is consistent with that reported in literature [13]. At calcination temperature of 900 °C, anatase phase nearly disappears, rutile phase Ti0.924O2is predominant and a small amount of rutile TiB0.024O2is present. The ratio between anatase and rutile extracted from XRD spectra, which is often used to quantify the anatase-to-rutile transformation, is calculated with the empirical relationship:
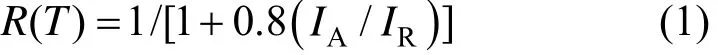
where R(T) is the percentage content of rutile at temperature T, IAis the intensity for the main anatase reflection, and IRis the intensity for the main rutile reflection. Table 1 presents basic characteristic parameters of the antimicrobial materials. It is well known that the crystalline and the crystal phase are crucial factors in the antimicrobial activity of TiO2, where the crystalline anatase phase is considered as the most active form of TiO2[16], while rutile and amorphous TiO2are believed to be relatively inactive.
Figure 1 also shows that the intensity and width of the diffraction peaks of anatase become higher and narrower as the calcination temperature increases from 600 to 900 °C, suggesting that the crystalline of anatase is greatly improved. According to the line width analysis of anatase (101) based on the Scherrer equation, the average grain sizes of the samples are estimated to be about 18 nm, 48 nm and 73 nm for B/TiO2-600, B/TiO2-700, B/TiO2-800, respectively. Apparently, the calcination temperature has a significant effect on the grain size of boron-doped titania particles.
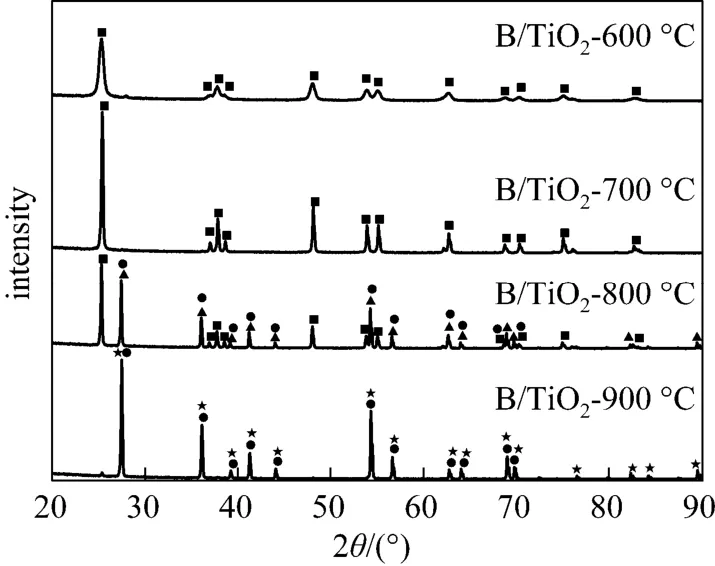
Figure 1 XRD patterns of B/TiO2calcinated at different temperatures■ anatase TiO2; ▲ rutile TiO2; ● rutile TiB0.024O2; ★ rutile Ti0.924O2
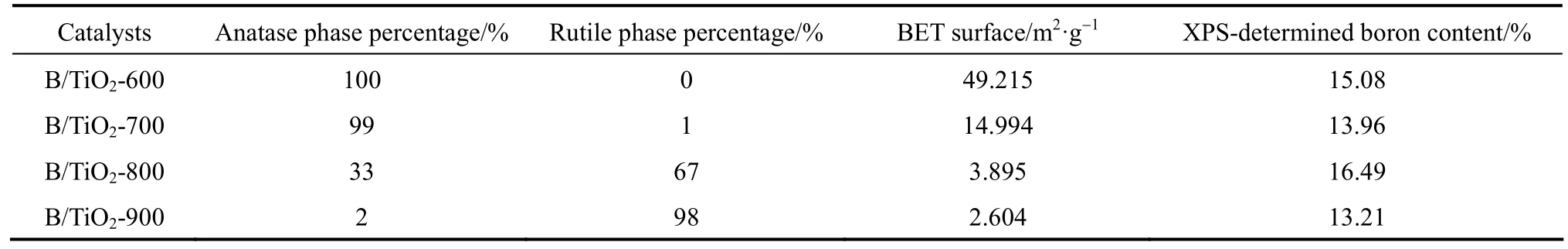
Table 1 Basic characteristic parameters of the antimicrobial materials
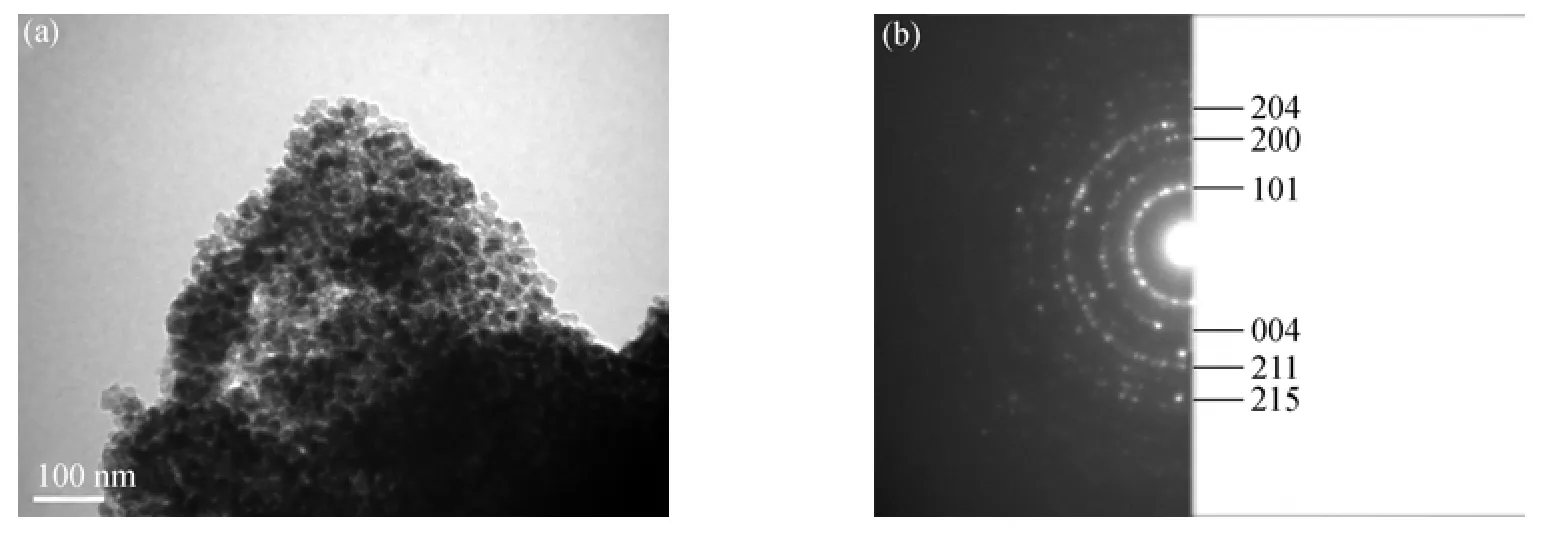
Figure 2 TEM images of B/TiO2-600 (a) and SADP of B/TiO2-600 (b)
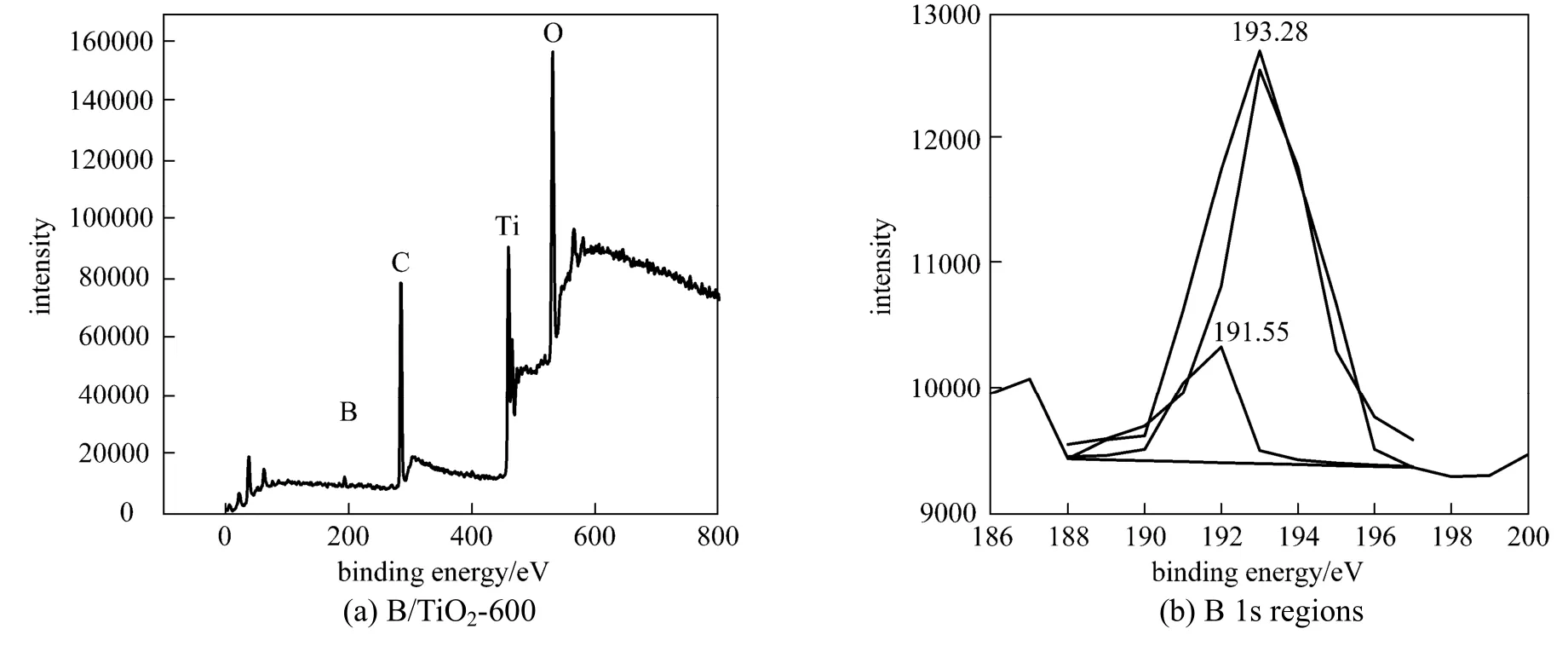
Figure 3 XPS spectra of B/TiO2-600
3.2 TEM analysis
Figure 2 shows the TEM images of the B-doped TiO2sample calcinated at 600 °C. The morphology, crystallite size and crystallographic planes of the particles are observed. The images show that the sample consists of large number of small particles with the size around 20 nm, which is in agreement with the XRD results calculated by the Scherrer equation. A corresponding selected area electron diffraction pattern (SADP) shows the Debye rings, exhibiting a polycrystalline nature of the particle. The Debye rings with d-values corresponding to 0.3512 nm (marked as 101), 0.2293 nm (marked as 004), 0.1841 nm (marked as 200), 0.1626 nm (marked as 211), 0.1442 nm (marked as 204), and 0.1297 nm (marked as 215) are assigned to the B/TiO2anatase phase.
3.3 XPS analysis
Figure 3 (a) shows the XPS spectra of B/TiO2-600. It contains only Ti, O, B, and C elements. Element C can be ascribed to the residual carbon from the precursor. Fig. 3 (b) shows the XPS B 1s spectrum of B/TiO2-600, which appears at around 191-193 eV. Based on previous study reported [13], the standard binding energy of B 1s in B2O3or H3BO3equals 193.0 eV (B O bond) and that in TiB2equals 187.5 eV (B Ti bond). The observed B 1s peak consists of two peaks. The first peak (191.55 eV) is related to TiO B bonds and the second peak (193.28 eV) is related to B O B bonds. XPS analysis confirms that boron ions partially incorporate into TiO2lattice after heat treatment to form a possible chemical environment like TiO B and the rest exist in the form ofB2O3. This is probably because the concentration of doped boron is beyond its solubility limit in the TiO2anatase structure. The boron ions expelled from the anatase structure could form nanoclusters on the surface of TiO2nanoparticles and grow slowly [13]. The B contents are given in Table 1. The influence of calcination temperature on boron content can be almost ignored. The average boron content in the surface layer is 14.5%.

Figure 4 Antimicrobial experiments of B/TiO2and pure TiO2nano-materials under visible light irradiation among different strains. (a) Candida albicans; (b) Staphylococcus aureus; (c) Escherichia coli; (d) B/TiO2nano-materials on Candida albicans in the dark
3.4 Study of antimicrobial effect of boron ions doped nano-TiO2
We tested the antimicrobial efficiency of B/TiO2by checking the ability of attached bacteria to form colonies in agar. Bacteria attached to a surface are able to duplicate, move and form colonies beyond the biofilm. Bacteria from the biofilm formed on the TiO2surface move and colonize the surrounding agar area forming a halo. From growing halo assays it is possible to have an indication about the viability of the bacteria attached to the surface [17].
The antimicrobial results are given in Fig. 4. Formation of inhibition zone confirms that all the B-doped TiO2nano-materials have antimicrobial activity, while pure TiO2nano-materials do not have. However, antimicrobial abilities of B-doped TiO2nano-materials prepared at different calcination temperatures are not the same. Calcinated at 600 and 700 °C, B/TiO2shows higher antimicrobial activity. B/TiO2-800 and B/TiO2-900 have moderate activity. Fig. 4 (d) shows that antimicrobial activity of B/TiO2nano-materials on Candida albicans in the dark is weaker than that in the visible light and the average inhibition zones against Candida albicans are 8.28, 7.31, 6.59, and 4.30 mm for B/TiO2-600, B/TiO2-700, B/TiO2-800, and B/TiO2-900, respectively.
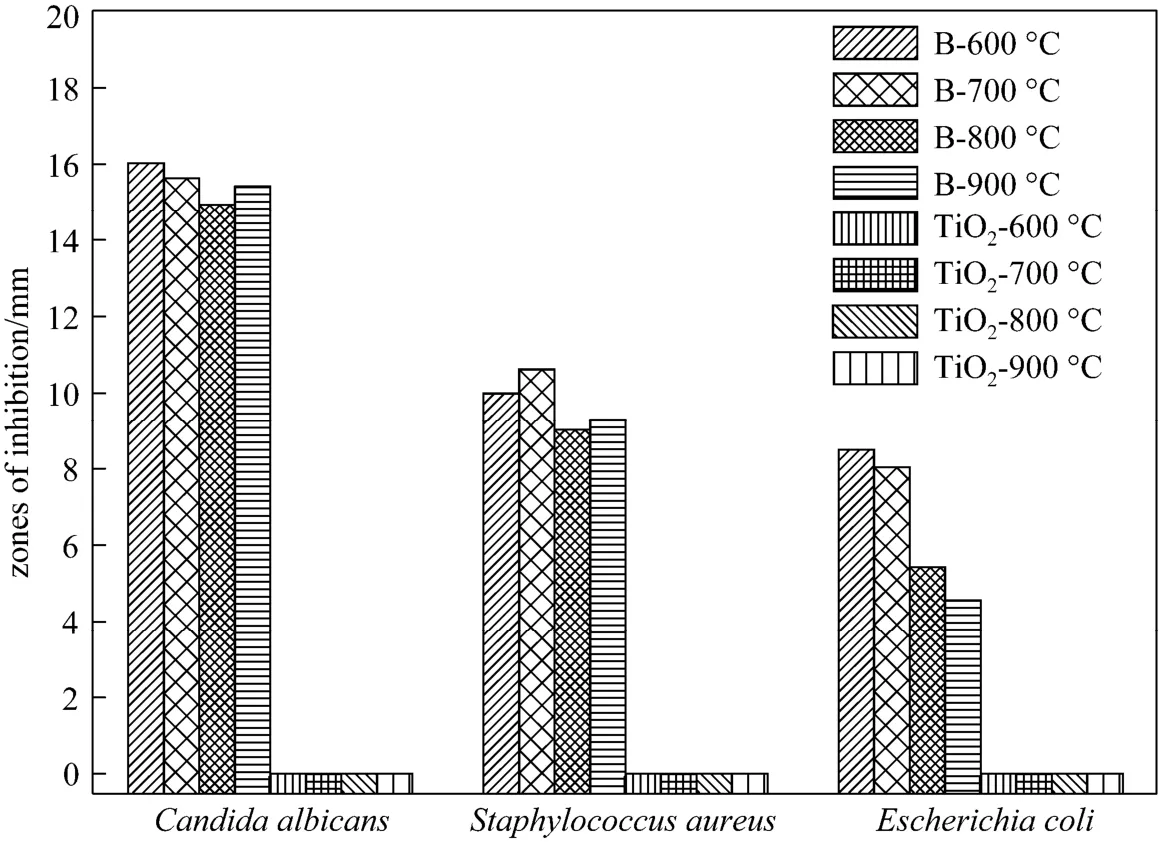
Figure 5 Antimicrobial experiment of B/TiO2nano-materials
For different strains, antimicrobial abilities of B/TiO2nano-materials are different (see Fig. 5). B/TiO2nano-materials present about 16 mm average inhibition zone against Candida albicans, 10 mm zone against Escherichia coli and 7 mm zone against Staphylococcus aureus. We can conclude that the antimicrobial action of the B/TiO2is more significant on fungal than on Gram-negative and Gram-positive bacteria. In one of the most important publications related to TiO2[18, 19], the reduction efficiency of TiO2coated Plexiglass depends on the cell wall thickness, with Gram-negative microorganisms killed easily and Gram-positive microorganisms more resistant. C. albicans is highly resistant to photocatalytic degradation due to its thick eukaryotic cell wall, while in our study, the antibacterial effect of boron-doped TiO2nano-materials on C. albicans is the best. This is a promising result especially for simple preparation route with incorporation of nano-materials.
In general, the bactericidal effect of TiO2is attributed to the decomposition of outer membranes of bacteria by reactive oxygen species, primarily hydroxyl radicals, which leads to phospholipid peroxidation and ultimately cell death. It is proposed that nano-materials that can physically attach to a cell are bactericidal when they contact with this cell. If the membrane of a bacterium is compromised, the cellmay repair itself; if the scratch is severe, the cell component may release and the cell will die eventually [20, 21]. capture electron hole pair and transform into free groups (·OH), causing the rupture of cell membrane [23]. (3) B atoms incorporated into TiO2lattice result in a charge imbalance and lattice distortion, so that TiO2generates surface oxygen vacancy, which also improves TiO2antimicrobial activity [24].
4 CONCLUSIONS
In this study, highly effective boron-doped TiO2antimicrobial materials were synthesized by a sol-gel method. The anatase is predominant in crystal planes of the TiO2calcinated at 600 and 700 °C, and rutile phases are predominant at calcination temperature of 900 °C. Part of boron ions incorporate into TiO2lattice, forming a possible chemical environment like TiO B and the rest exist in the form of B2O3. The B/TiO2nano-materials exhibit high antibacterial efficiency. Their antibacterial action is more significant on fungal than on Gram-negative and Gram-positive. Antimicrobial activities of B/TiO2materials on Candida albicans in the dark are weaker than that in the visible light.
REFERENCES
The boron-doped TiO2nano-particles under visible light have strong antimicrobial activity, probably due to the following points. (1) XPS show that part of B exists in the form of TiO B with boron atoms incorporated into the TiO2lattice, so B p and O 2p form with mixed valence band and the band gap narrows, leading to visible light response [22]. (2) Due to B3+electron deficient character, Lewis acid on the B/TiO2surface enhances and OH−increases. OH−can
1 Li, B., Wang, X., Chen, R.X., Hang, W.G., Xie, G.L., “Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima”, Carbohydr. Polym., 72 (2/5), 287-292 (2008).
2 Chi, G.J., Yao, S.W., Fan, J., Zhang, W.G., Wang, H.Z., “Antibacterial surface treatment of aluminum materials”, Chin. J. Chem. Eng., 10 (5), 622-624 (2002).
3 Zhang, J.L., Sun, D.H., Zhan, G.W., Lin, L.Q., Zheng, Y.M., Jing, X.L., Huang, J.L., Li, Q.B., “In-situ synthesis and antibacterial immobilized on aspergillus niger”, J. Chem. Ind. Eng., 63 (7), 2271-2278 (2012). (in Chinese)
4 Wang, F.M., Shi, Z.S., Gong, F., Jiu, J.T., Adachi, M., “Morphology control of anatase TiO2by surfactant-assisted hydrothermal method”, Chin. J. Chem. Eng., 15 (5), 754-759 (2007).
5 Zhou, Y.S., Jiang, W.G., “Study on properties of composite oxides TiO2/SiO2”, Chin. J. Chem. Eng., 10 (3), 349-353 (2002).
6 Ling, Q.C., Sun, J.Z., Zhou, Q.Y., “Preparation and characterization of visible-light-driven titania photocatalyst co-doped with boron and nitrogen”, Appl. Surf. Sci., 254 (10), 3236-3248 (2008).
7 Matsunaga, T., Tomoda, R., Nakajima, T., Wake, H., “Photoelectrochemical sterilization of microbial cells by semiconductor powders”, FEMS Microbiol. Lett., 29 (1/2), 211-214 (1985).
8 MacFarlanea, J.W., Jenkinsonb, H.F., Scotta, T.B., “Sterilization of microorganisms on jet spray formed titanium dioxide surfaces”, Appl. Catal., B: Environ., 106 (1/2), 181-185 (2011).
9 Ma, Y.F., Zhang, J.L., Tian, B.Z., Chen, F., Wang, L.Z., “Synthesis and characterization of thermally stable Sm, N co-doped TiO2with highly visible light activity”, J. Hazard. Mater., 182 (1/3), 386-393 (2010).
10 Gu, L.Y., Wang, Y.P., Peng, P.Y., Wang, L.J., “Preparation of S and metal co-doped TiO2and their photocatalytic activities”, Chin. J. Nonferrous Met., 19 (5), 911-918 (2009). (in Chinese)
11 Yang, K., Gu, J.H., Zhang, Y., “Visible light catalytical property and mechanism of carbon doped TiO2thin film”, Rare Met. Mater. Eng., 38(2), 759-761 (2009). (in Chinese)
12 Ding, H., Zhang, N., Rong, F., Fu, D.G., “Preparation, characterization and bactericidal activity of N-F-codoped TiO2Film”, J. Inorg. Mater., 26 (5), 517-522 (2011). (in Chinese)
13 Zhao, W., Ma, W.H., Chen, C.S., Zhao, J.C., Shuai, Z.G., “Efficient Degradation of Toxic organic pollutants with Ni2O3/TiO2-xBxunder visible irradiation”, J. Am. Chem. Soc., 126 (15), 4782-4783 (2004).
14 Chen, D.M., Yang, D., Wang, Q., Jiang, Z.Y., “Effects of boron doping on photocatalytic activity and microstructure of titanium dioxide nanoparticles”, Ind. Eng. Chem. Res., 45 (12), 4110-4116 (2006).
15 Zaleska, A., Grabowska, E., Sobczak, J. W., Gazda, M., Hupka, J.,“Photocatalytic activity of boron-modified TiO2under visible light: The effect of boron content, calcination temperature and TiO2matrix”, Appl. Catal. B: Environ., 89 (3/4), 469-475 (2009).
16 Mai, L.X., Wang, D.W., Zhang, S., “Synthesis and bactericidal ability of Ag/TiO2composite films deposited on titanium plate”, Appl. Surf. Sci., 257 (3), 974-978 (2009).
17 Flores, C.Y., Diaz, C., Rubert, A., Benítez, G.A., Moreno, M.S., Salvarezza, R.C., Schilardi, P.L., Vericat, C., “Spontaneous adsorption of silver nanoparticles on Ti/TiO2surfaces antibacterial effect on pseudomonas aeruginosa”, J. Colloid Interf. Sci., 350 (2), 402-408 (2010).
18 Kühn, K.P., Chaberny, I.F., Massholder, K., Stickler, M., Benz, V.W., Sonntag, H.G., Erdinger, L., “Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light”, Chemosphere, 53 (1), 71-77 (2003).
19 Sömena, M., Tatlıdil, I., Breenb, C., Cleggb, F., Buruk, C.K., Sivlima, T., Akkana, S., “A new nano-TiO2immobilized biodegradable polymer with self-cleaning properties”, J. Hazard. Mater., 187 (1/3), 199-205 (2011).
20 Rajakumara, G., Abdul Rahumana, A., Mohana Roopanb, S., Gopiesh Khannac, V., Elangoa, G., Kamaraja, C., Abduz Zahira, A., Velayuthama, K., “Fungus-mediated biosynthesis and characterization of TiO2nanoparticles and their activity against pathogenic bacteria”, Spectrochim. Acta Part A, 91, 23-29 (2012).
21 Haghighia, N., Abdia, Y., Haghighi, F., “Light-induced antifungal activity of TiO2nanoparticles/ZnO nanowires”, Appl. Surf. Sci., 257 (23), 10096-10100 (2011).
22 Grabowska, E., Zaleska, A., Sobczak, J.W., Hupka, J., “Boron-doped TiO2: Characteristics and photoactivity under visible light”, Procedia Chem., 1 (2), 1553-1559 (2009).
23 Long, H.J., Meng, Q.J., Yuan, J., Yang, W.S., Cao, Y.A., “Study on photocatalytic activity of boron doped TiO2catalyst (TiO2-xBx)”, Acta Chim. Sinica, 66 (6), 657-661 (2008). (in Chinese)
24 Wei, F.Y., Ni, L.S., “Photocatalytic performance and doping mechanism of B-S co-doped TiO2”, Chin. J. Catal., 28 (10), 905-909 (2007). (in Chinese)
2012-10-19, accepted 2013-02-27.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
- Effects of Assistant Solvents and Mixing Intensity on the Bromination Process of Butyl Rubber*
- Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF−in Six Alcohols*
